Carbapenem-resistant Gram-negative bacteria represent the most concerning problem in the field of antimicrobial resistance. Carbapenem-resistant Enterobacterales (CRE) can cause a range of hospital-acquired infection types, the most common of which are bloodstream infection (BSI), hospital-acquired and ventilator-associated bacterial pneumonia, intra-abdominal infection (IAI), and urinary tract infection (UTI) [].
The emergence and spread of CRE have left limited therapeutic options for severe infections; accordingly, these organisms are one of the leading causes of morbidity and mortality in vulnerable patients. Patients with CRE have mortality rates 2- to 3-fold higher than those with infections caused by carbapenem-susceptible Enterobacterales. Higher mortality rates among patients with CRE is likely driven by delays in the administration of prompt, appropriate empiric therapy [].
Carbapenem resistance among Enterobacterales is primarily due to the production of carbapenemases. The carbapenemases produced by Enterobacterales can be divided in 2 groups for therapeutic purposes: the serine carbapenemases, such as Klebsiella pneumoniae carbapenemases (KPCs) and oxacillinase (OXA)-48-type, and the metallo-β-lactamases (MBLs). The KPC family of carbapenemases emerged on the East coast of the United States in the late 1990s and is now the most common carbapenemase detected globally []. In the mid-2010s, a KPC was observed in more than 90% of carbapenemase-producing Enterobacterales (CPE) isolated in US hospitals []. However, because the epidemiology of resistance mechanisms is very dynamic, continuing to monitor CRE in US medical centers is critical to plan therapeutic strategies and infection control measures.
Although many β-lactamase inhibitor combinations have been approved recently by the US Food and Drug Administration (FDA) for use in combination with various β-lactams, none of the current, clinically available compounds are active against MBL-producing Enterobacterales [].
One strategy to overcome MBL-derived resistance is to combine an MBL-stable β-lactam with a serine carbapenemase inhibitor, because MBL-producing Enterobacterales isolates generally coproduce extended-spectrum β-lactamases (ESBLs), AmpC β-lactamases, and/or serine carbapenemases []. Therefore, the combination of aztreonam with avibactam has showed great potential for successfully treating infections caused by MBL-producing Enterobacterales []. We assessed the in vitro activity of aztreonam-avibactam and comparators, including ceftazidime-avibactam and meropenem-vaborbactam, against a large collection of clinical Enterobacterales isolates from US hospitals. We also evaluated the epidemiology of carbapenemases among CRE isolates identified in this collection.
METHODS
Organism Collection
Bacterial isolates were collected via the INFORM Antimicrobial Surveillance Program and sent to JMI Laboratories (North Liberty, IA) for susceptibility testing. Each participating center was asked to collect consecutive bacterial isolates from patients hospitalized with the following infection types: BSI, pneumonia, skin and skin structure infection (SSSI), UTI, and IAI. Isolates could be from any specimen type and were determined to be significant by local criteria as the reported probable cause of infection.
A total of 27 834 Enterobacterales isolates were collected consecutively in 2019–2021. Sixty-nine medical centers participated in the study in 2019 and 2020, whereas 68 centers participated in 2021. Moreover, 61 medical centers contributed isolates all 3 years, 8 medical centers participated for 2 years, and 5 medical centers contributed isolates for only 1 year. When a medical center could not continue to participate, it was replaced by another center in the same US Census Division, preferably from the same state. Overall, a total of 74 medical centers distributed throughout 36 states contributed to this investigation. Only isolates determined to be significant by local criteria as the reported probable cause of infection were included in the program. The most common Enterobacterales species were Escherichia coli (n = 9573; 34.4%), K pneumoniae (n = 5791; 20.8%), Enterobacter cloacae species complex (n = 2432; 8.7%), Proteus mirabilis (n = 2215; 8.0%), Klebsiella oxytoca (n = 1694; 6.1%), indole-positive Proteeae (n = 1652; 5.9%), Serratia marcescens (n = 1244; 4.5%), and Klebsiella aerogenes (n = 1048; 3.8%). Species identification was confirmed by using standard biochemical tests and/or a MALDI Biotyper (Bruker Daltonics, Billerica, MA), when necessary.
Susceptibility Testing
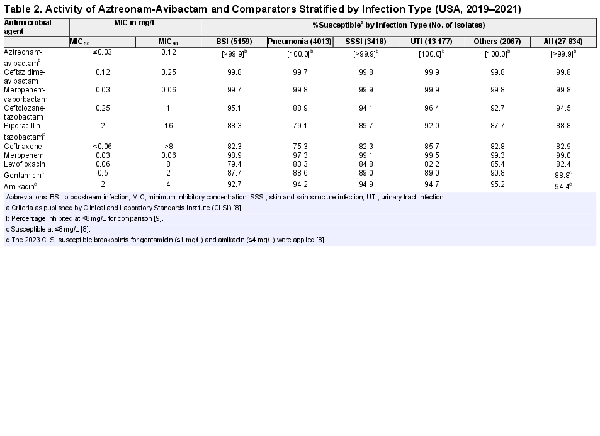
All isolates were susceptibility tested by the reference broth microdilution method specified by Clinical and Laboratory Standards Institute (CLSI) standards []. The antimicrobial susceptibility and frequency of key resistance phenotypes were assessed and stratified by year and infection type: BSI (5159 isolates; 18.5%), pneumonia (4013; 14.4%), SSSI (3418; 12.3%), UTI (13 177; 47.3%), or other (2067; 7.4%).
Aztreonam/avibactam was tested with avibactam at a fixed concentration of 4 mg/L. A tentative aztreonam/avibactam pharmacokinetic/pharmacodynamic-susceptible breakpoint of ≤8 mg/L was applied for comparison []. Moreover, the susceptible/resistant breakpoints approved by CLSI (M100 33rd edition; 2023) for gentamicin (≤1/≥4 mg/L) and amikacin (≤4/≥16 mg/L) were applied []. Carbapenem-resistant Enterobacterales was defined as displaying imipenem or meropenem minimum inhibitory concentration (MIC) values of ≥4 mg/L. Imipenem was not applied to P mirabilis or indole-positive Proteeae due to their intrinsically elevated MIC values. All tests were conducted in a central monitoring laboratory (JMI Laboratories). Multidrug resistant (MDR) was defined as nonsusceptible (CLSI breakpoints) to at least 3 antimicrobial classes and extensively drug resistant (XDR) as susceptible to ≤2 classes []. Concurrent quality control testing was performed to ensure proper test conditions and procedures.
β-Lactamase Screening and Molecular Characterization of Isolates With Decreased Susceptibility to Aztreonam/Avibactam
All CRE isolates (n = 261) were tested for β-lactamase-encoding genes by applying genome sequencing and in silico screening, as previously described []. Total genomic deoxyribonucleic acid (DNA) was extracted and purified using the KingFisher Cell and Tissue DNA kit (Thermo Fisher Scientific, Waltham, MA) in a KingFisher Flex Magnetic Particle Processor (Thermo Fisher Scientific) workstation. Total genomic DNA was used as input material for library construction and sequencing using either the Nextera XT library construction protocol and index kit on a MiSeq Sequencer (Illumina, San Diego, CA) with a MiSeq Reagent Kit v3 (600 cycles) or the Illumina DNA library construction protocol and index kit on a NextSeq 1000 Sequencer (Illumina) using NextSeq1000/2000 P2 Reagents (300 cycles).
FASTQ format files for each sample set were assembled independently using the de novo assembler SPAdes 3.15.3 with K-values of 21, 33, 55, 77, and 99 plus careful mode on to reduce the number of mismatches. This process produced a FASTA format file of contiguous sequences with the best N50 value. An in-house proprietary bioinformatics pipeline and a JMI-curated resistance gene database based on the National Center for Biotechnology Information Bacterial Antimicrobial Resistance Reference Gene Database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047) was used for the in silico screening of β-lactamase genes. These genes were used as queries to align β-lactamase resistance determinants against the target assembled sequences. Hits with identities greater than 94% and 40% minimum coverage length were selected for further analysis and the final assignment of β-lactamase alleles [12,13].
RESULTS
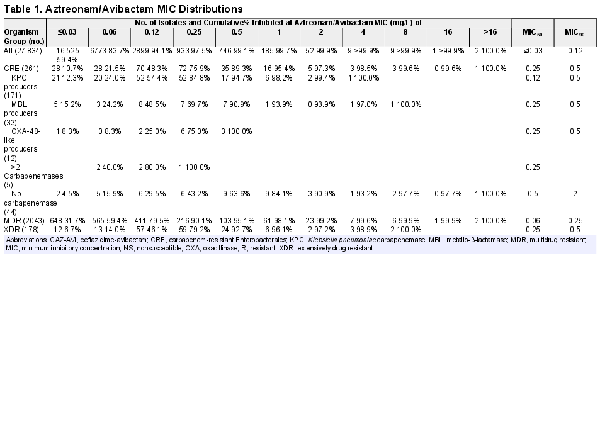
Aztreonam-avibactam (MIC50/90, ≤ 0.03/0.12 mg/L) inhibited >99.9% of isolates at ≤8 mg/L (99.9% at ≤2 mg/L), including 99.6% of CRE (MIC50/90, 0.25/1 mg/L; 97.3% inhibited at ≤2 mg/L), 99.9% of MDR (MIC50/90, 0.06/0.5 mg/L), and 100.0% of XDR (MIC50/90, 0.25/0.5 mg/L) isolates (Table 1). Only 3 isolates (0.01%) had an aztreonam-avibactam MIC >8 mg/L: an E coli from a SSSI collected in New York in 2019, an E coli from a BSI collected in Texas in 2020, and a K aerogenes from a BSI collected in Kentucky in 2021.
Ceftazidime-avibactam (MIC50/90, 0.12/0.25 mg/L) and meropenem-vaborbactam (MIC50/90, 0.03/0.06 mg/L) were also very active against this collection of Enterobacterales, with 99.8% susceptibility overall for both compounds (Table 2). The only other compounds active against >90% of these Enterobacterales isolates were meropenem (MIC50/90, 0.03/0.06 mg/L; 99.0% susceptible), ceftolozane-tazobactam (MIC50/90, 0.25/1 mg/L; 94.5% susceptible), and amikacin (MIC50/90, 2/4 mg/L; 94.4% susceptible at ≤4 mg/L) (Table 2). Susceptibility rates for the β-lactam compounds were lower among isolates from pneumonia compared with other infection types. In contrast, the lowest susceptibility rates for levofloxacin, gentamicin, and amikacin were observed among isolates from BSI (Table 2).
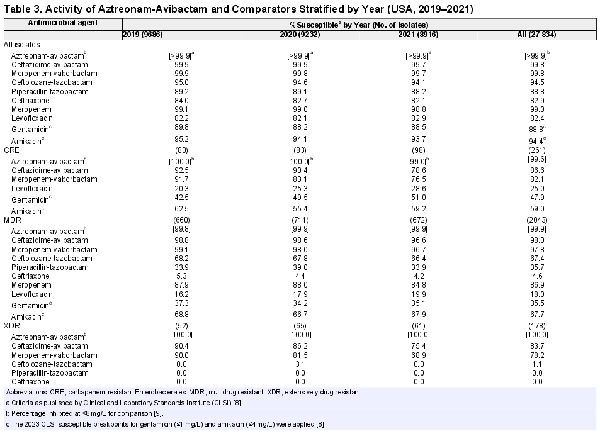
Aztreonam-avibactam activity remained stable, but susceptibility to other compounds, except for levofloxacin, decreased slightly during the period of the investigation (Table 3). Carbapenem-resistant Enterobacterales, MDR, and XDR rates stratified by infection type and year are shown in Supplementary Table 1. Carbapenem-resistant Enterobacterales rates increased from 0.8% in 2019 to 1.1% in 2021 (Supplementary Table 1), but yearly changes in carbapenem resistance varied markedly among Enterobacterales species (Figure 1). The highest increases in carbapenem resistance were observed with K aerogenes (from 0.6% in 2019 to 2.8% in 2021) and E cloacae (from 1.1% in 2019 to 1.9% in 2021) (Figure 1). Carbapenem resistance also increased among E coli and K pneumoniae, remained somewhat stable among K oxytoca and S marcescens, and decreased markedly among Citrobacter freundii (Figure 1). It is notable that CRE susceptibility to ceftazidime-avibactam and meropenem-vaborbactam decreased from 92.5% and 91.7% in 2019 to 78.6% and 76.5% in 2021, respectively (Table 3). Moreover, CRE susceptibility to amikacin was 81.2% when the 2022 CLSI breakpoint (≤16 mg/L) was applied (data not shown) but declined to only 59.0% when the 2023 CLSI breakpoint (≤4 mg/L) was used (Table 3).
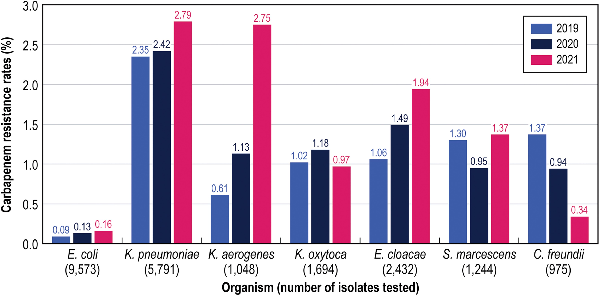
Figure 1
Carbapenem resistance rates stratified by species. C freundii, Citrobacter freundii; E cloacae, Enterobacter cloacae; E coli, Escherichia coli; K aerogenes, Klebsiella aerogenes; K pneumoniae, Klebsiella pneumoniae; K oxytoca, Klebsiella oxytoca; S marcescens, Serratia marcescens.
The MDR and XDR rates increased from 6.8% and 0.5% in 2019 to 7.5% and 0.7% in 2021, respectively (Supplementary Table 1). Aztreonam-avibactam, ceftazidime-avibactam, and meropenem-vaborbactam were the most active compounds against MDR and XDR isolates. Aztreonam-avibactam showed almost complete activity against these organism subsets (99.9%–100.0% susceptibility). Ceftazidime-avibactam and meropenem-vaborbactam retained potent activity against MDR isolates, with susceptibility rates of 98.0% and 97.8%, respectively (Table 3). In contrast, the activity of these 2 β-lactamase inhibitor combinations against XDR isolates decreased markedly from 2019 (90.0%–90.4% susceptible) to 2021 (68.9%–75.4% susceptible). Amikacin was active against 67.7% of MDR and 36.5% of XDR isolates when the CLSI revised breakpoint of ≤4 mg/L was applied (Table 3).
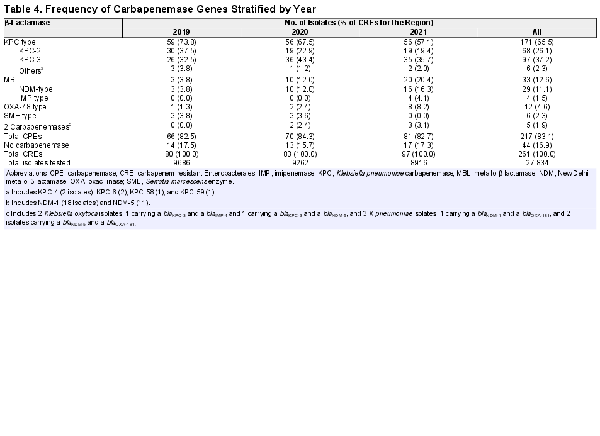
The most common carbapenemase among CRE isolates were KPC (65.5% of CRE), followed by New Delhi metallo-β-lactamase ([NDM] 11.1%), OXA-48-like (4.6%), Serratia marcescens enzyme ([SME] 2.3%), and imipenemase (1.5%). Five (1.9% of CRE) isolates had 2 carbapenemases; 44 (16.9% of CRE) isolates did not have carbapenemase genes identified (Table 4). It is interesting to note that the percentages of CRE-producing KPC carbapenemase decreased from 73.8% in 2019% to 57.1% in 2021, whereas the percentages of CRE isolates producing MBLs and OXA-48-like increased markedly from 3.8% and 1.3% in 2019 to 20.4% and 8.2% in 2021, respectively (Table 4 and Figure 2). The distribution of CPE types by species is shown in Supplementary Figure 1. The most noticeable increase in the frequency of MBLs was observed among carbapenem-resistant E cloacae where it increased from 0.0% in 2019 to approximately 40.0% in 2020 and 2021 (data not shown). Among carbapenem-resistant K pneumoniae, the frequency of MBLs increased from 2.0% in 2019 to 7.1% in 2020 and 12.2% in 2021, whereas the frequency of OXA-48-like increased from 2.0% in 2019 to 7.1% in 2020 and 17.4% in 2021. It is notable that the increase of carbapenem-resistant K aerogenes was mainly due to non-CPE-producing strains, which represented 87.5% of isolates (Supplementary Figure 1).
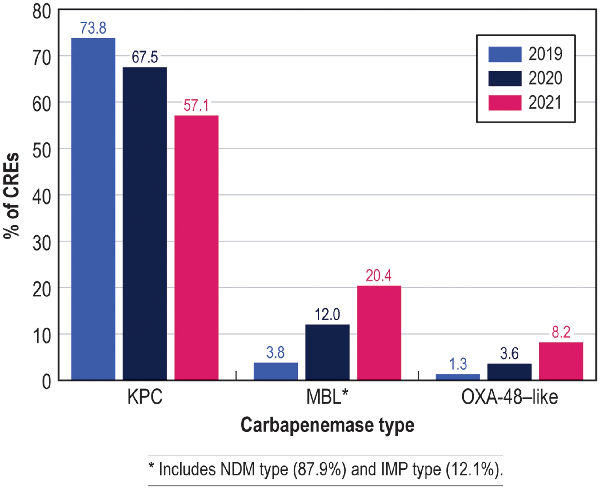
Figure 2
Frequencies of isolates producing Klebsiella pneumoniae carbapenemase (KPC), metallo-β-lactamase (MBL), and oxacillinase (OXA)-48 types of carbapenemases among carbapenem-resistant Enterobacterales (CRE) from US medical centers stratified by year (2019–2021). *Includes New Delhi metallo-β-lactamase (NDM) type (87.9%) and imipenemase (IMP) type (12.1%).
Sequence type (ST) 258 predominated among K pneumoniae; it was observed in 54.6% of KPC producers, but not noted among MBL or OXA-48-like producers. Among E cloacae, 18.2% of KPC producers belonged to ST171 and another 18.2% belonged to ST45, whereas STs 114 (36.4%), 171 (18.2%), and 270 (27.3%) predominated among MBL producers. There was no predominant ST among E coli. A summary of the predominant STs for the most frequent carbapenem-resistant species stratified by carbapenemase type and year is shown in Supplementary Table 2.
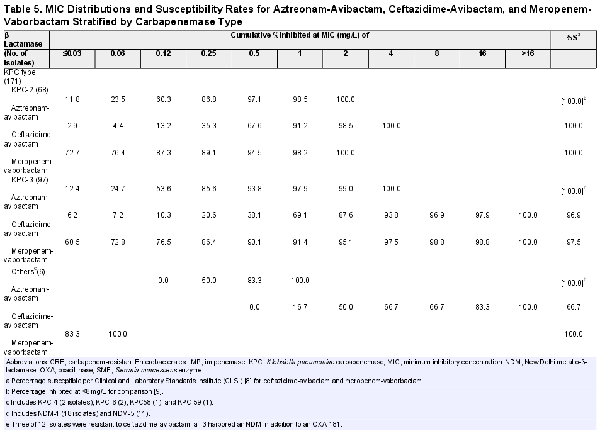
The MIC distributions and susceptibility rates for aztreonam-avibactam, ceftazidime-avibactam, and meropenem-vaborbactam stratified by carbapenemase type are shown in Table 5. Aztreonam/avibactam exhibited potent activity against isolates producing KPC (n = 171; MIC50/90, 0.12/0.5 mg/L; highest MIC, 4 mg/L), MBL (n = 33; MIC50/90, 0.25/0.5 mg/L; highest MIC, 8 mg/L), and/or OXA-48-like (n = 12; MIC50/90, 0.25/0.5 mg/L; highest MIC, 0.5 mg/L), as well as against noncarbapenemase-producing CRE (n = 44; MIC50/90, 0.12/0.5 mg/L; 97.7% inhibited at ≤8 mg/L and 90.3% inhibited at ≤2 mg/L) (Tables 1 and 5). Ceftazidime-avibactam and meropenem-vaborbactam retained good activity against KPC and SME producers but showed limited activity against MBL producers. Moreover, ceftazidime-avibactam exhibited greater activity than meropenem-vaborbactam against OXA-48-like producers (Table 5). Three of 12 OXA-48-like producers were resistant to ceftazidime-avibactam, and all 3 harbored an NDM in addition to an OXA-181.
Regarding the 3 isolates with elevated (>8 mg/L) aztreonam-avibactam MIC values, 2 E coli each contained blaCMY transferrable cephalosporinases and similar mutations in their OmpC and PBP3 proteins, among other resistance determinants. Both isolates shared numerous mutations throughout and an 8-residue deletion in OmpC. Each isolate contained a YRIK insertion at position Y333 in PBP3, among other mutations. One E coli (ST10886; aztreonam-avibactam MIC, >16 mg/L) possessed a CMY-16 (G176D) variant, a CTX-M-15, an OXA-181 carbapenemase, and a truncated OmpF outer membrane protein. The other E coli (ST410; aztreonam-avibactam MIC, 16 mg/L) expressed CMY-42, CTX-M-15, and OXA-1/30. The K aerogenes isolate (ST176; aztreonam-avibactam MIC, >16 mg/L) possessed multiple mutations in its chromosomal ampC and expressed a truncated OmpC.
DISCUSSION
The results of this investigation show a significant increase in the prevalence of CRE and a marked change in the epidemiology of CPE in US medical centers, with an important decline of KPC producers and a concurrent increase of MBL, mainly NDM type, and OXA-48-like producers. Many studies have demonstrated the predominance of KPC producers among CRE isolates from various US regions []. Satlin et al [] characterized patients with CRE bacteremia in 2013 at 8 New York/New Jersey medical centers and determined that 90% of the CRE were K pneumoniae and 92% produced a KPC (KPC-2, 44%; KPC-3, 48%). It is notable that the same group found a lower proportion of KPC producers when evaluating CRE isolates causing bacteremia in 2016–2018 []. Karlsson et al [] evaluated 419 CRE isolates collected from 8 US medical centers in 2011–2015 and showed that a KPC variant was observed in 97% of CPEs. The predominance of KPC has been attributed primarily to the spread of K pneumoniae isolates belonging to the successful clonal complex 258 [3,4]. It is remarkable that no predominant lineage among organisms that produced MBL or OXA-48-like was detected.
This reported shift of carbapenemase epidemiology may further limit the therapeutic options for CRE infections in US hospitals. The approval of ceftazidime-avibactam for clinical use in 2014 represented significant progress in the treatment of CRE infections. Ceftazidime-avibactam provides excellent coverage against CRE infections in geographic regions where serine carbapenemases, such as KPC and OXA-48-like, represent the main mechanism of carbapenem resistance [16,17]. Meropenem-vaborbactam and imipenem-relebactam, which were approved more recently, are very active against KPC-producing Enterobacterales, but they have limited activity against OXA-producing CRE []. Furthermore, none of the β-lactamase inhibitor combinations currently approved for clinical use are active against MBL-producing Enterobacterales [].
We evaluated the antimicrobial susceptibility of 27 834 Enterobacterales isolates consecutively collected from 74 medical centers in 36 states. The collection included 261 CRE isolates from 44 medical centers in 24 states. One of most remarkable findings of this investigation was the continued decrease in the activity of currently available β-lactamase inhibitor combinations, such as ceftazidime-avibactam and meropenem-vaborbactam, against CRE isolates from US medical centers in the last few years. Ceftazidime-avibactam and meropenem-vaborbactam had shown almost complete activity against CRE isolates from US hospital in recent investigations [17,19]. The decreased activity of these β-lactamase inhibitor combinations is related to the increased frequency of carbapenemases not inhibited by these inhibitors [3,18,20]. Cefiderocol is the only β-lactam compound with activity against MBL-producing strains, including those that coproduce ESBLs and/or serine carbapenemases, currently approved by the US FDA and European Medicines Agency []. The fact that cefiderocol was not tested as part of this investigation represents a limitation of the study, but cefiderocol powder was not available when this study was performed. Another limitation of the study is the fact that some medical centers did not participate in all 3 years of the investigation. However, when a medical center could not continue to participate in the program, it was replaced by a medical center located in the same region. Moreover, when medical centers that did not participate in all 3 years of the study were excluded from the analysis, yearly CRE rates were very similar (0.9%, 0.9%, and 1.2% for 2019, 2020, and 2021, respectively [data not shown]), and yearly susceptibility rates for the 3 new β-lactamase inhibitor combinations varied only by 0.0%–1.8% (data not shown). Thus, it is very unlikely that this limitation introduced significant bias to the study.
A decrease in the activity of newer β-lactamase inhibitor combinations has been observed in geographic regions where MBLs and OXA-48-like carbapenemases are more common, such as Eastern Europe, Asia, and Latin America []. We recently evaluated 1098 CRE isolates that were identified among 24 924 Enterobacterales from 69 medical centers in 36 countries located in Western Europe (W-EU), Eastern Europe (E-EU), Latin America (LATAM), and the Asia-Pacific region (APAC) []. Although the main mechanism of carbapenem resistance in all 4 geographic regions was the production of a carbapenemase, the type of carbapenemase varied substantially by region. The KPCs predominated in W-EU (66.5% of CRE) and LATAM (70.0% of CRE), whereas MBLs predominated in APAC (61.6% of CREs). A variety of carbapenemase types were observed in E-EU. Overall, KPC, MBL, and OXA-48-like represented 25.6%, 29.5%, and 31.7% of CRE isolates from E-EU, respectively. Consequently, meropenem-vaborbactam exhibited good activity against CRE isolates from W-EU (77.5% susceptible per CLSI) and LATAM (78.8% susceptible per CLSI) but limited activity against CRE isolates from both E-EU (50.1% susceptible per CLSI) and APAC (40.3% susceptible per CLSI). In contrast, aztreonam-avibactam was active against 99.6% of CRE isolates from all regions combined, including 100.0% of the MBL producers [].
It is also important to note that our results support the clinical use of ceftazidime-avibactam plus aztreonam for the treatment of infections caused by MBL producers although aztreonam-avibactam is not approved for clinical use. Falcone et al [] evaluated 102 patients with BSI caused by MBL-producing Enterobacterales and showed favorable impacts on their clinical outcomes when they were treated with ceftazidime-avibactam plus aztreonam.
CONCLUSIONS
In summary, aztreonam-avibactam demonstrated potent activity against a large collection of contemporary CRE isolates from US hospitals, including MBL producers and isolates resistant to ceftazidime-avibactam and/or meropenem-vaborbactam. The results of this investigation emphasize that resistance phenotypes and resistance mechanisms must continue to be monitored via large, well-designed surveillance programs, such as INFORM. Due to the clinical importance of these rapid fluctuations in the epidemiology of β-lactam resistance mechanisms, the results of comprehensive surveillance programs are critical to plan therapeutic strategies and infection control measures.
Acknowledgments
We thank all participants of the INFORM Antimicrobial Surveillance Program for their work in providing isolates. Editorial support was provided by Amy Chen at JMI Laboratories and was funded by AbbVie.
Financial support. This study was supported by AbbVie.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 7 October 2022.
- 2. Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis2018; 5:ofy150. https://doi.org/10.1093/ofid/ofy150
- 3. Bush K, Bradford PA. Epidemiology of beta-lactamase-producing pathogens. Clin Microbiol Rev2020; 33:e00047.
- 4. Karlsson M, Lutgring J, Ansari U, et al Molecular characterization of carbapenem-resistant enterobacterales collected in the United States. Microb Drug Resist2022; 28:389–97.
- 5. Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis2021; 40:2053–68.
- 6. Falcone M, Daikos GL, Tiseo G, et al Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by MBL-producing Enterobacterales. Clin Infect Dis2021; 72:1871–8.
- 7. Mauri C, Maraolo A, Di Bella S, Luzzaro F, Principe L. The revival of aztreonam in combination with avibactam against metallo-beta-lactamase-producing Gram-negatives: a systematic review of in vitro studies and clinical cases. Antibiotics (Basel)2021; 10:1012.
- 8. CLSI. M100. Performance standards for antimicrobial susceptibility testing. 33rd Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2023.
- 9. Cornely OA, Cisneros JM, Torre-Cisneros J, et al Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: results from the REJUVENATE study. J Antimicrob Chemother2020; 75:618–27.
- 10. Magiorakos AP, Srinivasan A, Carey RB, et al Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect2012; 18:268–81.
- 11. Mendes RE, Jones RN, Woosley LN, Cattoir V, Castanheira M. Application of next-generation sequencing for characterization of surveillance and clinical trial isolates: analysis of the distribution of beta-lactamase resistance genes and lineage background in the United States. Open Forum Infect Dis2019; 6(Suppl 1):S69–78.
- 12. Bankevich A NS, Antipov D, Gurevich AA, et al SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol2012; 19:455–77.
- 13. Camacho C CG, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics2009; 10:421.
- 14. Satlin MJ, Chen L, Patel G, et al Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother2017; 61:e02349.
- 15. Satlin MJ, Chen L, Gomez-Simmonds A, et al Impact of a rapid molecular test for Klebsiella pneumoniae carbapenemase and ceftazidime-avibactam use on outcomes after bacteremia caused by carbapenem-resistant enterobacterales. Clin Infect Dis2022; 75:2066–75.
- 16. Kazmierczak KM, Bradford PA, Stone GG, de Jonge BLM, Sahm DF. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance program from 2012 to 2015. Antimicrob Agents Chemother2018; 62:e00592.
- 17. Sader H, Flamm R, Carvalhaes C, Castanheira M. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017–2018). Diagn Microbiol Infect Dis2020; 96:114833.
- 18. Bush K, Bradford PA. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol2019; 17:295–306.
- 19. Carvalhaes CG, Shortridge D, Sader HS, Castanheira M. Activity of meropenem-vaborbactam against bacterial isolates causing pneumonia in patients in U.S. hospitals during 2014 to 2018. Antimicrob Agents Chemother2020; 64:e02177.
- 20. Castanheira M, Doyle TB, Collingsworth TD, Sader HS, Mendes RE. Increasing frequency of OXA-48-producing enterobacterales worldwide and activity of ceftazidime/avibactam, meropenem/vaborbactam and comparators against these isolates. J Antimicrob Chemother2021; 76:3125–34.
- 21. Timsit J, Paul M, Shields R, et al Cefiderocol for the treatment of infections due to metallo-beta-lactamase-producing pathogens in the CREDIBLE-CR and APEKS-NP phase 3 randomized studies. Clin Infect Dis2022; 75:1081–4.
- 22. Sader H, Castanheira M, Kimbrough J, Kantro V, Mendes R. Aztreonam-avibactam activity against a large collection of carbapenem-resistant enterobacterales (CRE) collected in hospitals from Europe, Asia, and Latin America (2019–2021). JAC Antimicrobial Resistance 2023 [In press].