Subjects infected with human immunodeficiency virus (HIV)-1 in the perinatal period now live with the virus for several decades due to progress in antiretroviral therapy. The immune system of these patients, born at the beginning of the epidemic, have developed in the presence of an immunosuppressive virus and chronic exposure to inflammatory stimuli and antiretroviral drugs. The immune status of perinatally infected patients who reach adulthood has been much less described than that of patients infected as adults. These patients are infected with viruses that were selected to escape CD8 T cells restricted by the human leukocyte antigen (HLA) molecules shared with their mothers []. Infants have CD8 T cells with lower antiviral activity during primary infection than adults. Human immunodeficiency virus-specific CD4 and CD8 T cells exert some control over viral replication, even in successfully treated patients, as shown by the acquisition of mutations in CD8 T-cell epitopes [], and by the evolution of the T-cell receptor Vβ repertoire []. Human immunodeficiency virus-specific T cells may play an important role in the destruction of infected cells after the reversal of viral latency in future therapeutic strategies that target the viral reservoir [, ].
We initiated the ANRS-EP38-IMMIP study to assess the immune and virological status of adolescents and young adults infected with HIV-1 during the perinatal period [, ]. We previously reported a modest frequency of youths with Gag-specific CD8 T-cell proliferation, which was greater in patients of black ethnicity []. Youths with Gag-specific CD8 T-cell proliferation who had undetectable plasma HIV-1 ribonucleic acid (RNA) at the time of the study (aviremic patients) had more recent exposure to viral replication. In addition to the level of cognate antigen, antiviral T cells are tightly regulated by activating and inhibitory effector cells and molecules, many of which are affected by HIV infection. We aimed to identify immune correlates of preserved HIV-specific T-cell proliferation by analyzing cytokines and T-cell subsets that may regulate Gag-specific CD8 T-cell proliferation in aviremic youths.
PATIENTS AND METHODS
Patients
The ANRS-EP38-IMMIP study included 93 youths between 15 and 24 years of age who had been infected with HIV-1 during the perinatal period [, ]. All patients, and their legal guardians for those under 18 years of age, received written information and signed an informed consent form. A single blood sample was collected for immunological evaluations. At the time of the study, 59 patients had a plasma HIV RNA level below the detection level and are referred to as aviremic. The threshold values used in HIV RNA assays depended on the volume of available plasma. The highest threshold used was 80 copies/mL, and this value was selected as the cutoff for HIV RNA detection []. Fifty-three combination anti-retroviral therapy (cART)-treated aviremic patients had a valid evaluation of their Gag-specific T-cell proliferation and were included in the present analysis. Their characteristics are presented in Supplementary Table 1. The 6 aviremic patients for whom the T-cell proliferation assay was not performed or not valid were not different from the other 53 patients (data not shown).
Biological Investigations
Gag-specific T-cell proliferation was assessed against peptide pools with a 5-6-carboxyfluorescein diacetate succimidyl ester (CFSE)-based proliferation assay []. Peripheral blood mononuclear cells (PBMCs) were isolated from blood by density centrifugation, immediately labeled with CFSE, and stimulated with a pool of Gag peptides (15-mer peptides covering the clade B consensus sequence; NIH AIDS Reagent program, Catalog number#8117). Peptide diluent (0.034% dimethyl sulfoxide) was used as a negative control, and enterotoxin B of Staphylococcus aureus ([SEB] 500 ng/mL) was used as a positive control. Forty-eight hours after stimulation, 25 µL of supernatant was collected from each P96 well and stored frozen at −20°C before quantification of the 25 cytokines by the Cytokine Human 25-Plex Panel (LHC0009; Invitrogen). On day 6, PBMCs were labeled with a combination of anti-CD3-ECD, anti-CD4-PC7, and anti-CD8β-PC5 antibodies (Beckman Coulter, Villepinte, France). The cells were analyzed on a FC500 flow cytometer (Beckman Coulter). The differences in the percentage of CFSElowCD8+ T cells between antigen-stimulated and mock-stimulated cells and the ratio of these percentages (stimulation index) were calculated. The PBMCs from 8 uninfected donors were tested with Gag peptides. The mean + 2 standard deviation antigen-specific T-cell proliferation for these control subjects was 0.8% for the difference and 4 for the ratio. These values were used as a dual cutoff criterion to define a positive response in the T-cell proliferation assay. Patients with positive/negative results in the Gag-specific CD8 T-cell assay are referred to as CD8 responders (CD8Rs) and CD8 nonresponders (CD8NRs).
Plasma samples were stored at −80°C and interferon (IFN)-γ, interleukin (IL)-1β, IL-1Rα, IL-10, IL-12p70, IL-17A, IL-18, IL-18BPA, IL-2, IL-21, IL-23, IL-6, transforming growth factor (TGF)-β1, and tumor necrosis factor (TNF)-α levels were quantified by enzyme-linked immunosorbent assay or Luminex technology-based assay according to the manufacturer’s instructions for each kit. The detection kits and quantification thresholds are presented in Supplementary Table 2. We used flow cytometry for the phenotypic study of frozen PBMCs and quantified CD4 regulatory T cells (Tregs), gut-homing CD4 and CD8 T cells, and activated and exhausted memory CD8 T cells. Supplementary Table 3 presents the combinations of antibodies used, and Supplementary Table 4 shows the phenotypic definitions of the lymphocyte subsets. Data were collected on an LSR II cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, OR). The gating strategies are shown in Supplementary Figures 1 to 6.
Statistical Analysis
Logistic regressions were performed to study the relationships between immune parameters and Gag-specific CD8 T-cell proliferation. Before carrying out multivariate analyses, we determined whether there were interactions between ethnicity and Gag-specific T-cell proliferation. We carried out an analysis of variance for continuous variables and assessed the variation of the odds ratio across the strata for categorical variables (data not shown and Supplementary Table 5). For multivariate analyses, ethnicity and duration of plasma HIV RNA <500 copies/mL were included in the model, because these variables were significantly associated with Gag-specific T-cell proliferation []. Other variables were included if associated with Gag-specific T-cell proliferation, with a P value ≤.10 in univariate analysis of the whole group (Table 1) or in at least one of the ethnic groups (Supplementary Table 5). We did not build a model with all immunological parameters, because these were not quantified for all patients, due to the lack of available samples for some. Mann-Whitney U and Fisher’s exact test were used to compare cytokine production by cell culture from CD8Rs and CD8NRs. Analyses were conducted using STATA software (version 12.1). A P value of <.05 was considered to indicate statistical significance.
RESULTS
Association of Gag-Specific CD8 T-Cell Proliferation With Higher Levels of Transforming Growth Factor-β1 in Plasma
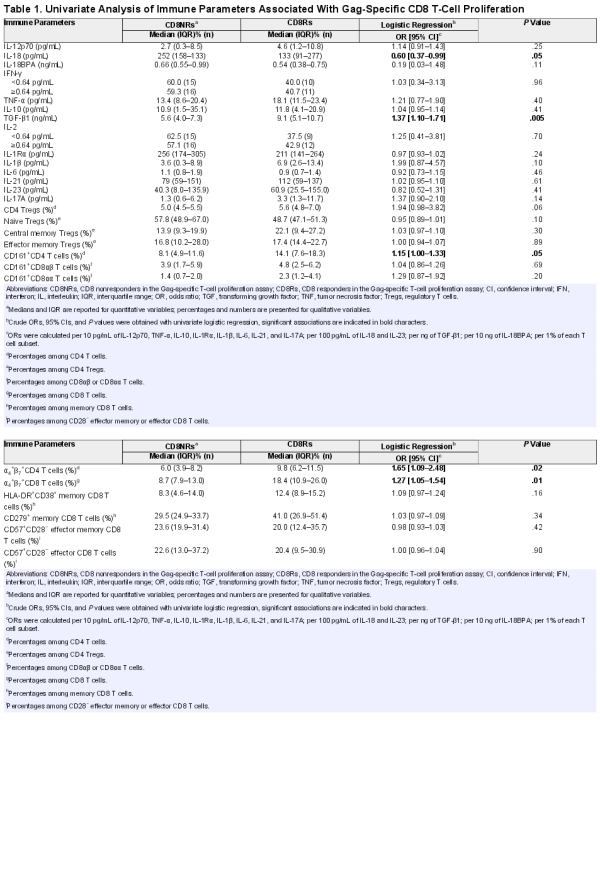
Twenty-two (42%) of 53 aviremic youths had detectable Gag-specific CD8 T-cell proliferation. The immune characteristics of CD8Rs were compared with those of CD8NRs by logistic regression. We searched for immune factors that may influence Gag-specific CD8 T-cell proliferation by first measuring levels of plasma cytokines associated with type 1 T-cell priming (IL-12p70, IL-18, IL-18BPa), effector function (IFN-γ and TNF-α), or suppressive activity (IL-10 and TGF-β1). The CD8Rs had significantly lower IL-18 levels and higher TGF-β1 levels than CD8NRs (Figure 1, Table 1). Plasma levels of IL-12p70, IL-18PBa, IFN-γ, TNF-α, and IL-10 were similar in CD8Rs and CD8NRs. We stratified the analyses on the basis of ethnicity because proliferative responses were more frequent in patients of black ethnicity than in those of other ethnicities []. The CD8Rs had lower IL-18 and higher TGF-β1 levels than CD8NRs in all ethnic groups (Supplementary Table 5).
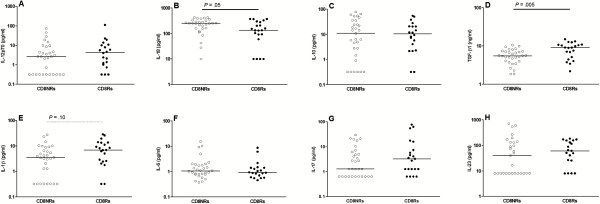
Figure 1
Gag-specific CD8 T-cell proliferation and plasma levels of cytokines. The levels of immune parameters are presented as a function of Gag-specific CD8 T-cell proliferation. Open and closed symbols represent CD8 non-responders (CD8NRs) and CD8 responders (CD8Rs) in the Gag-specific T-cell proliferation assay. Lines represent median values. A: plasma IL-12p70 (pg/mL), B: plasma IL-18 (pg/mL), C: plasma IL-10 (pg/mL), D: plasma TGF-β1 (ng/mL), E: plasma IL-1β (pg/mL), F: plasma IL-6 (pg/mL), G: plasma IL-17 (pg/mL), and H: plasma IL-23 (pg/mL).
Transforming growth factor-β1 is a pleiotropic cytokine. Higher levels of TGF-β1 in patients with Gag-specific CD8 T-cell proliferation are not consistent with the direct suppressive action of this cytokine on responding T cells. The action of TGF-β1 is modulated by the other locally produced cytokines during T-cell activation to regulate the Treg/Th17 differentiation pathway. We quantified 6 of these cytokines. The combination of IL-2 and TGF-β1 promotes the differentiation of naive CD4 T cells into CD4 Tregs. However, plasma IL-2 was detectable in a similar proportion of CD8Rs and CD8NRs (43% vs 57%, P = .78; Table 1). Transforming growth factor-β1 promotes the differentiation and/or expansion of Th17 cells in combination with IL-1β, IL-6, IL-21, and IL-23 []. Plasma IL-1β levels tended to be higher in CD8Rs than in CD8NRs (Figure 1). The IL-17 and IL-23 levels were higher in CD8Rs than in CD8NRs, but the differences were not significant. The CD8Rs and CD8NRs had similar plasma levels of IL-1Rα, IL-6, and IL-21. Overall, IL-18 and TGF-β1 are the only 2 plasma cytokines for which the levels were significantly different between CD8Rs and CD8NRs.
Association of Gag-Specific CD8 T-Cell Proliferation With Higher Regulatory T, T Helper 17, and T Cytotoxic 17 Cell Levels
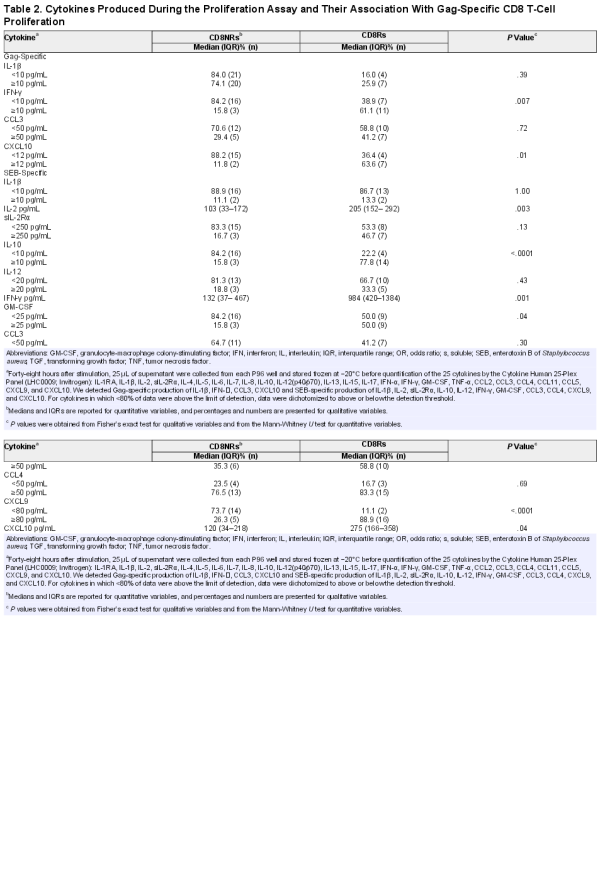
We next performed a phenotypic study of Treg, Th17, and Tc17 cells, which share a TGF-β1-dependent differentiation pathway for the 35 patients with available frozen PBMCs. The CD8Rs tended to have higher percentages of CD4 Treg and lower percentages of naive cells among Tregs than CD8NRs (Figure 2). The percentage of central memory Tregs was higher in CD8Rs than in CD8NRs, but the differences were not statistically significant.
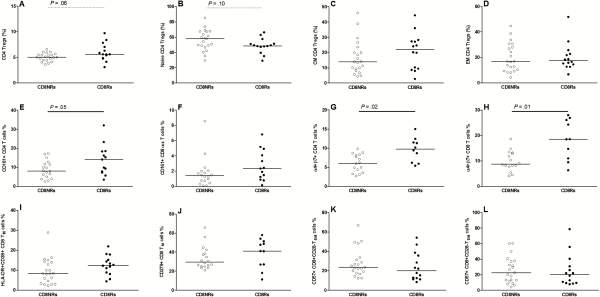
Figure 2
Gag-specific CD8 T-cell proliferation and T cell subsets. Data are presented as described in Figure 1. A: percentages of CD4 Tregs among total CD4 cells, B: percentages of naive cells among CD4 Tregs, C: percentages of central memory cells among CD4 Tregs, D: percentages of effector memory cells among CD4 Tregs, E: percentages of CD161+ CD4 T-cells among total CD4 T cells, F: percentages of CD161+ CD8αα T-cells among total CD8 T cells, G: percentages of α4+β7+ memory CD4 T-cells among total CD4 T cells, H: percentages of α4+β7+ memory CD8 T-cells among total CD8 T cells, I: percentages of HLA-DR+CD38+ cells among memory CD8 T cells, J: percentages of CD279+ cells among memory CD8 T cells, K: percentages of CD57+ T-cells among CD28- effector memory (CD45RA-CD197−) CD8 T cells, L: percentages of CD57+ T-cells among CD28- effector (CD45RA+CD197−) CD8 T cells.
We used several markers to define T-cell populations that comprise IL-17-producing cells. We assessed CD161 expression on CD4, CD8αβ, and CD8αα T cells. The CD8Rs had higher percentages of CD161+CD4 and CD161+CD8αα T-cell subsets than CD8NRs (Figure 2E and 2F). The levels of memory T cells expressing the gut-homing α4β7 integrin were significantly higher in CD8Rs than in CD8NRs (Figure 2G and 2D). We also used CXCR3, CCR4, and CCR6 expression on memory CD4 T cells to study T-cell polarization. The CD8Rs had higher levels of Th17 (CXCR3−CCR4−CCR6+) and Th1/17 (CXCR3+CCR4−CCR6+) cells than CD8NRs, and both groups had similar levels of Th1 and Th2 cells (data not shown). Thus, CD8Rs have higher levels of T cells with a type 17/gut-homing phenotype.
No Association Between Gag-Specific CD8 T-Cell Proliferation and the Activation or Exhaustion Status of Total CD8 T Cells
We assessed whether CD8Rs had lower levels of activated HLA-DR+CD38+, exhausted CD279/PD-1+, or senescent CD57+ cells among the total memory CD8 T cells, because Gag-specific CD8 T-cell proliferation was associated with increased levels of CD4 Tregs. Both groups had similar levels of HLA-DR+CD38+ and CD279+ cells among the memory CD8 T cells as well as similar proportions of CD57+ among CD28− CD8 TEM and TE (Figure 2). Furthermore, we observed no associations when considering CD4 T-cell activation or differentiation status (data not shown).
Gag-Specific CD8 T-Cell Proliferation, Cytokine Production, and Other Proliferative Responses
During the proliferation assay, supernatants were collected for the quantification of cytokines and chemokines (Table 2). Of the 4 cytokines and chemokines induced by Gag-peptide stimulation, IFN- and CXCL10 were more frequently detected in cultures from CD8Rs than in those from CD8NRs, whereas rates of IL-1β and CCL3 detection were not different between the 2 groups. The levels of several Th1-related cytokines and chemokines produced after SEB stimulation were significantly higher in cultures from CD8Rs than from CD8NRs (Table 2). Neither Th2 nor Th17-related cytokines were detectable after Gag-peptide or SEB stimulation. The production of TGF-β by antigen-specific T cells could not be detected due to high levels of TGF-β in the culture medium. Overall, Gag-specific CD8 T-cell proliferation was associated with type 1 cytokines and IL-10 production in response to either Gag peptides or a superantigen.
Then, we investigated whether the immunoregulatory cytokines and cells were associated with other proliferative responses. We observed trends for associations between plasma cytokine/Tregs levels and Gag-specific CD4 T-cell proliferation (Supplementary Table 6). However, the analysis of CD4 responses was underpowered due to the small number of patients with Gag-specific CD4 T-cell proliferation []. The percentage of proliferating CD8 T cells in response to SEB were positively correlated with the levels of CD161+CD8αα T-only (data not shown).
Multivariate Logistic Regression Analysis of Immune Factors Associated With Gag-Specific T-Cell Proliferation
We adjusted for the 2 parameters associated with CD8 T-cell proliferation in our previous study [], ie, ethnicity and duration of the last period with plasma HIV RNA <500 copies/mL (Figure 3), for each parameter with a P value ≤.1 in univariate analysis of either the whole group (Table 1) or at least one of the ethnic groups (Supplementary Table 5). Plasma levels of TGF-β1, the percentages of naive Treg, CD161+CD4, CD161+CD8αα, and α4+β7+ CD8 T cells were significantly associated with Gag-specific CD8 T-cell proliferation. Ethnicity and the duration of viral suppression remained associated with Gag-specific CD8 T-cell proliferation in these multivariate analyses.
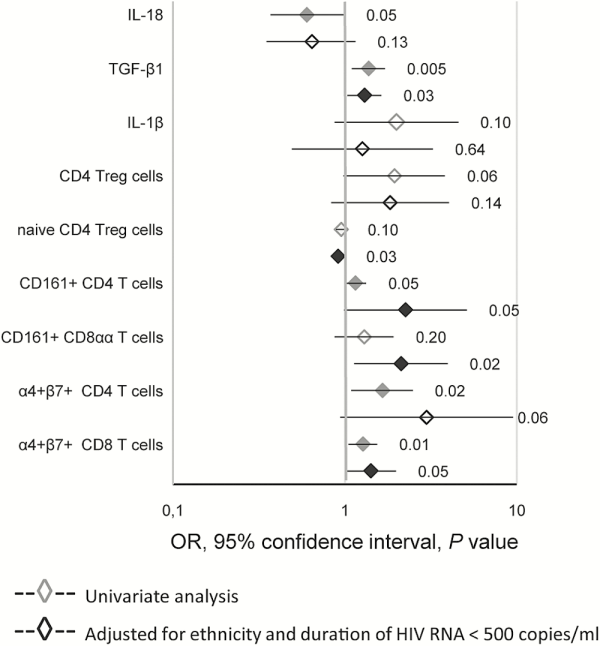
Figure 3
Logistic regression analysis of the association between Gag-specific CD8 T-cell proliferation and immune parameters. Results from univariate and multivariate logistic regression are presented as ORs and 95% confidence intervals. P values are indicated and black symbols correspond to P values < .05. Multivariate analysis included ethnicity and the duration of plasma HIV RNA suppression as covariables. The ORs are given per 100 pg/mL of IL-18, per ng of TGF-β1, per 10 pg/mL of IL-1β, and per 1% of each T-cell subset.
DISCUSSION
Our study aimed to identify immune correlates of preserved HIV-specific T-cell proliferation after long-term perinatally acquired HIV-1 infection. Gag-specific CD8 T-cell proliferation in treated youths with viral control was associated with higher levels of plasma TGF-β1 and lower levels of naive Tregs, as well as a higher proportion of CD4 and CD8 T cells with a gut-homing and/or IL-17-producing phenotype. The presence of higher levels of TGF-β1 in youths who maintained Gag-specific CD8 T-cell proliferation appears to be at odds with many previous observations on the immunosuppressive action of this cytokine. Two issues should be considered when interpreting our data. First, we assessed the proliferative capacity of Gag-specific CD8 T cells, not their immediate effector functions. In patients with suppressed viral replication, most of these HIV-specific T cells belong to the long-term memory pool and were in a resting state at the time of in vitro stimulation. Reduced immune activation favors the generation of such long-lived memory cells at the expense of short-lived effector cells []. Second, the opposite roles of immunoregulatory effector cells and molecules on HIV disease progression have been well described: they reduce both beneficial antiviral responses and deleterious immune activation []. Here, we studied patients whose immune systems had been exposed to HIV replication and antiviral drugs over 15 to 24 years. In this setting, higher levels of immune suppression may have limited the exhaustion and/or deletion of HIV-specific T cells, resulting in the long-term maintenance of these virus-specific immune cells.
The CD8Rs had higher plasma levels of TGF-β1 than CD8NRs. In vivo, TGF-β1 may have favored the development of long-lived memory T cells, resulting in higher in vitro HIV-specific T-cell proliferation []. In murine models, TGF-β1 increases expression of antiapoptotic molecules in memory T cells after clearance of the antigen [] and the prosurvival action of TGF-β1 is specific for memory cells []. Transforming growth factor-β1 also downregulates KLRG1 expression, a marker of terminally differentiated CD8 T cells, on both human and murine cells []. In addition to its T-cell intrinsic effects, TGF-β1 may act through T-cell extrinsic pathways involving antigen-presenting cells [].
The CD8Rs had higher levels of Tregs and of T cells with a gut-homing phenotype than CD8NRs. Associations between Gag-specific CD8 T-cell proliferation and T-cell subsets were restricted to the Tregs and Th17 subsets. There was no difference between the T-cell differentiation, activation, or exhaustion in CD8Rs and CD8NRs ([] and this study), and we also observed no differences in Th1 and Th2 cell levels (data not shown). The observed differences are consistent with elevated plasma TGF-β1 levels because this cytokine stimulates the differentiation of both Tregs and type 17 T-cell subsets []. Furthermore, TGF-β1 mediates the conversion of conventional T cells into Tregs in HIV-infected patients, leading to the selective accumulation of Tregs []. T-regulatory cells suppress HIV-specific T-cell activation both directly [, ] and indirectly by dampening viral replication. In adults, the suppressive activity of Tregs is associated with lower viral load and higher CD4/CD8 ratios [].
Our results support that preservation of the proliferative capacity of HIV-specific T cells may result from their reduced exposure to inflammatory molecules through better immune control at mucosal sites by Th17 cells. The Th17 and Tc17 cells play a key role in the defense against fungal and bacterial infections. Mucosal CD4 T-cell depletion and the reduction of both Th17 and Tc17 cells in the gut of HIV-infected patients leads to decreased antimicrobial function and the persistence of increased immune activation. Combination therapy partially restores these populations []. Our data are consistent with previous reports in adults. During primary infection, higher Th17 levels are associated with increased functionality of HIV-specific T cells []. After long-term highly-active antiretroviral therapy, efficient gut-associated lymphoid tissue (GALT) CD4 T restoration is associated with both higher polyfunctionality of HIV-specific CD4 and CD8 T cells and an increased number of GALT CD4 T cells producing IL-17 in response to mitogens []. In simian immunodeficiency virus (SIV)-infected macaques, enhanced SIV-specific T-cell responses and higher levels of Th17 cells were concomitantly observed [, ].
Our work raises the question of whether the observed pattern of immune restoration is specific to infection during the perinatal period. Indeed, infants differ from adults by having higher levels of Tregs and lower mucosal immunity []. A seminal study showed the ability of cord blood Tregs to suppress HIV-specific T-cell responses []. In SIV-infected macaques, Tregs are present at a higher frequency and possess higher in vitro suppressive activity in infants than in adults []. Acute SIV infection leads to the rapid loss of Tregs and increases immune activation in neonatal intestinal mucosa []. In infected children, the proportion of Tregs increases with immune suppression [, ]. To the best of our knowledge, only a single study has stated that the frequency of IL-17-producing cells was strongly reduced in the peripheral blood of HIV-infected pediatric patients, with a stronger reduction in those with active replication than in those who were successfully treated []. Further studies are clearly needed to describe and understand the Th17 compartment in pediatric HIV infection.
The patients we studied experienced long-term chronic HIV infection, because cART became available when they were between 3 and 10 years of age. Moreover, most received bitherapy with nucleoside reverse-transcriptase inhibitor and could have had adherence problems over the first years of infection. Most of them experienced severe immune suppression followed by treatment-induced viral control and immune restoration []. For patients with such disease histories, the concomitant increase of both Th17 and Treg cells is a relevant parameter of functional restoration of Gag-specific T-cell proliferation. Indeed, Th17 and Treg percentages were positively correlated, and their ratio was not associated with Gag-specific T-cell proliferation (data not shown). This association is different from the loss of the Th17/Treg balance seen in untreated adults during primary infection [, ]. These differences may be related to the evolution of the immune equilibrium from acute to chronic HIV infection. T-regulatory cell frequencies are indeed lower in primary than in chronic infection in adults [, ]. In nonhuman primates, non-pathogenic SIV infections are characterized by early and transient anti-inflammatory responses, including the elevation of TGF-β1, whereas delayed and persistent elevation of this cytokine is associated with pathogenic infection [].
The strength of our study was in performing an exploratory analysis on a large number of immune parameters. Nevertheless, we were limited by the number of patients with available samples for T-cell phenotyping. The proliferation assay was carried out using PBMCs, and T-cell proliferation depends on the functional status of both responding and bystander cells. We indeed observed an association between blood dendritic cell levels and HIV-specific CD4 T-cell proliferation []. Based on the observed associations between Gag-specific CD8 T cells and peripheral blood levels of Tregs, Th17, and Tc17, it would have been of interest to perform new assays to compare the proliferation of purified CD8 T cells to that of PBMCs. Assays combining functional (proliferation) and physical (tetramer) detection would have provided information about the physical absence versus the inhibition of proliferation of Gag-specific T cells in samples from CD8NRs. However, we lacked samples for these analyses. Finally, the reported associations may not reflect direct interactions between the immune factors but instead their common temporal or mechanistic pathways of restoration under suppressive cART.
CONCLUSIONS
Our work has demonstrated the association between the presence of Gag-specific CD8 T cells that have maintained their proliferative capacity, higher levels of suppressive T cells and cytokines, and better preservation of T cells involved in protective mucosal immunity. These results represent a step ahead in our understanding of the dynamics of these antiviral cells that may help in the design of future therapeutic strategies. Our results are consistent with the increasingly recognized deleterious impact of persistent immune activation on immune restoration.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Acknowledgments
Peptides were provided by the National Institutes of Health AIDS Research and Reference Reagent Program.
We thank all the patients who agreed to participate in this study. We also thank the nurses and staff members from the various clinical sites. We thank Sandrine Leveillé (Hôpital Robert Debré), Geneviève Vaudre (Hôpital Trousseau), Sylvie Tassi (Hôpital Jean Verdier), Nora Boudjoudi (Hôpital Port Royal), Marie-Christine Mourey (Hôpital Necker), Thierry Wack (CESP INSERM U1018), Yassine Benmebarek, and Naima Bouallag-Bonnet (former members of CESP INSERM U1018). We thank Yves Rivière for his role in this collaborative work. We also thank Elisabeth Monchâtre, Pauline Louche, and Céline Clairet for expert technical assistance. This text has been verified by a native English speaker.
Disclaimer. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Financial support. This work was funded by the 2006–232 and 2009–165 grants from the Agence Nationale de Recherche sur le SIDA et les Hépatites (ANRS) and by the Fondation AREVA.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev2013; 254:143–69.
- 2. Shiu C, Cunningham CK, Greenough T, et al. Identification of ongoing human immunodeficiency virus type 1 (HIV-1) replication in residual viremia during recombinant HIV-1 poxvirus immunizations in patients with clinically undetectable viral loads on durable suppressive highly active antiretroviral therapy. J Virol2009; 83:9731–42.
- 3. King DJ, Larsson-Sciard EL. Clonal evolution of CD8+ T-cell expansions in HIV-infected patients on long-term HAART. Clin Exp Immunol2001; 126:280–6.
- 4. Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity2012; 36:491–501.
- 5. Deeks SG, Autran B, Berkhout B, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol2012; 12:607–14.
- 6. Avettand-Fenoel V, Blanche S, Le Chenadec J, et al. Relationships between HIV disease history and blood HIV-1 DNA load in perinatally infected adolescents and young adults: the ANRS-EP38-IMMIP study. J Infect Dis2012; 205:1520–8.
- 7. Blanche S, Scott-Algara D, Le Chenadec J, et al. Naive T lymphocytes and recent thymic emigrants are associated with HIV-1 disease history in French adolescents and young adults infected in the perinatal period: the ANRS-EP38-IMMIP study. Clin Infect Dis2014; 58:573–87.
- 8. Le Chenadec J, Scott-Algara D, Blanche S, et al. Gag-specific CD4 and CD8 T-cell proliferation in adolescents and young adults with perinatally acquired HIV-1 infection is associated with ethnicity—the ANRS-EP38-IMMIP study. PLoS One2015; 10:e0144706.
- 9. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol2012; 30:531–64.
- 10. Scott-Algara D, Warszawski J, Le Chenadec J, et al. Gag-specific CD4 T-cell proliferation, plasmacytoid dendritic cells and ethnicity in perinatally HIV-1-infected youths—The ANRS-EP38-IMMIP Study. AIDS Res Hum Retrovir2016; doi: 10.1089/AID.2016.0177.
- 11. Hamilton SE, Jameson SC. CD8 T cell quiescence revisited. Trends Immunol2012; 33:224–30.
- 12. Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood2013; 121:29–37.
- 13. Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol2014; 32:51–82.
- 14. Ma C, Zhang N. Transforming growth factor-β signaling is constantly shaping memory T-cell population. Proc Natl Acad Sci U S A2015; 112:11013–7.
- 15. Filippi CM, Juedes AE, Oldham JE, et al. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes2008; 57:2684–92.
- 16. Schwartzkopff S, Woyciechowski S, Aichele U, et al. TGF-β downregulates KLRG1 expression in mouse and human CD8 T cells. Eur J Immunol2015; 45:2212–7.
- 17. Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. J Virol2012; 86:10262–9.
- 18. Kinter AL, Hennessey M, Bell A, et al. CD25CD4 regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4 and CD8 HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med2004; 200:331–43.
- 19. Weiss L, Donkova-Petrini V, Caccavelli L, et al. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood2004; 104:3249–56.
- 20. He Y, Li J, Zheng Y, et al. A randomized case-control study of dynamic changes in peripheral blood Th17/Treg cell balance and interleukin-17 levels in highly active antiretroviral-treated HIV type 1/AIDS patients. AIDS Res Hum Retroviruses2012; 28:339–45.
- 21. Jenabian MA, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One2013; 8:e78146.
- 22. Allers K, Puyskens A, Epple HJ, et al. The effect of timing of antiretroviral therapy on CD4+ T-cell reconstitution in the intestine of HIV-infected patients. Mucosal Immunol2016; 9:265–74.
- 23. Falivene J, Ghiglione Y, Laufer N, et al. Th17 and Th17/Treg ratio at early HIV infection associate with protective HIV-specific CD8+ T-cell responses and disease progression. Sci Rep2015; 5:11511.
- 24. Macal M, Sankaran S, Chun TW, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol2008; 1:475–88.
- 25. Hartigan-O’Connor DJ, Abel K, Van Rompay KK, et al. SIV replication in the infected rhesus macaque is limited by the size of the preexisting TH17 cell compartment. Sci Transl Med2012; 4:136ra69.
- 26. Baker CA, Swainson L, Lin DL, et al. Exposure to SIV in utero results in reduced viral loads and altered responsiveness to postnatal challenge. Sci Transl Med2015; 7:300ra125.
- 27. Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One2006; 1:e102.
- 28. Hartigan-O’Connor DJ, Abel K, McCune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J Exp Med2007; 204:2679–92.
- 29. Wang X, Xu H, Shen C, et al. Profound loss of intestinal Tregs in acutely SIV-infected neonatal macaques. J Leukoc Biol2015; 97:391–400.
- 30. Freguja R, Gianesin K, Mosconi I, et al. Regulatory T cells and chronic immune activation in human immunodeficiency virus 1 (HIV-1)-infected children. Clin Exp Immunol2011; 164:373–80.
- 31. Argüello RJ, Balbaryski J, Barboni G, et al. Altered frequency and phenotype of CD4+ forkhead box protein 3+ T cells and its association with autoantibody production in human immunodeficiency virus-infected paediatric patients. Clin Exp Immunol2012; 168:224–33.
- 32. Ndhlovu LC, Chapman JM, Jha AR, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS2008; 22:990–2.
- 33. Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med2010; 2:32ra36.
- 34. Chevalier MF, Petitjean G, Dunyach-Rémy C, et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog2013; 9:e1003453.
- 35. Kared H, Lelièvre JD, Donkova-Petrini V, et al. HIV-specific regulatory T cells are associated with higher CD4 cell counts in primary infection. AIDS2008; 22:2451–60.
- 36. Simonetta F, Lecuroux C, Girault I, et al. Early and long-lasting alteration of effector CD45RAFoxp3high regulatory T-cell homeostasis during HIV infection. J Infect Dis2012; 205:1510–9.
- 37. Kornfeld C, Ploquin MJ, Pandrea I, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest2005; 115:1082–91.
APPENDIX
This study was approved by the Comité de Protection des Personnes Ile-de-France II” (registration number 06-09-08), authorized by the “Direction Générale de la Santé (authorization number 2006-AOO142-49), and registered as an observational study at www.clinicaltrials.gov under the identifier NCT01055873. The institutions and investigators of the ANRS-EP38-IMMIP Study were as follows: Pédiatrie-Néonatologie, Hôpital Louis Mourier, Colombes (Corinne Floch-Tudal); Gynécologie-Obstétrique, Groupe Hospitalier Cochin Tarnier-Port-Royal, Paris (Ghislaine Firtion); Pédiatrie-Centre Hospitalier Intercommunal, Créteil (Sophie Lemerle); Pédiatrie-Centre Hospitalier Intercommunal, Villeneuve Saint-Georges (Anne Chace); Immuno-Hématologie Pédiatrique, Groupe Hospitalier Necker-Enfants Malades, Paris (Stéphane Blanche, Florence Veber); Pédiatrie, Centre Hospitalier Sud-Francilien, Evry (Adrien May); Maladies Infectieuses, Hôpital Jean Verdier, Bondy (Vincent Jeantils); Onco-Hématologie Pédiatrique Hôpital Trousseau, Paris (Catherine Dollfus); Pédiatrie-Hôpital Robert Debré, Paris (Martine Levine, Albert Faye); Centre de Diagnostic et de Thérapeutique, Hôpital de l’Hôtel-Dieu, Paris (Jean-Paul Viard).