Introduction
Esophageal cancer (EC) is an increasing entity with an incidence of 7,200 newly diagnosed patients prognosticated in 2016 in Germany []. The effective 5-year overall survival (OS) is 20% []. Most of the cases of EC are either squamous cell carcinomas (ESCC) arising from the squamous cell epithelial lining of the esophagus, or adenocarcinomas (EAC). In EAC, it is thought that the squamous cells are replaced by metaplastic columnar epithelium from which the adenocarcinoma arises. Over the last 20 years the incidence of ESCC has been stable, while the number of patients presenting with EAC has dramatically risen in Europe and North America.
Although multidisciplinary treatment of patients with clinically non-metastasized disease offers the chance for cure in EC, nearly 50% of these curatively treated patients develop recurrence due to distant metastasis within 5 years [,,]. The most significant transit mechanism of this distant tumor recurrence is hematogenous distribution of circulating tumor cells (CTC) from the primary tumor to the location of distant metastasis. These CTC are rare cells found in the peripheral and central blood of cancer patients []. These cells are genetically and phenotypically heterogeneous []. In the past, their presence and quantity during treatment regimens has been correlated with poor oncological outcome in patients with various solid tumors including EC [,]. CTC are also referred to as circulating epithelial cells (CEC) when phenotypically benign and found in patients without a known tumor diagnosis, and circulating tumor microemboli (CTM) when seen in clusters [,]. These cells in transit, obtained by a simple blood draw, not only offer the hope of early tumor diagnosis and possible treatment decisions , they may also help to improve our understanding of tumor biology and ultimately to improve treatment options. This review focuses on the biology of CTC in ESCC and EAC and highlights the potential for CTC to serve as liquid tumor biopsies. It also discusses the problem of heterogeneous and incomparable data due to inconsistent isolation techniques. An English language literature search in PubMed/Medline was performed using the search terms circulating tumor cells, CTCs, CTC detection methods, esophageal cancer, esophageal adenocarcinoma , esophageal squamous cell carcinoma (up to November 2016).
Epidemiology and Histological Types
Malignant tumors of the esophagus are constituted by the main subtypes ESCC and EAC. With a prognosticated current yearly incidence of 7,200 newly diagnosed patients, EC is a proportionally rare tumor in Germany []. Over the last 2 decades the incidence especially of EAC has been rapidly rising in Europe and North America. In the USA the incidence of EAC has exceeded ESCC since 1997 []. EC is especially rare in young people and increases in incidence with age, peaking in the seventh and eighth decades of life. EC is 3-5 times more common in men than in women with a median age at onset of 67 years in men and 73 years in women.
Current Staging and Management
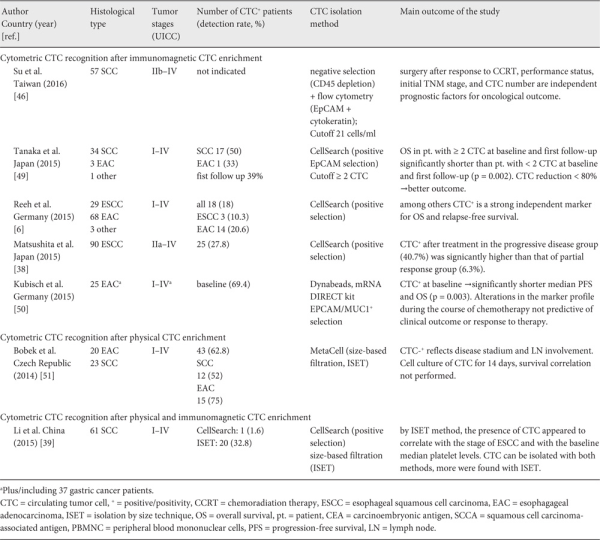
Clinically, EC presents with symptoms of progressive dysphagia, anemia or weight loss. An increasing percentage of diagnoses is discovered in screening and surveillance endoscopy programs especially for Barret's metaplasia of the esophagus. Macroscopic and histological confirmation of the diagnosis of EC is performed by endoscopy with biopsy. Current management of EC is stage dependent and often carried out multidisciplinarily by surgical, interventional endoscopic, chemotherapeutic and radio-chemotherapeutic means. Pre-therapeutically it is essential to generate an exact picture of the local and systemic extent of the tumor disease. Computed tomography (CT) provides information not only on the local extent of the tumor but also on local and distant nodal involvement and distant metastasis in liver, lung and bone. Endoscopic ultrasound offers the highest preoperative clinical accuracy concerning the local tumor infiltration of the esophageal wall (UICC T-Stage) and the local nodal involvement (UICC N-Stage) []. Positron emission tomography (PET) and PET-CT scans are complementary diagnostic modalities in EC staging, which provide the possibility to detect previously unseen distant metastasis in 5% of cases []. PET-CT is generally considered an effective method for post-neoadjuvant assessment []. As tumor recurrence especially in the distant compartment is frequent in approximately 50% of curatively treated patients, it has to be assumed that small metastatic or micro-metastatic disease is missed by current staging modalities [,,].
Novel tools and staging systems for early tumor detection, adequate prognostic staging, and accurate treatment monitoring and selection, in particular in the neo-adjuvant setting, are also needed. The seventh edition of the AJCC Cancer Staging Manual introduced the new category M0(i+) for breast cancer []. It is defined by the presence of disseminated tumor cells not exceeding 0.2 mm detectable in bone marrow, circulating blood or other non-regional tissues of the non-(macro)metastatic patient. The development and utilization of reliable methods for the detection of this minimal metastatic disease, like CTC detection techniques, and the introduction of new staging categories, such as M0 (i+), could significantly improve prognostic and predictive value of clinical cancer staging not only in breast cancer but also in gastrointestinal cancers such as EC.
CTC Enrichment and Detection Techniques in EC
CTC were first described in 1869 by Thomas Ashwoth []. Since then several techniques for detection, isolation and enumeration of CTC have been developed. As CTC are present in the blood of patients with solid tumors such as EC in concentrations of 1 cell per 5-10 × 106 white blood cells, detecting them is like looking for a needle in a haystack []. For this reason, very sensitive and specific analytical techniques are needed first to ensure reliable enrichment and second to allow sure detection of CTC []. The methods that have been developed for enrichment of CTC from other blood cells can be grouped into technologies that select CTC based on their antigenic surface structure and methods that enrich CTC by physical properties of the cells. For antigenic surface structure-based enrichment procedures, immune-magnetic cell selection procedures are mostly applied []. For enrichment of CTC based on physical properties, different approaches can be used: CTC cell size for membrane filter-based enrichment procedures, specific density for gradient centrifugation enrichment, cellular electric charge properties in dielectrophoretic procedures and the deformation ability of the cell in chip-based microfluidic CTC enrichment methods [,,,]. After enrichment of CTC, detection is carried out either by nucleic acid-based methods or by methods that apply different techniques of whole cell recognition.
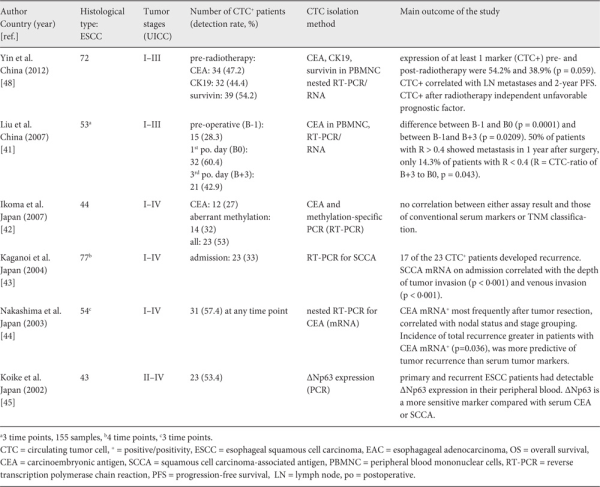
For nucleic acid-based CTC recognition, reverse transcription polymerase chain reaction (RT-PCR) is primarily applied and markers, such as cytokeratin (CK) and carcinoembryonic antigen (CEA), and mRNA are used to detect CTC in EC patients (table 1). New biomarkers such as p63 and survivin are now also being used in RT-PCR detection techniques (table 1). Since at least the conventional biomarkers like CK and CEA are also expressed in physiological peripheral blood cells and non-malignant epithelial cells, the specificity of nucleic acid-based CTC recognition is impaired by significant false-positive results caused by biomarker expression by physiological and non-malignant circulating cells [].
In cytometric-based CTC detection, immunocytometric techniques like immunocytochemical visualization, immunofluorescent visualization or flow cytometry are utilized []. Cytometric detection methods isolate individual cells based on their surface antigen expression using, for example, monoclonal antibodies directed against epithelium-specific antigens. The clear advantage of cytometric methods over nucleic acid-based methods is the possibility to further characterize the cells as the target cells are not lysed in the detection procedure. Subsequent morphological identification and molecular characterization of CTCs are, therefore, possible. The major limitation is the current lack of tumor-specific antibodies for most solid tumors including EC. The commonly used CK antigens are also expressed on white blood cells and nucleated hematopoietic precursor cells [].
Up to now different methods for enrichment and consecutive cytometric detection of CTC have been developed, including immunocytochemistry, flow cytometry, and the isolation by size technique (ISET) [,,]. However, most of these methods are predisposed to technical variation and have not been widely available commercially. More recently, technical developments in the methods of CTC capture and enumeration have led to the development of an FDA-approved CTC detection platform: the established CellSearch® system (Janssen Diagnostics, Raritan, NJ USA). Through immunomagnetic cell separation with the epithelial surface antigen EpCAM, this system enables consistent isolation and quantification of CTC from the blood of patients with different solid tumors [,]. Studies evaluating the CellSearch® platform have found wide variation in the detection of CTC across different tumor types [,]. In these studies, CTC are usually defined as EpCAM+/CK+/CD45- cells with a round or oval intracellular nucleus. A large study including patients with cancer, patients with non-malignant diseases, and healthy volunteers found that CTC are present in 11-57% of patients with cancer but cannot be found in healthy individuals []. The CellSearch® platform has also been used in prospective studies of CTC in EC []. Although several studies have shown the utility of the CellSearch® method, there is emerging evidence that there are cells in transit that escape the immunomagnetic mechanism of CTC enrichment due to different, more mesenchymal surface antigens [,,]. Thus, alternative, technically simple, less costly, and also commercially available methods such as the ISET (e.g. ScreenCell®, Paris, France) have been developed. Our study group recently reported a study on CTC isolated with the ScreenCell® method in pancreatic cancer [,], which found CTC in 73% of patients, with a combination of cytology and genetic evaluation. Several studies have identified size-based CTC isolation as a valid accompanying technique to epithelial marker-dependent CTC isolation [,,,].
CTC in EC
Compared to other solid tumor entities, only few studies have explored the meaning of CTC for progression and prognosis in EC. Investigations performed in the first decade of this century, which were mainly carried out in Asian EC patients, used nucleic-acid based CTC detection by RT-PCR. These studies revealed varying CTC detection rates ranging from 2 to 57% in patients with ESCC subtype (table 2). The relatively broad range may be explained by inconsistent definition of positive CTC status in RT-PCR, different nucleic acid-extraction protocols and the utilization of different molecular markers. A recent meta-analysis examined 13 eligible studies with a total of 979 ESCC patients. In this meta-analysis, 11 of the 13 studies (821 of 979 patients) used RT-PCR for CTC detection. The meta-analysis revealed positive CTC in patients' blood to be associated with both worse progression-free/disease-free survival (PFS/DFS) with a hazard ratio (HR) of 2.32 (95% confidence interval (CI) 1.57-3.43, p < 0.001) and poorer OS with an HR of 2.64 (95% CI 1.69-4.14, p < 0.001) [].
Recent technical advancements in both CTC enrichment and detection methods have been centered on cytometric-based CTC detection assays rather than nucleic acid-based CTC detection methods, not only in EC but also in many other solid cancer entities. In 2014, a first pilot study on immunomagnetic CTC detection with the FDA-approved CellSearch® platform in 18 patients with either metastasized gastric cancer or EC showed ≥ 2 CTC/7.5 ml blood (EpCAM+/CK+/CD45-) in 8 of the patients []. After systemic chemotherapy, the tumor response rate was 60% versus 38% in patients with < 2 CTC/7.5 ml blood versus ≥ 2 CTC/7.5 ml blood. Median PFS and OS were 6.1/10.5 months in the groups of patients with < 2 CTC and 5.2/6.1 months in those with ≥ 2 CTC.
Using the same platform, another study showed that 28% of the patients (25/90) with advanced non-treated ESCC were positive for CTC (EpCAM+/CK+/CD45-). OS was significantly shorter in CTC-positive patients. After chemotherapy or chemoradiotherapy, CTC follow-up was obtained from 71 patients. After treatment, CTC positivity was significantly higher in patients with clinical progressive disease (n = 27) compared to patients with clinical partial response to therapy (n = 32; 40.7 vs. 6.3%). Patients with no CTC at baseline and patients with a change in CTC status from positive to negative showed a favorable prognosis (fig. 1) [].
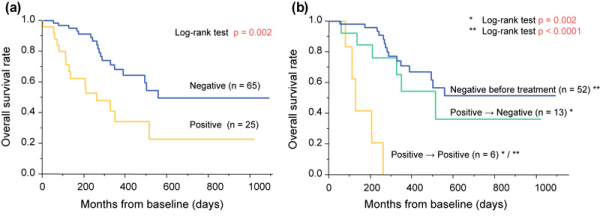
Fig. 1
Overall survival in 90 patients with esophageal squamous cell carcinoma (ESCC). circulating tumor cell (CTC) detection was carried out pre-therapeutically (n = 90) and after treatment (n = 71). From [].
A larger prospective examination of CTC in ESCC (n = 29) and EAC (n = 68) was carried out in 100 chemotherapy-naive patients with resectable EC and pre-operative immunomagnetic CTC (EpCAM+/CK+/CD45-) detection. Notably, 10% (3/29) of the ESSC patients and 21% (14/68) with EAC were CTC positive. The study revealed that CTC-positive patients with non-metastatic disease had a significantly shorter OS and PFS than CTC-negative patients [].
As the EpCAM+/CK+/CD45- definition of CTC used by the CellSearch® platform only refers to epithelial surface markers, it is likely to miss many CTC subpopulations especially if they have undergone epithelial-mesenchymal transition. There is emerging evidence that there are cells in transit that escape the immunomagnetic mechanism of CTC enrichment due to a different, mainly mesenchymal surface antigenic profile [,,]. Thus, alternative, technically simple and also commercially available methods such as ISET have been developed.
In 2015, Li et al. [] compared the marker-dependent immunomagnetic CellSearch method and the epithelial marker-independent ISET method in 61 non-treated ESCC patients. CTC were detected in 33% (20/61) by ISET and in less than 2% (1/61) by the immunomagnetic method. Moreover, circulating tumor microemboli (CTM) were observed in 5% (3/61) patients using ISET, but were undetectable in any of the patients by immunomagnetic method. Therefore, further studies are certainly needed to assess the potential clinical relevance of the different CTC detection technologies in EC. With this goal, the immunomagnetic CellSearch method and the epithelial marker-independent ISET method are currently being compared in a multicenter clinical trial of multimodal treatment of non-metastasized EAC in more than 400 patients [].
Future Clinical Implications of CTC in EAC
One main clinical aim of CTC research is to assess CTC as a prognostic and predictive biomarker for EAC to stratify patients into defined prognostic and therapeutic subgroups. The key aim of future studies is to determine whether pre- and intra-therapeutic CTC detection can accurately indicate prognosis in EAC patients and may improve preoperative staging, differential indication for specific neoadjuvant therapeutic modalities and indication for adjuvant therapy []. With regard to multimodal treatment protocols, CTC detection could improve selection for either protocols with stronger systemic effects such as perioperative chemotherapy or protocols with a predominantly locoregional effect on the tumor disease such as neoadjuvant chemoradiation. The addition of CTC detection to intra-therapeutic re-staging procedures and the comparison of the results to pre-therapeutic CTC detection values could also possibly identify patients with either strong or limited response to neoadjuvant treatment. If the presence of CTC following neoadjuvant chemotherapy or chemoradiation could contribute to the currently available prognostic information, it will help to identify non-metastatic EAC patients at high risk for disease progression who may derive benefit from additional adjuvant therapies or inclusion into clinical trials of novel therapies for high-risk patients [].
Conclusion
Appropriate staging systems are essential for determining treatment strategies, especially those involving multimodal treatments in EC. Despite the availability of several diagnostic techniques, current pre-treatment staging remains inconsistent. Novel tools for adequate prognostic staging and accurate therapy monitoring in EC are urgently needed. CTC have been found to be independent risk factors for worse prognosis in patients with EC. Pre- and intra-therapeutic CTC enrichment and detection has the potential to improve preoperative staging, differential indication for neoadjuvant therapeutic modality and indication for adjuvant therapy.
Disclosure Statement
The authors report no competing financial interests.
References
- 1. Krebs in Deutschland, 10. Ausgabe, Robert Koch Institut, Berlin, 2015.
- 2. Sudarshan M, Alcindor T, Ades S, et al.: Survival and recurrence patterns after neoadjuvant docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophagogastric adenocarcinoma. Ann Surg Oncol 2015;22:324-330.
- 3. Makowiec F, Baier P, Kulemann B, et al.: Improved long-term survival after esophagectomy for esophageal cancer: Influence of epidemiologic shift and neoadjuvant therapy. J Gastrointest Surg. 2013;17:1193-1201.
- 4. Hoeppner J, Zirlik K, Brunner T, et al.: Multimodal treatment of locally advanced esophageal adenocarcinoma: Which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J Surg Oncol. 2014;109:287-293.
- 5. Kulemann B, Liss AS, Warshaw AL, et al.: KRAS mutations in pancreatic circulating tumor cells: A pilot study. Tumour Biol 2016;37:7547-7554.
- 6. Reeh M, Effenberger KE, Koenig AM, et al.: Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg 2015;261:1124-1130.
- 7. Cristofanilli M, Budd GT, Ellis MJ, et al.: Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-791.
- 8. Li H, Song P, Zou B, et al.: Circulating tumor cell analyses in patients with esophageal squamous cell carcinoma using epithelial marker-dependent and -independent approaches. Medicine (Baltimore) 2015;94:e1565.
- 9. Cauley CE, Pitman MB, Zhou J, et al.: Circulating epithelial cells in patients with pancreatic lesions: clinical and pathologic findings. J Am Coll Surg 2015;221:699-707.
- 10. Pohl H, Welch HG: The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-146.
- 11. van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al.: Staging investigations for oesophageal cancer: A meta-analysis. Br J Cancer 2008;98:547-557.
- 12. Van Westreenen HL, Westerterp M, Sloof GW, et al.: Limited additional value of positron emission tomography in staging oesophageal cancer. Br J Surg 2007;94:1515-1520.
- 13. Anderegg MC, de Groof EJ, Gisbertz SS, et al.: 18F-FDG PET-CT after neoadjuvant chemoradiotherapy in esophageal cancer patients to optimize surgical decision making. PLoS One 2015;10:e0133690.
- 14. Edge SB, Compton CC: The American Joint Committee on Cancer: The 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol 2010;17:1471-1474.
- 15. Ashworth TR: ‘A case of cancer in which cells similar to those in the tumours were seen in the blood after death'. Australian Med J 1869;14:146-147.
- 16. Allard WJ, Matera J, Miller MC, et al.: Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-6904.
- 17. Alix-Panabières C, Pantel K: Circulating tumor cells: Liquid biopsy of cancer. Clin Chem 2013;59:110-118.
- 18. Vona G, Sabile A, Louha M, et al.: Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000;156:57-63.
- 19. Gascoyne PR, Noshari J, Anderson TJ, Becker FF: Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009;30:1388-1398.
- 20. Moon HS, Kwon K, Kim S, et al.: Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011;11:1118-1125.
- 21. Nagrath S, Sequist LV, Maheswaran S, et al.: Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-1239.
- 22. Wicha MS, Hayes DF: Circulating tumor cells: Not all detected cells are bad and not all bad cells are detected. J Clin Oncol 2011;29:1508-1511.
- 23. Mostert B, Sleijfer S, Foekens JA, Gratama JW: Circulating tumor cells (CTCs): Detection methods and their clinical relevance in breast cancer. Cancer Treat Rev 2009;35:463-474.
- 24. Sun YF, Yang XR, Zhou J, et al.: Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol 2011;137:1151-1173.
- 25. Van der Auwera I, Peeters D, Benoy IH, et al.: Circulating tumour cell detection: A direct comparison between the CellSearch system, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br J Cancer 2010;102:276-284.
- 26. Khoja L, Backen A, Sloane R, et al.: A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508-516.
- 27. Giuliano M, Giordano A, Jackson S, et al.: Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res 2014;16:440.
- 28. Goldkorn A1, Ely B, Quinn DI, et al.: Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:1136-1142.
- 29. Gorges TM, Tinhofer I, Drosch M, et al.: Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012;12:178.
- 30. Yu M, Bardia A, Wittner BS, et al.: Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-584.
- 31. Kulemann B, Pitman MB, Liss AS, et al.: Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas 2015;44:547-550.
- 32. Krebs MG, Hou JM, Sloane R, et al.: Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-315.
- 33. Adams DL, Stefansson S, Haudenschild C, et al.: Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the cellsearch(®) CTC test. Cytometry A 2015;87:137-144.
- 34. Kaifi JT, Kunkel M, Das A, et al.: Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: A prospective trial with different detection techniques. Cancer Biol Ther 2015;16:699-708.
- 35. Hofman V, Ilie MI, Long E, et al.: Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-1660.
- 36. Wang S, Du H, Li G: Significant prognostic value of circulating tumor cells in esophageal cancer patients: A meta-analysis. Oncotarget 2017;8:15815-15826.
- 37. Sclafani F, Smyth E, Cunningham D, et al.: A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer 2014;13:94-99.
- 38. Matsushita D, Uenosono Y, Arigami T, et al.: Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol 2015;22:3674-3680.
- 39. Li H, Song P, Zou B, et al.: Circulating tumor cell analyses in patients with esophageal squamous cell carcinoma using epithelial marker-dependent and -independent approaches. Medicine (Baltimore) 2015;94: e1565.
- 40. Hoeppner J, Lordick F, Brunner T, et al.: ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503.
- 41. Liu Z, Jiang M, Zhao J, Ju H: Circulating tumor cells in perioperative esophageal cancer patients: Quantitative assay system and potential clinical utility. Clin Cancer Res 2007;13:2992-2997.
- 42. Ikoma D, Ichikawa D, Ueda Y, et al.: Circulating tumor cells and aberrant methylation as tumor markers in patients with esophageal cancer. Anticancer Res 2007;27:535-539.
- 43. Kaganoi J, Shimada Y, Kano M, et al.: Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg 2004;91:1055-1060.
- 44. Nakashima S, Natsugoe S, Matsumoto M, et al.: Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery 2003;133:162-169.
- 45. Koike M, Hibi K, Kasai Y, et al.: Molecular detection of circulating esophageal squamous cell cancer cells in the peripheral blood. Clin Cancer Res 2002;8:2879-2882.
- 46. Su PJ, Wu MH, Wang HM, et al.: Circulating tumour cells as an independent prognostic factor in patients with advanced oesophageal squamous cell carcinoma undergoing chemoradiotherapy. Sci Rep 2016;6:31423.
- 47. Glatz T, Bronsert P, Schäfer M, et al.: Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: Postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol 2015;41:1300-1307.
- 48. Yin XD, Yuan X, Xue JJ, et al.: Clinical significance of carcinoembryonic antigen-, cytokeratin 19-, or survivin-positive circulating tumor cells in the peripheral blood of esophageal squamous cell carcinoma patients treated with radiotherapy. Dis Esophagus. 2012;25:750-756.
- 49. Tanaka M, Takeuchi H, Osaki Y, et al.: Prognostic significance of circulating tumor cells in patients with advanced esophageal cancer. Esophagus 2015;12:352-359.
- 50. Kubisch I, de Albuquerque A, Schuppan D, et al.: Prognostic role of a multimarker analysis of circulating tumor cells in advanced gastric and gastroesophageal adenocarcinomas. Oncology 2015;89:294-303.
- 51. Bobek V, Matkowski R, Gürlich R, et al.: Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol 2014;52:171-177.