Introduction
Endometrial cancer (EC) is the most widespread malignancy of the female reproductive system, especially in postmenopausal women []. In Turkey, the incidence of adenocarcinoma of the endometrium is 5% and the 5-year overall survival (OS) rate is 90% []. According to previous studies, the incidence of International Federation of Gynecology and Obstetrics (FIGO) stage I endometrial cancer is 75%, and the 5-year survival rate is 85% [,,].
Low-risk EC has been defined as patients with disease confined to the uterine corpus, histologic grade 1 or 2, endometrioid histologic subtype, and less than 50% myometrial invasion (MMI) [,,]. Low-risk EC has a minimal risk for pelvic lymph node (LN) metastasis (≤ 5%), vaginal recurrence (1-3%), and pulmonary metastases (< 1%) []. A number of known prognostic factors for EC have been defined by many studies, including stage, histological type and grade, depth of MMI, and lymphovascular space invasion (LVSI) [,,,,]. However, because low-risk EC has an excellent prognosis with a very low rate of recurrence, risk factors for recurrence in women with low-risk EC have not been clearly delineated.
The aim of this study was to address the risk factors for recurrence in patients with low-risk EC.
Patients and Methods
This retrospective study was performed using 10 gynecological oncology department databases. All surgical specimens were examined and interpreted by gynecological pathologists. Low-risk EC (grade 1 or 2 endometrioid EC with < 50% MMI) was diagnosed after examination of permanent sections. Architectural grading was defined by standard FIGO criteria. Tumor size was macroscopically measured on fresh tissue by gynecologic pathologists who noted the size in the 3 largest dimensions. The largest of these 3 dimensions of the tumor was defined as the primary tumor diameter (PTD) []. LVSI was defined as the presence of adenocarcinoma of any extent in endothelium-lined channels of uterine specimens extracted at the time of surgery []. All tumors were staged according to the 2009 FIGO staging system. In patients treated before 2009, the stage was determined retrospectively based on a surgical and pathologic assessment.
Patients who met the following criteria were included in the study: (a) endometrioid-type histology, (b) histological grade 1 or 2, (c) no or < 50% MMI, (d) no intraoperative evidence of extrauterine spread, (e) diagnosed between January 1, 1998 and December 31, 2015, and (f) underwent at least a pelvic lymphadenectomy. Patients with non-endometrioid-type tumors, women with grade 3 histology, and patients with FIGO stage IB, II, III, or IV disease were excluded. This study was approved by the Institutional Review Boards. It was conducted in accordance with the ethical standards of the Declaration of Helsinki. At admission, all patients provided informed consent regarding research use of their medical information.
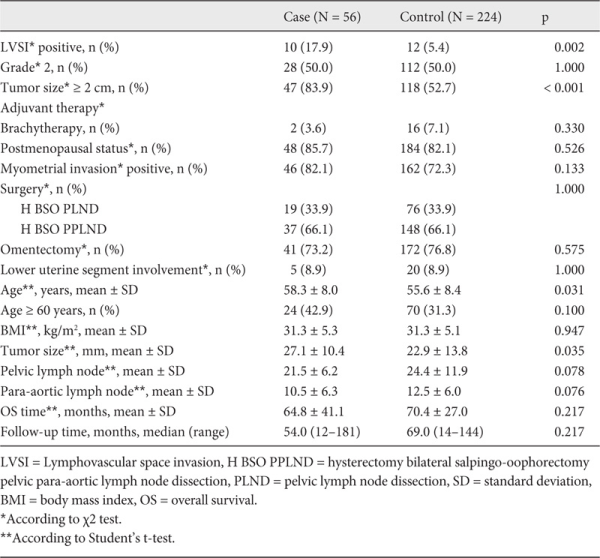
Recurrence was detected in 56 patients who were histologically diagnosed with low-risk EC, and these patients made up the case group. Using a dependent random sampling method, 224 patients with low-risk EC having no recurrence were selected as a control group with complete data. In the case and control groups, pairs were matched in terms of grade, stage, and operative technique, such as hysterectomy plus bilateral salpingo-oophorectomy with pelvic and para-aortic lymphadenectomy or only pelvic lymphadenectomy.
Clinical and pathological data were obtained from the patients' files and original pathology reports. During the study period, the management of endometrioid EC varied among the institutions participating in the study, particularly with respect to the role of lymphadenectomy; no LNs were sampled in some of the patients, only the pelvic LN or para-aortic LNs were sampled in some patients, complete staging with bilateral pelvic LNs was applied in some patients, and some patients underwent complete staging with bilateral pelvic and para-aortic lymphadenectomy. The individual practitioners were responsible for these variations over the study period.
Depth of MMI (no MMI or < 50% MMI as dichotomous options), PTD, LVSI, number of removed pelvic and para-aortic LNs, lower uterine segment involvement, and total number of retrieved LNs were evaluated from the original pathology reports. Brachytherapy was administered to some cases in the case and control groups as adjuvant therapy (2-6 cycles, 5-8 Gy). External radiotherapy or chemotherapy was not administered to any patients. Age, menopausal status, body mass index (BMI), adjuvant therapy and type (if applicable), disease-free survival (DFS), OS, type of primary treatment (such as surgery, surgery plus adjuvant radiotherapy), recurrence, and treatment of recurrence were also investigated.
The site of recurrence was grouped into 4 categories: (i) vaginal relapse: recurrence within vaginal walls or vaginal cuff [], (ii) nodal failure: recurrence in pelvic, para-aortic node regions or other node-bearing areas (i.e., groin, axilla, supraclavicular, mediastinal) as the primary site of failure [], (iii) peritoneal (abdominal) failure: disease recurring in the upper abdomen or involving the pelvic peritoneum (or both) generally manifested by ascites, peritonitis carcinomatosa, or intestinal obstruction [], (iv) hematogenous dissemination: lung, liver, or other sites (i.e., adrenals, breast, brain, bone, or skin via hematogenous spread) []. In case of several concomitant recurrence localizations, the patient was included in the group with the most advanced disease.
We also classified tumor relapses at the surgical vaginal cuff, vagina, pelvic sidewall, or pelvic LNs as locoregional, and all other recurrences (peritoneal, hematogenous, and LN recurrences outside the pelvis), as extrapelvic.
After completion of treatment, the patients entered a routine surveillance program and were scheduled for follow-up every 3 months for the first 2 years, every 6 months until 5 years, and annually thereafter. Surveillance consisted mainly of questioning the patients about symptoms, a physical examination, and serum cancer antigen 125 level determination. Tumor recurrence was confirmed via a clinical pelvic exam, which was further supplemented with biopsies in case of suspicious findings or imaging studies in case of suspicion of distant metastases during a regular visit or following the occurrence of symptoms, such as vaginal bleeding or abdominal discomfort. After diagnosis of recurrence, all the hematoxylin-and-eosin (H-E)-stained slides of the primary tumor were reviewed by a gynecologic pathologist at each participating institution before initiating treatment for recurrence and the primary diagnosis of low-risk EC was confirmed.
DFS was defined as the time from the date of primary surgery to the detection of recurrence or the latest observation. OS was defined as the time from the date of primary surgery to death or the latest observation. The survival analysis was based on the Kaplan-Meier method, and the results were compared using the log-rank test. Logistic regression analysis was used to define the factors for recurrence. The χ2 test and Student's t-test were used to analyze the unpaired data. All statistical analyses were performed using MedCalc software (version 16.0; Mariakerke, Belgium). A p-value < 0.05 was considered significant.
Results
Demographic and clinicopathologic data of the case and the control groups with low-risk EC are presented in table 1. The patterns of the recurrences were detected as vaginal in 28 patients (50.0%), peritoneal in 13 patients (23.2%), hematogenous in 9 patients (16.1%), and lymphogenous in 6 patients (10.7%). The most frequently involved organ was the vaginal cuff in 28 patients (50.0%). Pelvic peritoneal recurrence was detected in 8 patients (14.3%), colon recurrence in 2 (3.6%), retroperitoneal LN metastases in 4 (7.2%), lung recurrence in 2 (3.6%), and multiple organ metastases in 9 (16%) patients. 1 patient (1.8%) had skin metastasis, 1 patient (1.8%) had recurrence in inguinal LNs, and 1 woman (1.8%) had a relapse in mediastinal LNs. For the treatment of recurrence, 3 (5.4%) patients had only surgical resection, 12 (21.4%) patients had only chemotherapy, 8 (14.3%) patients had only radiotherapy, 6 (10.7%) patients had surgery plus chemotherapy, 7 (12.5%) patients had surgery plus radiotherapy, 9 (16.1%) patients had surgery plus chemoradiotherapy, and 11 (19.6%) patients had chemoradiotherapy.
The 5-year OS rate was 65.5% in the case group and 89.8% in the control group (hazard ratio (HR) 1.1, 95% confidence interval (CI) 0.8-1.6; p = 0.336). The median time of recurrence was 24.5 (CI 16.1-32.6) months. 28 (50%) patients developed recurrence before 24 months and 46 (82.1%) patients had a relapse before 60 months.
In the case group, 39 (69.6%) patients had a locoregional recurrence (vaginal cuff, pelvic recurrence, or pelvic LN), and 17 (30.4%) patients had an extrapelvic recurrence. None of the patients had both pelvic and extrapelvic metastases. All multiple recurrences were located in the extrapelvic region.
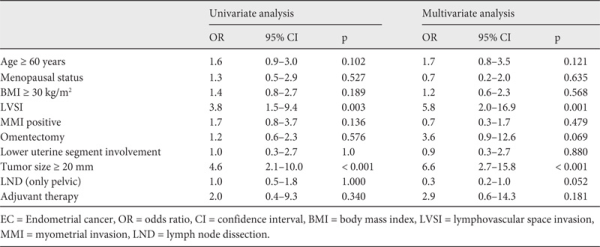
Risk factors for recurrence in patients with low-risk EC are shown in table 2. LVSI and PTD ≥ 20 mm were found to be significant prognostic factors for recurrence in both univariate and multivariate analyses. According to the risk factors detected at the end of univariate regression analysis, patients with both positive LVSI and PTD ≥ 2 cm were 27 times more likely to have recurrent disease (odds ratio (OR) 27.5, 95% CI 6.9-109.6). However, low-risk EC patients carrying only 1 of these risk factors (positive LVSI or PTD ≥ 2 cm) were 4 times more likely to have relapse (OR 3.9, 95% CI 1.7-8.7).
Discussion
Survival in cases with endometrial adenocarcinoma is generally good; however, at least 7% of patients eventually develop a recurrence []. Prognostic factors have been established to stratify cases at risk for recurrence, which is associated with a poor prognosis. However, risk factors and prognostic implications for recurrence of low-risk EC are uncertain. This multicenter case-control study examined the risk factors for recurrence in patients with low-risk EC. To the best of our knowledge, this is the first study to investigate this topic.
Consistent with the literature regarding the recurrence seen in patients with all stages of endometrioid EC, our data indicated that the most frequent recurrence region was the vaginal cuff, and the second one was the pelvis [,]. Similarly, those studies were not different from the current data that half of the recurrences are vaginal, followed by peritoneal, hematogenous, and lymphatic spread []. A significant portion of the recurrences in patients with endometrioid EC occur within the first 2 years (37.5% of patients recurred within the first year, 54.2% within the second year, and 8.3% within the third year) []. The median duration of recurrence in our study was 24.5 (CI 16.1-32.6) months. In the current study, half of the patients developed a recurrence before 24 months.
Prognostic factors have been investigated previously between heterogeneous groups for the development of a recurrence in patients with EC (all stages or early stages (stage I and II simultaneously) or in groups with grade 1, 2, 3 or endometrioid and non-endometrioid coexisting groups). LVSI and lower uterine segment involvement in early-stage well-differentiated endometrioid EC could be a highly significant predisposing factor for recurrence [,,]. Our findings showed that the presence of LVSI was an independent risk factor for recurrence, whereas lower segment involvement was not. The standard method for assessing LVSI is light microscopic examination of H-E-stained sections. However, many difficulties arise in identifying lymphatic vessel walls by H-E staining. Immunostaining with D2-40 (an anti-podoplanin antibody) significantly increased the detection rate of LVSI as compared to H-E staining, underlining the importance of D2-40 immunohistochemistry (IHC) as an investigative modality [].
Furthermore, PTD ≥ 20 mm was a significant risk factor for recurrence in patients with low-risk EC in univariate and multivariate analyses. There are also contrasting studies showing that tumor size > 20 mm and LVSI are not significant risk factors for recurrence or duration of DFS [,]. The most important limitations of these studies are the inclusion of advanced-stage patients and the presence of patients with different grades and different myometrial invasion depths. The presence of MMI, which has been reported to be a significant risk factor in some studies [], was not a significant risk factor in our study. The incidence of < 50% MMI was 82.1% whereas the patients with no MMI constituted 17.9% of women in the case group, and the incidence of < 50% MMI was 72.3% while disease confined only to the endometrium comprised 27.7% of women in the control group. However, these findings were not significantly different between the case and control groups.
Although the majority of isolated vaginal cuff recurrences in early-stage EC can be salvaged by adjuvant radiotherapy, a 2012 Cochrane meta-analysis of 8 trials evaluating adjuvant radiotherapy in patients with stage I EC reported no significant difference in OS []. The recurrence rates in patients with low-risk (stage I and grade 1 or 2 and endometrioid histology) EC after hysterectomy and bilateral salpingo-oophorectomy have been reported as 3.2% and 3.6%, for observation and adjuvant radiotherapy groups, respectively []. Patients receiving adjuvant radiotherapy have been reported to be less likely to have a recurrence []. In our study, only 18 (5.7%) patients received adjuvant brachytherapy after initial surgery. Although our population size was small, the current study showed that 2 patients (3.5%) had recurrence (vaginal cuff recurrence and colon recurrence) although they were treated with adjuvant brachytherapy following initial surgery.
According to the Surveillance, Epidemiology and End Results (SEER) data, low-risk EC patients represent approximately 61% of endometrioid ECs grossly confined to the uterus [,]. Although the recurrence rate is very low in this subgroup of patients (1-7%), the absolute number of recurrences in low-risk EC seems to be actually considerable as approximately 70% of women with EC are given a diagnosis while the disease is confined to the uterus []. Given the rapidly increasing incidence of EC worldwide, clinicians seem to be obliged to deal with more cases with recurrent low-risk EC in the future. Against this background, among women with low-risk EC, the subgroup of patients with unknown negative prognostic factors leading them to recurrence will seem to gain importance.
However, our study had several limitations. First, the retrospective nature of the study cannot exclude any bias. Second, many different clinical approaches were used. Our data were collected from 10 different institutions with potential differences in surgical and clinical management. Possibly, analyzing clinical outcomes among patients with low-risk EC may not be equally balanced and objectively represented. Third, although all surgical specimens were evaluated by a specialized single gynecological pathologist at each institution, the absence of a centralized pathological slide review is another limitation of the current study. However, given the low rate of recurrence in low-risk EC, the study design used here was necessary to achieve a satisfactory sample size. Despite the above limitations, this study represents one of the largest series of recurrent cases with low-risk EC. Moreover, the availability of good follow-up data increased the validity of the results and mitigated the weaknesses.
In conclusion, the presence of LVSI and PTD ≥ 20 mm seem to be significant risk factors for recurrence in women with low-risk EC. These parameters should be examined carefully in women with low-risk EC and patients having these features should be followed up closely because of increased risk for recurrence. We should emphasize that low-risk EC patients carrying both risk factors (positive LVSI and PTD ≥ 20 mm at the same time) seem to be 27 times more likely to have recurrence when compared to patients with none of these risk factors.
Disclosure Statement
The authors declare that there is neither financial nor academic support of relationships that may pose potential conflicts of interest.
References
- 1. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al.: Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 1991;40:55-65.
- 2. Gungorduk K, Ozdemir A, Ertas IE, Sahbaz A, Asicioglu O, Gokcu M, et al.: A novel preoperative scoring system for predicting endometrial cancer in patients with complex atypical endometrial hyperplasia and accuracy of frozen section pathological examination in this context: a multicenter study. Gynecol Obstet Invest 2015;79:50-56.
- 3. Carey MS, O'Connell GJ, Johanson CR, Goodyear MD, Murphy KJ, Daya DM, et al.: Good outcome associated with a standardized treatment protocol using selective postoperative radiation in patients with clinical stage I adenocarcinoma of the endometrium. Gynecol Oncol 1995;57:138-144.
- 4. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC: Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol 2000;182:1506-1519.
- 5. Chi DS, Barakat RR, Palayekar MJ, Levine DA, Sonoda Y, Alektiar K, et al.: The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer 2008;18:269-273.
- 6. Gupta V, McGunigal M, Hayes MP, Kalir T, Liu J: Adjuvant radiation therapy is associated with improved overall survival in high-intermediate risk stage I endometrial cancer: a national cancer data base analysis. Gynecol Oncol 2017;144:119-124.
- 7. Kaewpangchan P, Cheewakriangkrai C: Relapse patterns and outcomes following recurrence of endometrial cancer in northern Thai women. Asian Pac J Cancer Prev 2015;16:3861-3866.
- 8. Roma AA, Rybicki LA, Barbuto D, Euscher E, Djordjevic B, Frauenhoffer E, Kim I, Hong SR, Montiel D, Ali-Fehmi R, Malpica A, Silva EG: Risk factor analysis of recurrence in low-grade endometrial adenocarcinoma. Hum Pathol 2015;46:1529-1539.
- 9. Iavazzo C, Gkegkes ID, Vrachnis N: Early recurrence of early stage endometrioid endometrial carcinoma: possible etiologic pathways and management options. Maturitas 2014;78:155-159.
- 10. Huijgens ANJ, Mertens HJMM: Factors predicting recurrent endometrial cancer. Facts Views Vis Obgyn 2013;5:179-186.
- 11. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al.: A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744-751.
- 12. Mariani A, Dowdy SC, Keeney GL, Haddock MG, Lesnick TG, Podratz KC: Predictors of vaginal relapse in stage I endometrial cancer. Gynecol Oncol 2005;97:820-827.
- 13. Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC: Predictors of lymphatic failure in endometrial cancer. Gynecol Oncol 2002;84:437-442.
- 14. Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC: Endometrial cancer: predictors of peritoneal failure. Gynecol Oncol 2003;89:236-242.
- 15. Mariani A, Webb MJ, Keeney GL, Calori G, Podratz KC: Hematogenous dissemination in corpus cancer. Gynecol Oncol 2001;80:233-238.
- 16. Topfedaisi NO, Meydanlı MM, Sarı ME, Demirkiran F, Kahramanoglu I, Bese T, et al.: Factors associated with survival after relapse in patients with low-risk endometrial cancer treated with surgery alone. J Gynecol Oncol 2017;28:e65.
- 17. Bendifallah S, Canlorbe G, Huguet F, Coutant C, Hudry D, Graesslin O, et al.: A risk scoring system to determine recurrence in early-stage type 1 endometrial cancer: a French multicentre study. Ann Surg Oncol 2014,21:4239-4245.
- 18. Weber SK, Sauerwald A, Pölcher M, Braun M, Debald M, Serce NB, Kuhn W, Brunagel-Walgenbach G, Rudlowski C: Detection of lymphovascular invasion by D2-40 (podoplanin) immunoexpression in endometrial cancer. Int J Gynecol Cancer 2012;22:1442-1448.
- 19. Han KH, Kim HS, Lee M, Chung HH, Song YS: Prognostic factors for tumor recurrence in endometrioid endometrial cancer stages IA and IB. Medicine (Baltimore) 2017;96:e6976.
- 20. Kong A, Johnson N, Kitchener HC, Lawrie TA: Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst 2012;104:1625-1634.
- 21. Yoney A, Yildirim C, Bati Y, Unsal M: Low risk stage I endometrial carcinoma: prognostic factors and outcomes. Indian J Cancer 2011;48:204-210.
- 22. Mahdi H, Munkarah AR, Ali-Fehmi R, Woessner J, Shah SN, Moslemi-Kebria M: Tumor size is an independent predictor of lymph node metastasis and survival in early stage endometrioid endometrial cancer. Arch Gynecol Obstet 2015;292:183-190.
- 23. Vargas R, Rauh-Hain JA, Clemmer J, Clark RM, Goodman A, Growdon WB, et al.: Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: a SEER analysis. Gynecol Oncol 2014;133:216-220.
- 24. Robbins JR, Yechieli R, Laser B, Mahan M, Rasool N, Elshaikh MA: Is time to recurrence after hysterectomy predictive of survival in patients with early stage endometrial carcinoma? Gynecol Oncol 2012;127:38-42.