Established Facts
The prognosis of patients with post-myelofibrosis (MF) acute myeloid leukemia (AML) is poor, and treatment choices are limited and not sufficiently effective.
Novel Insights
This case report offers a new treatment choice for patients with post-MF AML with the combination of the JAK2 inhibitor ruxolitinib and the hypomethylating agent 5-azacytidine. This combination is being actively studied in clinical trials, but results are still pending. Our case suggests that ruxolitinib in combination with 5-azacytidine may be a safe and effective choice for patients with post-MF AML.
Introduction
Acute leukemia is an unfavorable event emerging in a minority of patients with myelofibrosis (MF). Usually, leukemic transformation (LT) is of myeloid origin (acute myeloid leukemia [AML]); however, leukemias of other lineage have been described. Circulating blasts ≥3% and a platelet count <100,000/μL at diagnosis were identified as independent predictors of LT in 311 patients with MF []. Survival after LT is generally poor [-].
There is no standard of care for the management of LT and no randomized trials have been carried out. The treatment decision is thus individualized, and patients may be treated with curative intent (intensive induction chemotherapy with or without hematopoietic stem cell transplantation) [] or with noncurative intent (low-dose cytarabine, hypomethylating agents [HMAs], or supportive care). In general, HMAs have limited activity, with overall survival (OS) rates of about 8–10 months [, ]. The JAK kinase inhibitor ruxolitinib also was used in a phase II study as monotherapy in patients with post-myeloproliferative neoplasm (MPN) AML, with modest antileukemic activity [].
In this report, we present the case of a 67-year-old female patient with post-polycythemia vera (PV) MF who developed AML and was successfully treated with a combination of 5-azacytidine and ruxolitinib.
Case Presentation
A 67-year-old lady had been diagnosed at the age of 57 years with PV (Hb 15.4 g/dL, trilineage bone marrow hyperplasia, and a V617F JAK2 mutation). The patient’s medical history was remarkable for beta-thalassemia heterozygosity. She was classified as high risk per the International Prognostic Scoring System (IPSS) for OS in PV [] and treated with hydroxyurea and acetylsalicylic acid in the following years with an initial optimal response for 3 years, but, for personal reasons, she was poorly monitored thereafter.
Eight years after the initial diagnosis, she complained of weight loss, malaise, and fatigue. Upon evaluation she had developed splenomegaly (23 cm by an abdominal ultrasound), anemia (Hb 9.3 g/dL), leukocytosis (WBC 18.4 × 109/L), and thrombocytopenia (PLT 131 × 109/L). A peripheral blood smear examination revealed leukoerythroblastosis with teardrop poikilocytosis along with nucleated RBC precursors and immature myeloid cells, without any blasts. A trephine bone marrow biopsy confirmed grade 3 fibrosis with 2% marrow blasts. Cytogenetic analysis showed 46,XX with 20q deletion.
The patient was started on ruxolitinib at a dose varying between 5 and 15 mg b.i.d., and within the following 6 months, she achieved substantial amelioration of her symptoms, normalization of her blood counts except for persisting leukocytosis, and improvement of splenomegaly (19 cm by ultrasound), without significant toxicity. Eleven months after ruxolitinib initiation, she presented with anemia, thrombocytopenia, and leukocytosis with 75% peripheral blood blasts (Fig. 1A–C). A bone marrow aspiration revealed 80% myeloid blasts, along with a complex karyotype.
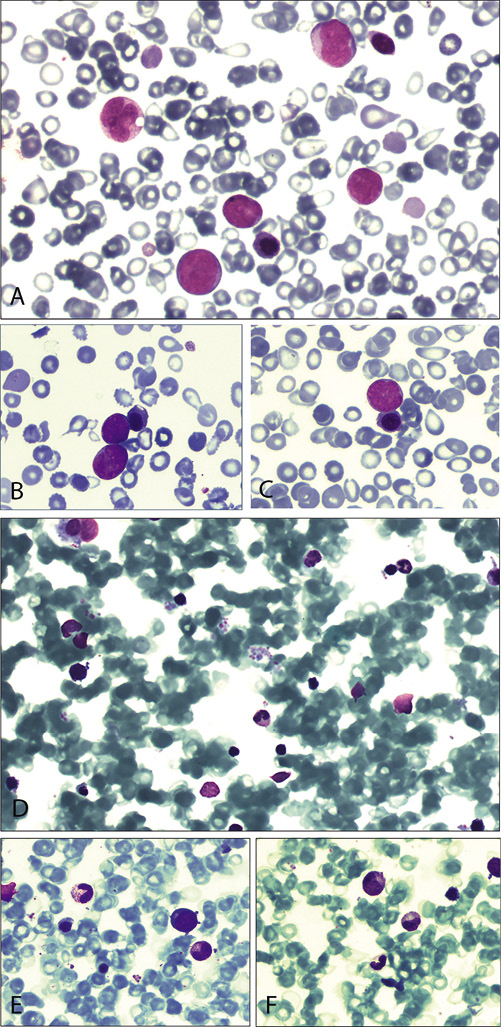
Fig. 1
May-Grünwald-Giemsa staining of peripheral blood smears at diagnosis of acute myeloid leukemia (A–C) and bone marrow smears at complete remission (D–F). A Peripheral blood smear (×40) showing the concurrent presence of myeloblasts and erythroblastosis. B, C Peripheral blood smear (×100) showing myeloblasts, erythroblasts, and several teardrop-shaped erythrocytes. D Bone marrow smear (×10) with pronounced peripheral blood admixture (difficult aspiration due to myelofibrosis) showing the absence of myeloblasts with normal maturation of myeloid cells. E, F Bone marrow smear (×100) showing maturing erythroid and myeloid precursors.
She was started on 5-azacytidine at a dose of 75 mg/m2/day for 7 consecutive days in 28-day cycles. Due to the persistence of splenomegaly (21 cm on ultrasound), ruxolitinib was not discontinued (at a dose of 5 mg b.i.d.). After 2 treatment cycles, she achieved a complete remission (CR) with bone marrow blasts <1% (Fig. 1D–F). Her karyotype was once again 46,XX,del(20q), and her spleen size was 18 cm on ultrasound. After 6 cycles of 5-azacytidine, due to the emergence of thrombocytosis, the ruxolitinib dose was increased to 15 mg b.i.d., and acetylsalicylic acid was added at a dose of 100 mg q.d. After 11 cycles of treatment, her spleen size is still reducing, and her platelet count has returned to normal while she is still in CR.
Discussion
5-Azacytidine is widely used for the treatment of de novo or secondary AML in patients ineligible for intensive chemotherapy. Its use in LT of MF offers modest responses and short OS rates. In a study on the use of 5-azacytidine in post-MPN AML or myelodysplastic syndrome, only 8% of the patients with AML achieved a CR []. These low response rates have led to the design of trials with treatment combinations of HMAs with several agents, ruxolitinib being one of them.
Our patient has had PV for about 8 years that was poorly controlled during the last 3 years at least, followed by MF transformation that was adequately managed with intermediate doses of ruxolitinib for 1 year, but which, unfortunately, transformed into AML. The patient had thrombocytopenia with a low bone marrow blast count and no circulating blasts at MF diagnosis (thrombocytopenia and circulating blasts have been proposed as independent predictors of LT) []. Interestingly, the disease course of the patient passed through all stages (from PV to MF and AML), but remarkably reversed to a myeloproliferative state after 6 months of 5-azacytidine and ruxolitinib combination. Not only did she achieve a CR – with a complex karyotype reversing to 46,XX,del(20q), which is a cytogenetic abnormality characteristic of MF – but she also developed thrombocytosis that was successfully managed with increased doses of ruxolitinib.
Ruxolitinib combinations in MF have emerged due to the short-term and incomplete responses to the drug. The rationale for ruxolitinib combinations with HMAs relies mainly on in vitro data supporting the hypothesis that several genes potentially important for the suppression of the JAK/STAT pathway (SOCS) and the prevention of the abnormal cell trafficking of CD34+ cells to sites of extramedullary hematopoiesis (CXCR4) are hypermethylated and thus silenced in patients with MF [, ]. Moreover, it has been shown that HMAs are effective in reducing the number of circulating malignant progenitor cells in MF [].
The combination of HMAs and ruxolitinib has not yet been approved either for MF or for LT, but there are two clinical trials and another two small case series for the use of this combination. A phase II study (NCT01787487) [] evaluated the combination of ruxolitinib and azacytidine in 41 patients with MF, using a sequential approach with single-agent ruxolitinib for 3 months followed by the addition of 5-azacytidine. The preliminary results of this trial show that objective responses per the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) 2013 criteria were noted in 69% of the patients, with 5% partial remissions and no patients achieving a CR. Moreover, a phase I study (NCT02076191) [] evaluating the safety and efficacy of combined ruxolitinib and decitabine in 21 patients with blast-phase MPN (52%) and post-MPN AML (48%) showed that the combination was safely administered to the patients, median OS was 7.9 months, and the overall response rate was 53% (with CR in only 2 patients [10%]). There was no mention of whether CR was achieved in patients with blast-phase MPN or those with post-MPN AML. Both studies are still ongoing.
Moreover, 2 patients with post-MF AML have been reported to be successfully managed with a combination of ruxolitinib and 5-azacytidine, achieving stable disease []. Moreover, another 3 patients with blast-phase MPN (marrow blasts <20%) were treated with a combination of ruxolitinib and low-dose decitabine or 5-azacytidine, achieving partial remission, stable disease, and clinical improvement, respectively []. There was no mention of the post-treatment cytogenetic status of the patients in any of the studies referenced above. Finally, ruxolitinib has been efficiently used in combination with intensive chemotherapy in 6 patients with blast-phase MPN prior to allogeneic hematopoietic stem cell transplantation [].
To our knowledge, this is the first report of a patient with a full-blown post-MF AML achieving a CR with the combination of ruxolitinib and 5-azacytidine. This impressive result was not only rapidly achieved (after only 2 cycles of treatment), but, moreover, was not accompanied by any grade 3/4 toxicities and lasted for at least 11 months since initiation of the combination regimen.
Acknowledgements
We would like to thank Ms. Evita Alexopoulos for copy-editing the final manuscript.
Statement of Ethics
The patient’s personal information was kept confidential throughout the process of data collection and reporting. The patient provided her consent for the case report to be published by signing an informed consent form.
Disclosure Statement
P.T.D. reports personal fees for presentations and advisory roles from Novartis, Sandoz, and Roche. N.-A.V. reports personal fees for presentations and advisory roles from Novartis, Celgene, Janssen, and Roche. The remaining authors have no conflict of interest to report.
Author Contributions
P.T.D., N.G., and S.H. wrote the manuscript; N.-A.V. critically revised the manuscript; P.T.D. and N.-A.V. approved the final version of the manuscript.
References
- 1. Huang J, Li CY, Mesa RA, Wu W, Hanson CA, Pardanani A, et al Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112(12):2726–32.
- 2. Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol. 2012;30(24):2981–7.
- 3. Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901.
- 4. Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–7.
- 5. Kennedy JA, Atenafu EG, Messner HA, Craddock KJ, Brandwein JM, Lipton JH, et al Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121(14):2725–33.
- 6. Mascarenhas J, Navada S, Malone A, Rodriguez A, Najfeld V, Hoffman R. Therapeutic options for patients with myelofibrosis in blast phase. Leuk Res. 2010;34(9):1246–9.
- 7. Thepot S, Itzykson R, Seegers V, Raffoux E, Quesnel B, Chait Y, et alGroupe Francophone des Myelodysplasies (GFM). Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood. 2010;116(19):3735–42.
- 8. Eghtedar A, Verstovsek S, Estrov Z, Burger J, Cortes J, Bivins C, et al Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119(20):4614–8.
- 9. Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–81.
- 10. Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A, et alItalian Registry of Myelofibrosis with Myeloid MetaplasiaMyeloproliferative Disorders Research Consortium. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007;38(3):280–6.
- 11. Mascarenhas J. Rationale for combination therapy in myelofibrosis. Best Pract Res Clin Haematol. 2014;27(2):197–208.
- 12. Shi J, Zhao Y, Ishii T, Hu W, Sozer S, Zhang W, et al Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res. 2007;67(13):6417–24.
- 13. Daver N, Cortes JE, Pemmaraju N, Jabbour EJ, Bose P, Zhou L, et al Ruxolitinib (RUX) in Combination with 5-Azacytidine (AZA) As Therapy for Patients (pts) with Myelofibrosis (MF). Blood. 2016;128:1127.
- 14. Rampal RK, Mascarenhas JO, Kosiorek HE, Price L, Berenzon D, Hexner E, et al Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast-phase myeloproliferative neoplasms. Blood Adv. 2018;2(24):3572–80.
- 15. Mwirigi A, Galli S, Keohane C, Raj K, Radia DH, Harrison CN, et al Combination therapy with ruxolitinib plus 5-azacytidine or continuous infusion of low dose cytarabine is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2014;167(5):714–6.
- 16. Tabarroki A, Saunthararajah Y, Visconte V, Cinalli T, Colaluca K, Rogers HJ, et al Ruxolitinib in combination with DNA methyltransferase inhibitors: clinical responses in patients with symptomatic myelofibrosis with cytopenias and elevated blast(s) counts. Leuk Lymphoma. 2015;56(2):497–9.
- 17. Devillier R, Raffoux E, Rey J, Lengline E, Ronchetti AM, Sebert M, et al Combination therapy with ruxolitinib plus intensive treatment strategy is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2016;172(4):628–30.
