Introduction
Abdominal wall defects are caused by either abdominal wall tumors or huge incisional hernia. Tumors arising from the abdominal wall have variable histological types and biological behaviors, and surgical resection with adequate free margins is considered the main line of treatment []. Fibromas, lipomas, and hemangiomas are the most common benign tumors and constitute about 26–40% of all abdominal wall tumors. Malignant neoplasms, including fibrosarcomas, leiomyosarcomas, and lymphangiosarcomas, are soft tissue sarcomas and account only for 5% of abdominal wall neoplasms and 1% of all tumors [].
Desmoid tumors, desmoid-type fibromatosis, or aggressive fibromatosis are subtypes of a benign mesenchymal neoplasm with monoclonal proliferation, which belong to a family of myofibroblastic fibromatosis characterized by aggressive local infiltration of surrounding tissues, with high rates of local recurrence, despite no distant metastatic potentials []. Resection of large anterior abdominal wall tumors will cause sizable full-thickness abdominal wall defects that need reconstruction and it remains a challenging point for most surgeons. There are many varieties in the methods used in reconstruction of such complex defects, including the mesh reinforcement, autologous flaps, and component separation techniques (CST) [].
Meshes have played an important role in the process of reconstruction of the anterior abdominal wall. Synthetic meshes such as polypropylene (PP) could significantly reduce the hernia development; however, its nonabsorbable characteristic may lead to many complications such as infections, chronic pain, bowel adhesions, obstruction, and fistula formation in severe conditions [].
The omental flap was first described by Senn in 1880 and has been used for abdominal wall reconstruction []. The omental flap has unique protective characteristics against infection and has regenerative properties against ischemia []. Omental flaps can be used in massive abdominal wall reconstruction with the synthetic meshes, thus facilitating the process of mesh integration into tissues and preventing further infections []. This study aims to review our center experience with the omental flap use in the immediate mesh reinforcement for reconstruction of the abdominal wall defects.
Patients and Methods
Between July 2016 and February 2021, we retrospectively reviewed the internal database registry of the Oncology Center, Mansoura University (OCMU) Egypt, for patients with type II (myofascial) abdominal wall defects after abdominal wall tumor resection and were reconstructed with omental flap and synthetic PP mesh. Approval from the Institutional Research Board (IRB) of the Faculty of Medicine, Mansoura University, code (R.21.03.1273) was obtained.
Inclusion Criteria
Patients with type II (myofascial) defects after abdominal wall tumor resection and deserve reconstruction.
Exclusion Criteria
Patients with abdominal wall defects type I and type III that needed skin flaps after abdominal wall tumor resection. Thirty-two patients met the inclusion criteria and we collected demographic data including age, gender, the body mass index (BMI) of the patients, history of previous tumor resections, and the pathological type of the tumors resected. Operative data, i.e., the size of the defect, the mesh size, intra-abdominal tumor extension, postoperative outcomes, and complications were collected and analyzed.
Surgical Techniques
Computerized tomography (CT) abdomen and pelvis was done for all patients prior to surgery to assess any intra-abdominal extension of the tumors. General anesthesia was applied to all patients. Longitudinal skin ellipse surrounding the tumor was done to reach adequate margins and all layers of the anterior abdominal wall were resected en bloc with the tumor.
The abdominal wall defects created after tumor resection were of myofascial type. The high BMI of our patients with redundant abdomen facilitated the approximation of the edges of the defects. However, in patients with small-sized tumors, the defects were closed without any significant tension. Approximation of the defect edges was done by mobilization of the abdominal wall muscles from the subcutaneous tissue (Fig. 1), then the greater omentum was mobilized and dissected to have a long vascularized omental flap. The omental flap was sutured meticulously to the defect edge using Vicryl sutures (Fig. 2). A synthetic PP mesh was then inserted, and the mesh size varies according to the defect size in every case. A bridged repair with mesh placement in the pro-rectus planes was done, after achieving the maximal advancement of the defect (Fig. 3). We did not utilize in this study any antiadhesive meshes or biologic meshes. The meshes were sutured to the edges of the defect using Prolene sutures. A subcutaneous suction drain (Redivac, 18 Fr) was inserted for drainage of any serous fluid formed after mesh application and an intra-abdominal drain was inserted in cases with bowel resection.
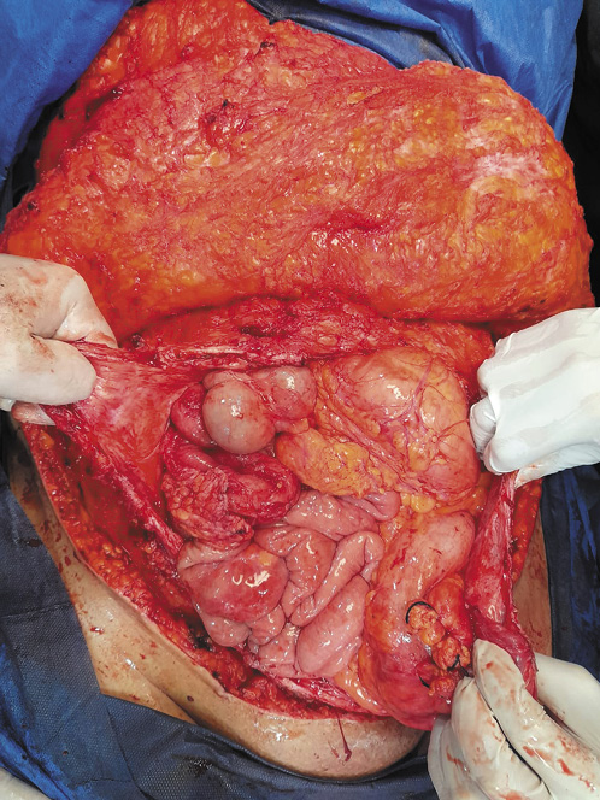
Fig. 1
Approximation of the edge of the defect after tumor resection.
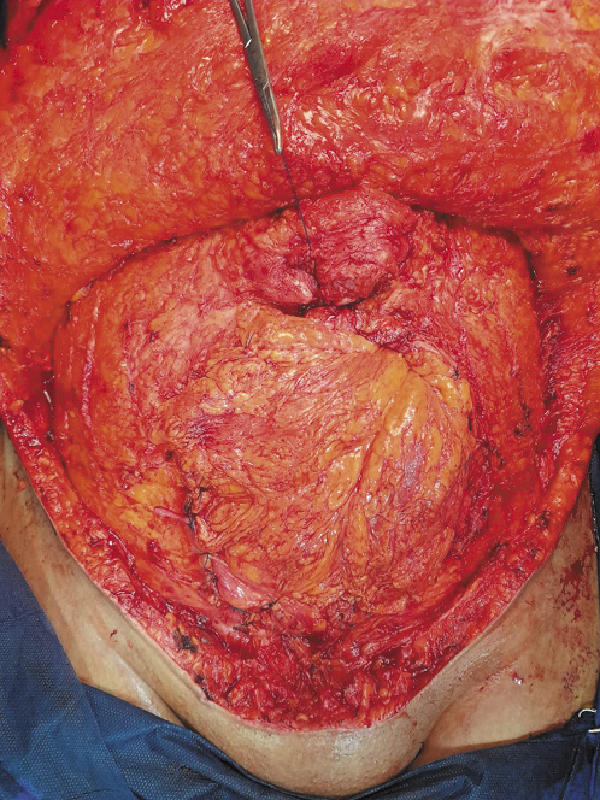
Fig. 2
Suturing of the omental flap to the edge of the defect.
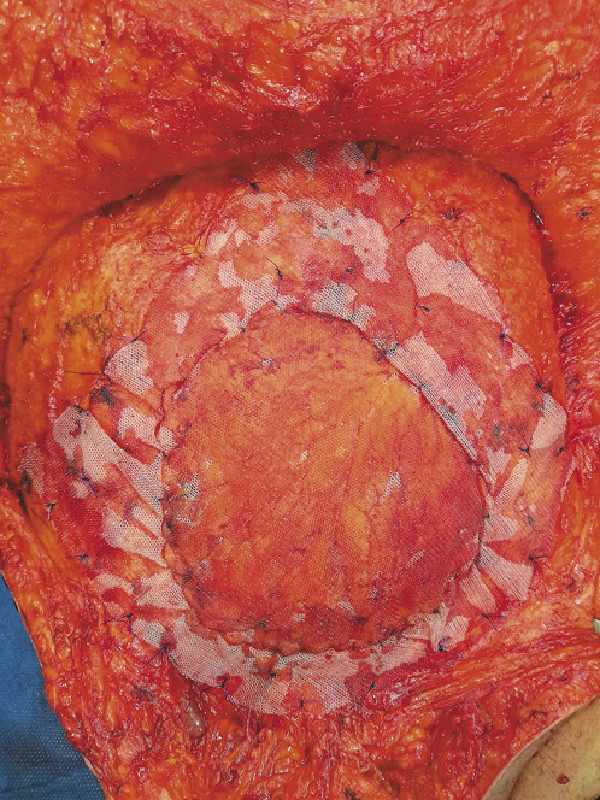
Fig. 3
Mesh placement in the pro-rectus planes after fixation of the omental flap.
An abdominal binder was applied to all patients postoperatively, for 3–6 months, and prophylactic antibiotics were also given postoperatively for 7 days. Patients started oral intake when the bowel functions returned. Drains were removed when their discharge was <20 mL/day for 2 successive days.
Follow-Up
We followed up with all the patients in the outpatient clinic. Abdominal ultrasonography and computerized tomography scans were done to assess tumor recurrence and subtle hernia at intervals of 6 months post-resection.
Statistical Analysis
Analysis of data was performed using Statistical Package for Scientific Studies (SPSS) v.26 for macOS v11.3. Numerical data were expressed as means ± standard deviation (SD).
Results
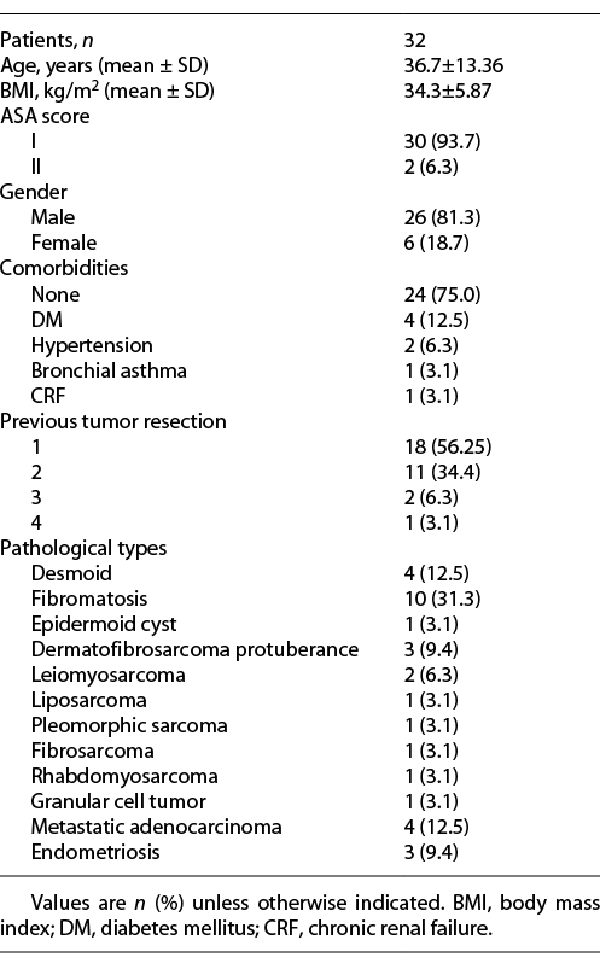
Between July 2016 and February 2021, 32 patients with abdominal wall neoplasms underwent resection in our center and the defects were closed with an omental flap and synthetic PP mesh. The mean age of the patients in this study was 36.7 ± 13.36 years. Most of the cases were males (81.3%). The BMI was 34.3 ± 5.87 kg/cm2. In addition, most of the patients (93.7%) had an ASA score I (according to the American Society of Anesthesiologists). Pathological findings of the resected tumors are reported in Table 1. Regarding the previous tumor resection, 18 cases had one previous resection, 11 cases had two previous resections, 2 cases had three previous resections, and only 1 case of dermatofibrosarcoma protuberance had a history of 4 previous resections.
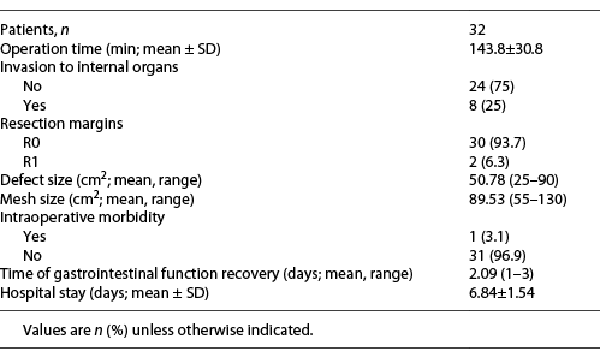
The surgical characteristics are shown in Table 2, the mean operative time was 143.8 ± 30.8 min. We reported 8 cases with intra-abdominal extension, small intestinal resections were done for four of them (2 cases with fibromatosis, 1 case with leiomyosarcoma, and another case with pleomorphic sarcoma), 1 patient with fibromatosis had nephrectomy of a transplanted kidney, and another patient with colonic adenocarcinoma was invading the anterior abdominal wall. Two patients with (liposarcoma and leiomyosarcoma) had incomplete (R1) resection due to infiltration of vital intra-abdominal organs, while complete (R0) resection was achieved for the rest of the 30 patients. The mean size of the abdominal wall defect was 50.8 cm2 (range: 25–90 cm2) and the meshes used in reconstruction had a mean size of 89.5 cm2 (range: 55–130 cm2). Nephrectomy of the transplanted kidney was the only intraoperative morbidity reported.
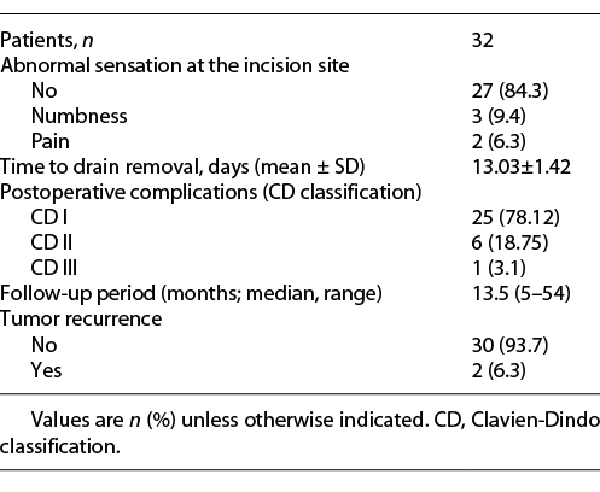
The gastrointestinal function was regained within 2 days (range: 1–3 days). The mean hospital stay was 6.8 ± 1.5 days. Drains were removed after 13 ± 1.42 days postoperatively. We had 6 cases with postoperative complications grade II (according to Clavien-Dindo classification) (Table 3), 3 cases with wound infection in the early postoperative period were managed conservatively, 1 case developed hematoma, and 2 cases developed seroma that needed no intervention. One case had grade III complication in the form of wound infection that developed 3 months after the operation and required drainage only without the need for mesh removal. Five patients developed abnormal sensations (numbness in 3 cases and pain over the incisional line in 2 cases). The median follow-up period was 13.5 months (range: 5–54 months), tumor recurrence was reported in 2 cases with fibromatosis. No cases developed incisional/ventral hernias during the follow-up period and 2 patients were reported dead due to cancer progression.
Discussion
Abdominal wall defects have many causes such as trauma, neoplasms, or surgical resection. For abdominal wall neoplasms, resection with safety margins is the main line of treatment and usually results in a complex abdominal wall defect. Large abdominal wall defects are challenging to most surgeons, as they necessitate in their reconstruction the use of autologous flaps or synthetic mesh [].
Abdominal wall neoplasms are classified into benign, borderline, and malignant types and the latter one may be primary or secondary []. Fibromatosis was the most common tumor type in our study (n = 32, 31.3%); it belongs to myofibroblastic tumors with a high rate of local recurrence but still with no metastatic potentials. Primary malignant tumors of the abdominal wall are rare, while secondaries of a distant primary are more common comparatively; however, the abdominal wall metastasis is generally uncommon []. In this study, we reported 4 cases of metastatic adenocarcinoma (the primary was originating from the colon, stomach, endometrium, and hepatobiliary tree), while Zhao et al. [] reported 6 cases of metastatic carcinoma (two of gastrointestinal tract origin, one of hepatic origin, two of endometrial origin, and one of unknown origin).
We have also experienced multiple varieties of other abdominal wall neoplasms such as (desmoid tumors, fibromatosis, dermatofibrosarcoma protuberance, endometriosis, liposarcoma, leiomyosarcoma, pleomorphic sarcoma, fibrosarcoma, rhabdomyosarcoma, and granular cell tumor). Generally, abdominal wall tumors are variable and include benign tumors such as neurofibromas, and malignant tumors such as liposarcomas and rhabdomyosarcomas [, ].
The reconstruction of the abdominal wall defects after complete resection of large abdominal wall tumors with the surrounding normal safety margin is difficult and needs surgical experience with the use of variable types of tissue grafts and flaps, CST, or mesh repairs. Flap reconstruction for abdominal wall defects is a well-known technique with multiple varieties of the donor sites and has the best outcomes when combined with mesh reinforcement []. However, this tissue transfer has some limitations, including the morbidity of the donor site, patient recovery, difficulty to attain desirable flap size, and surgeon’s experience with such procedures [].
CST (Ramirez technique) was used for primary closure of the midline defects through mobilization of the abdominal wall muscles medially []. It is a commonly used procedure that allows closure of the midline defects successfully in 80% of the cases. It was combined with biologic mesh placement in a study that included 80 patients with abdominal hernia and has improved the hernia recurrence rate []. However, CST is associated with wound complications due to creation of large subcutaneous space, which accumulate fluid and potentially cause devascularization of the skin flaps which lead to skin loss [].
Omental flaps had been used a long time ago in variable surgical procedures to decrease the operative morbidities such as seroma, hematoma, and infection by obliterating the dead space with a well-vascularized tissue []. Moreover, omental flaps may show resistance to the effects of radiation therapy; because it is a fatty organ that makes the flap very soft and less atrophic after the radiotherapy.
Mesh reinforcement has been established for many decades for anterior abdominal wall defects. We used the omental flap with mesh reinforcement to avoid the complications associated with isolated synthetic mesh repair []. All patients in this study had an omental flap with mesh reinforcement immediately after tumor resection, and the mesh was placed in the pro-rectus plane (bridged Onlay mesh) according to the defect size and the degree of mobilization of anterior abdominal wall muscles.
The high BMI of our patients and their redundant abdominal wall have made the defect closure easier with adequate mobilization of abdominal wall muscles. The BMI reported in another study was less than ours as it was about 22.89 ± 4.09 kg/cm2 []. Although all patients were with recurrent tumors after previous resections, most of them (56.25%) had a previous single-time resection, which made this method of reconstruction easier and more feasible as it did not need any myocutaneous flap. We used synthetic PP mesh in all patients enrolled in this study as there was no direct contact between the mesh and the abdominal viscera in the presence of an omental flap. So, there was no need to use antiadhesive or biologic mesh with its high cost.
It is well known that bridged onlay mesh when used alone associated with a high incidence of complications such as tissue adhesion, intestinal fistula, and hernia development []; despite underlay (preperitoneal/retromuscular) mesh is the preferred position because it is associated with reduced wound complications and low rates of hernia recurrence, but it requires longer duration for retrorectus muscle dissection and associated with chronic pain. Moreover, it causes dense adhesions, enterocutaneous fistula, and needs prolonged drainage []. In patients with recurrent abdominal surgery or with abdominal wall tumors, it is not preferred to use sublay mesh as the muscles will be severely lacerated. Approximation to the midline with placement of onlay mesh [] has been shown to be effective, with less incidence of complications [].
The patients in the current study did not experience any postoperative distension as the gastrointestinal function was returned within 2 days after surgery and the patients initiated oral intake despite the dissection of the greater omentum for flap formation. Postoperative morbidities in this study were in the form of wound infection in 4 cases; none of them required mesh removal. Infection may be attributed to the use of synthetic meshes with a high rate of contamination. Moreover, patients’ medical history of diabetes or receiving chemotherapy or other immunosuppressive drugs may be a contributing factor to infection, so we used prophylactic antibiotics for 7 days postoperatively. A meta-analysis has discussed the risk factors for mesh-related infections after hernia surgery and found that the crude rate of infection is 5% [].
Seroma is considered a common complication with mesh reinforcement after abdominal wall tumor resection due to foreign body reaction to the synthetic mesh and large subcutaneous space formation []. Only 2 cases (6.25%) in our study have developed mild seroma that was treated conservatively as the suction drains were removed within 13 days postoperatively to give enough time for drainage of any serous fluid accumulation. The rate of seroma formation after incisional hernia repairs with mesh was 21% despite the postoperative drainage [].
Postoperative hematoma has developed in 1 patient, and it was managed conservatively as the patient was receiving anticoagulant drugs and had a large dead space after tumor resection. We had 5 patients with abnormal sensations in the wound and they were reassured without the need for any intervention as it did not interfere with their daily life activities. It was thought to be due to the use of synthetic PP mesh with tissue trauma during mesh fixation and dissection of abdominal wall muscle for approximation and narrowing of the defects. Sorour [] has reported the interposition of the omentum or peritoneum during repair of large abdominal hernia using synthetic mesh and reported wound infection in 5.6% of their cases, seroma formation in 11.4%, chest infection in 7.6%, and deep vein thrombosis in 1.9% of the cases with no incidence of chronic pain, intestinal obstruction, or enterocutaneous fistula.
The most common and concerning complication after resection of abdominal wall tumors is the tumor recurrence, negative resection margins is one of the factors that decrease the rate of recurrence, as well as the tumor behavior and aggression []. The recommended safety margin for resection of abdominal wall tumors is >2 cm of the normal tissue surrounding the palpable tumor []. However, it is difficult sometimes to reach an adequate margin in some cases, due to the invasion of vital irresectable structures. We had 2 patients in this study with incomplete resection; one of them had a fungating liposarcoma, infiltrating multiple intra-abdominal structures. The other patient had recurrent leiomyosarcoma, for the third time, which has progressed on adjuvant chemoradiotherapy.
Moreover, we had also a 20-year-old male patient with a history of renal transplant 2 years ago presented to our center with anterior abdominal wall fibromatosis, and with exploration, the mass was infiltrating the transplanted kidney, and nephrectomy of the grafted kidney was done after intraoperative consultation of urology surgeons. The patients were followed up in this study within 13.5 months and tumor recurrence was reported in 2 cases with fibromatosis after 8 and 12 months after surgery and they were treated with redo surgical resection. During the follow-up period, no cases developed ventral hernia or fistula in comparison with other studies [].
The defects in our study were type II (myofascial); type I abdominal wall defects are easily closed primary; type III defects (including myofascial and skin) need flaps, with or without mesh and if there is wound contamination, biological mesh or flaps are the best choices. Common myocutaneous flaps used in repair of type III abdominal wall defects are tensor fascia lata, latissimus dorsi flap, external oblique flap, internal oblique flap, rectus femoris flap, or omental flap []. We think that there is no standard technique for abdominal wall reconstruction after tumor resection as it depends on the defect size, defect type, tumor pathology, and patient acceptance for flap reconstruction.
Conclusions
Surgical resection of abdominal wall neoplasms with a safety margin is the mainstay of treatment. Reconstruction of anterior abdominal wall defects remains a great challenge with different techniques. Our study suggested that the use of omental flaps with synthetic mesh reinforcement in abdominal wall defects’ reconstruction is a feasible method and avoids the complications associated with the use of the synthetic mesh alone.
Acknowledgments
The authors are grateful to their patients and colleagues at the Oncology Center, Mansoura University.
Statement of Ethics
An approval was obtained from the Institutional Research Board (IRB) of the Faculty of Medicine, Mansoura University, code (R.21.03.1273). A written informed consent was obtained from all the patients included in this study.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
There is no fund to be reported.
Author Contributions
Amr Abouzid made substantial contributions to the methodology, validation, formal analysis, investigation, and writing of the original draft; Mosab Shetiwy reviewed and edited the submitted version; Amr Hossam revised the manuscript; and Mohamed Abd Elghaffar had made the visualization and project administration. All authors have approved the submitted version of the article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Stojadinovic A, Hoos A, Karpoff HM, Leung DH, Antonescu CR, Brennan MF, et al. Soft tissue tumors of the abdominal wall: analysis of disease patterns and treatment. Arch Surg. 2001;136(1):70–9. http://dx.doi.org/10.1001/archsurg.136.1.70.
- 2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. http://dx.doi.org/10.3322/canjclin.57.1.43.
- 3. Singer S, Maki RG, O’Sullivan B. Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: principles and practice of oncology. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. p. 1533–77.
- 4. Song Z, Yang D, Yang J, Nie X, Wu J, Song H, et al. Abdominal wall reconstruction following resection of large abdominal aggressive neoplasms using tensor fascia lata flap with or without mesh reinforcement. Hernia. 2018;22(2):333–41. http://dx.doi.org/10.1007/s10029-018-1738-8.
- 5. Abdollahi A, Maddah GH, Mehrabi BM, Jangjoo A, Forghani MN, Sharbaf N. Prosthetic incisional hernioplasty: clinical experience with 354 cases. Hernia. 2010;14(6):569–73. http://dx.doi.org/10.1007/s10029-010-0685-9.
- 6. Senn N. An experimental contribution to intestinal surgery, with special reference to the treatment of intestinal obstruction (continued). Ann Surg. 1888;7(3):171–86.
- 7. Kiricuta I. L’emploi du grand epiploon dans la chirugie du sein cancereus. Press Med. 1963;71:15–7. French.
- 8. Wong CH, Tan BK, Koong HN, Lim CH, Chia SJ, Song C. Use of the omentum flap as additional soft-tissue cover for abdominal wall defects reconstructed with Gore-Tex. Plast Reconstr Surg. 2005;116(6):1715–20. http://dx.doi.org/10.1097/01.prs.0000185664.33079.5d.
- 9. Shestak KC, Edington HJ, Johnson RR. The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg. 2000;105(2):731–9. http://dx.doi.org/10.1097/00006534-200002000-00041.
- 10. Koshariya M, Shukla S, Khan Z, Vikas V, Pratap Singh A, Baghel P, et al. Giant desmoid tumor of the anterior abdominal wall in a young female: a case report. Case Rep Surg. 2013;2013:780862. http://dx.doi.org/10.1155/2013/780862.
- 11. Meshikhes AN, Al-Badr SH, Sulais EA, Al-Qudaihi HM. Late metastatic endometrial carcinoma at the repair site of an abdominal wall incisional hernia. Saudi Med J. 2017;38(5):546–8. http://dx.doi.org/10.15537/smj.2017.5.17395.
- 12. Zhao X, Cao Z, Nie Y, Liu J, Yuan X, Chen J, et al. Retrospective analysis of defect reconstruction after abdominal wall tumor resection in 30 patients. Hernia. 2021;25(2):375–81. http://dx.doi.org/10.1007/s10029-020-02219-1.
- 13. Patel M, Rani KU, Sharma M, Bhatnagar A. A rare case of giant solitary neurofibroma of abdominal wall masked by pregnancy. J Clin Diagn Res. 2017;11(8):QD08–9. http://dx.doi.org/10.7860/JCDR/2017/25629.10482.
- 14. Kovačević P, Veličkov AV, Stojiljković D, Veličkov AI, Ćeranić Z. [Reconstruction of full thickness abdominal wall defect following tumor resection: a case report]. Srpski arhiv za celokupno lekarstvo. 2014;142(5–6):347–50. Serbian. http://dx.doi.org/10.2298/sarh1406347k.
- 15. Bodin F, Dissaux C, Romain B, Rohr S, Brigand C, Bruant-Rodier C. Complex abdominal wall defect reconstruction using a latissimus dorsi free flap with mesh after malignant tumor resection. Microsurgery. 2017;37(1):38–43. http://dx.doi.org/10.1002/micr.22434.
- 16. Gu Y, Tang R, Gong DQ, Qian YL. Reconstruction of the abdominal wall by using a combination of the human acellular dermal matrix implant and an interpositional omentum flap after extensive tumor resection in patients with abdominal wall neoplasm: a preliminary result. World J Gastroenterol. 2008;14(5):752–7. http://dx.doi.org/10.3748/wjg.14.752.
- 17. Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–26.
- 18. Itani KM, Rosen M, Vargo D, Awad SS, DeNoto G III, Butler CE, et al. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery. 2012;152(3):498–505. http://dx.doi.org/10.1016/j.surg.2012.04.008.
- 19. Cornette B, De Bacquer D, Berrevoet F. Component separation technique for giant incisional hernia: a systematic review. Am J Surg. 2018;215(4):719–26. http://dx.doi.org/10.1016/j.amjsurg.2017.07.032.
- 20. Welten VM, Fields AC, Lu P, Goldberg JE, Irani J, Bleday R, et al. Omental flaps in patients undergoing abdominoperineal resection for rectal cancer. Int J Colorectal Dis. 2019;34(7):1227–32. http://dx.doi.org/10.1007/s00384-019-03319-w.
- 21. Atema JJ, Furnée EJ, Maeda Y, Warusavitarne J, Tanis PJ, Bemelman WA, et al. Major complex abdominal wall repair in contaminated fields with use of a non-cross-linked biologic mesh: a dual-institutional experience. World J Surg. 2017;41(8):1993–9. http://dx.doi.org/10.1007/s00268-017-3962-2.
- 22. Kapiris SA, Brough WA, Royston CMS, O’Boyle C, Sedman PC. Laparoscopic transabdominal preperitoneal (TAPP) hernia repair. Surg Endos. 2001;15(9):972–5.
- 23. Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–14. http://dx.doi.org/10.1007/s10029-009-0518-x.
- 24. Chevrel JP. [The treatment of large midline incisional hernias by “overcoat” plasty and prothesis (author’s transl)]. La Nouvelle presse medicale. 1979;8(9):695–6. French.
- 25. Sailes FC, Walls J, Guelig D, Mirzabeigi M, Long WD, Crawford A, et al. Synthetic and biological mesh in component separation: a 10-year single institution review. Ann Plast Surg. 2010;64(5):696–8. http://dx.doi.org/10.1097/SAP.0b013e3181dc8409.
- 26. Mavros MN, Athanasiou S, Alexiou VG, Mitsikostas PK, Peppas G, Falagas ME. Risk factors for mesh-related infections after hernia repair surgery: a meta-analysis of cohort studies. World J Surg. 2011;35(11):2389–98. http://dx.doi.org/10.1007/s00268-011-1266-5.
- 27. Dudai M, Gilboa Ittah K. Intraoperative hypertonic saline irrigation preventing seroma formation and reducing drain secretion in extended endoscopic hernia and linea alba reconstruction glue. Hernia. 2019;23(6):1291–6. http://dx.doi.org/10.1007/s10029-019-01956-2.
- 28. Tuveri M, Tuveri A, Nicolò E. Repair of large abdominal incisional hernia by reconstructing the midline and use of an onlay of biological material. Am J Surg. 2011;202(1):e7–11. http://dx.doi.org/10.1016/j.amjsurg.2010.06.005.
- 29. Sorour MA. Interposition of the omentum and/or the peritoneum in the emergency repair of large ventral hernias with polypropylene mesh. Int J Surg. 2014;12(6):578–86. http://dx.doi.org/10.1016/j.ijsu.2014.04.009.
- 30. Majors J, Stoikes NF, Nejati R, Deneve JL. Resection and abdominal wall reconstruction of a desmoid tumor with endometrioma features. Case Rep Surg. 2016;2016:9453450. http://dx.doi.org/10.1155/2016/9453450.
- 31. Wanjeri JK, Opeya CJ. A massive abdominal wall desmoid tumor occurring in a laparotomy scar: a case report. World J Surg Oncol. 2011;9(1):35–4. http://dx.doi.org/10.1186/1477-7819-9-35.
- 32. Yezhelyev MV, Deigni O, Losken A. Management of full-thickness abdominal wall defects following tumor resection. Ann Plast Surg. 2012;69(2):186–91. http://dx.doi.org/10.1097/SAP.0b013e31821d0715.
- 33. Ramasastry SS, Futrell JW, Williams SL, Hurwitz DJ. Internal oblique muscle pedicle flap for coverage of a major soft tissue defect of the groin. Ann Plast Surg. 1985;15(1):57–60. http://dx.doi.org/10.1097/00000637-198507000-00007.