Introduction
Ovarian cancer is the leading cause of death from gynecologic cancer worldwide, and epithelial ovarian cancer (EOC) accounts for over 90% of all ovarian cancers and affects more than 200,000 women, with 125,000 deaths every year []. The patients generally have a poor prognosis due to high chemotherapy resistance and late-stage disease diagnosis.
The current standard treatment for EOC remains surgery and platinum-based cytotoxic chemotherapy. Although significant progress has been made in the treatment, most women with advanced disease will develop platinum-resistant disease, and progression-free survival (PFS) is fairly constant at about 18 months. Approximately 80% of women with advanced ovarian cancer will have tumor progression and more commonly a recurrence within 1-2 years [].
Nowadays, tumor recurrence followed by chemoresistance is still an enormous clinical challenge. It is clear that EOC is a heterogeneous disease, and a platinum-based chemotherapy is not the optimal regimen for all patients. In general, patients with disease progression during first-line platinum-based chemotherapy or within 6 months of the end of treatment are identified as ‘platinum-resistant' and characterized by a very poor prognosis. However, such patients receive routine treatment like that initially given to ‘platinum-sensitive' patients after surgery, which is usually of little or no benefit and inevitably leads to short-term recurrence, increased morbidity, and undue costs [,].
Therefore, it is imperative to discover a proven method to identify patients who are resistant to platinum-based treatment and at high risk of early progression. If identified early, platinum-resistant EOC patients could benefit from alternate and/or additional therapeutic schedules in first-line therapy.
The nutritional and immunological status before surgery plays an indispensable role in the occurrence of postoperative complications and for OS rates of patient with malignant tumors [,]. The prognostic nutritional index (PNI), which can be calculated based on the serum albumin concentration and peripheral blood lymphocyte count, was originally proposed by Onodera et al. [ ]to evaluate the nutritional and immunological status of patients who undergo gastrointestinal surgery. The index has attracted a lot of interest as a novel prognostic marker in various cancers including renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, pancreatic cancer, and gastric carcinoma [,,,,]. However, to our knowledge, evidence for the use of PNI as a direct predictor of chemotherapy outcome in patients with EOC is still lacking. Therefore, we retrospectively studied the relationship between PNI and clinicopathological characteristics of EOC patients who were treated with platinum-based chemotherapy, in an attempt to determine whether the preoperative PNI can be used as a marker for predicting chemotherapy response and survival outcome in patients with EOC.
Patients and Methods
Patient Selection
This retrospective study reviewed the medical records of 344 patients pathologically proven to have EOC between 2005 and 2010, with a follow-up of more than 60 months. For each patient, the following clinical, biochemical, and pathological variables were collected: age, body mass index (BMI), disease characteristics (histology, stage, surgery, residual tumor, lymph nodes metastasis), and relapse (progression-free interval, location, and treatment). The patients underwent comprehensive surgical staging or tumor debulking as clinically indicated, including total hysterectomy, bilateral oophorectomy, peritoneal washing, omentectomy, pelvic/para-aortic nodal biopsy, and multiple peritoneal biopsies. Optimal debulking was defined as a procedure that left a maximum residual tumor of < 1 cm in diameter. All cancer patients underwent the postoperative 6 cycles of adjuvant chemotherapy performed every 3 weeks according to the scheme carboplatin (area under the curve (AUC) = 5) and paclitaxel 175 mg/m2; 2 patients received a 20% reduced dosage due to grade 3/4 toxicity. Written informed consent was obtained from all patients prior to blood collection and surgery.
All patients underwent a radiologic evaluation after 6 cycles. If patients experienced an increase in carbohydrate antigen (CA)-125 during follow-up, radiologic evaluation was anticipated. Response to treatment and diagnosis of progression were determined according to the Response Evaluation Criteria In Solid Tumors (RECIST).
Patients were divided into 2 groups in accordance with the length of the progression-free interval. Patients with disease progression within 6 months of completion of first-line platinum-based chemotherapy were defined as the platinum-resistant group (P-R group), while all others were defined as the platinum-sensitive group (P-S group). Differences in clinical features and prognosis were compared between the 2 groups.
Blood samples were collected before surgery to measure albumin, lymphocyte percentage, lymphocyte count, and CA-125 in the peripheral blood. The PNI was calculated according to the following formula: 10 × serum albumin value (g/dl) + 0.005 × lymphocyte count (per mm3) in the peripheral blood.
Statistical Analysis
All statistical analyses were performed with SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Chi-square or Fisher's exact test were used for comparative analysis of categorical variables. Demographic characteristics and known risk factors of tumor recurrence were analyzed according to chemotherapy response using the Student's t-test for continuous variables. The Man-Whitney U-test was used for continuous variables without normal distribution. A receiver operating characteristic (ROC) curve was used to assess the discriminative role of the PNI level, and the best cut-off value was determined. Log-rank test statistics for the analysis of equality of survival distribution were performed. PFS was calculated from the date of the start of treatment to the date of recurrence or progression. OS was calculated from the date of the start of treatment to the date of death or last follow-up. The cumulative survival curve and median PFS time were estimated by use of the Kaplan-Meier method. Comparison between survival curves was carried out using the log-rank test. The relative importance of variables as independent predictors of PFS and OS was analyzed with multivariate Cox proportional hazard regression, and its associated 95% confidence interval (CI) was calculated. A two-tailed p value of < 0.05 was considered as statistically significant.
Results
Patient Characteristics
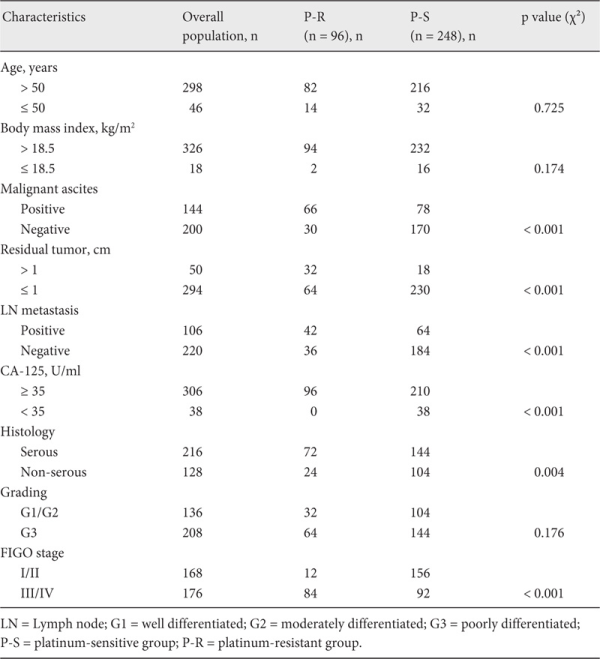
A total of 344 patients were enrolled. At the 6th month of follow-up, by the end of platinum-based chemotherapy, 96 (28%) patients were classified as platinum-resistant and 248 (72%) as platinum-sensitive. The median age of the patients was 55 years (range 45-84 years). Most of the patients presented with serous papillary histology (n = 216, 62.8%) and FIGO stage III (n = 126, 48.8%) at initial diagnosis. Table 1 shows the association of patients' clinicopathologic features with chemotherapy response. The median follow-up period was 72 months (range 61-97 months).
Predictive Value of PNI for Determining Response to Platinum-Based Chemotherapy
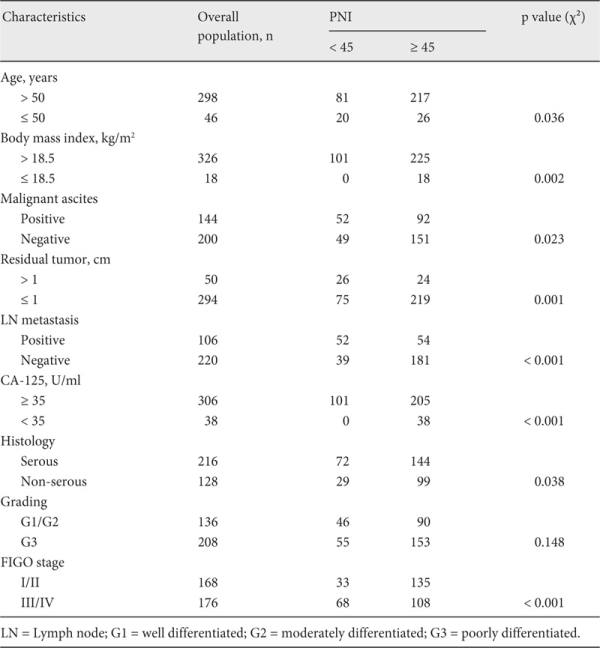
Features such as malignant ascites, residual tumor, lymph node metastasis, preoperative CA-125 value, histology, grading, and FIGO stage were found to be related to response to chemotherapy in EOC patients. The respective mean levels of serum albumin and peripheral lymphocyte count were 41.04 ± 4.53 g/dl and 1.73 ± 0.49/mm3 in the P-S group, and 38.95 ± 4.83 g/dl and 1.45 ± 0.37/mm3 in the P-R group. The mean levels of PNI were 49.70 ± 5.35 in the P-S group and 46.19 ± 6.21 in the P-R group. PNI levels were significantly different between the 2 groups (p < 0.001). Correlations were further observed between the preoperative PNI and clinicopathological factors. The PNI level was significantly associated with age, BMI, residual tumor, lymph node metastasis, CA-125, histology, and FIGO stage (p = 0.036, 0.002, 0.001, < 0.001, < 0.001, 0.038, and < 0.001, respectively) (table 2). ROC curves for platinum-based chemotherapy outcome prediction were plotted to verify the optimum cut-off point for PNI. AUC, sensitivity, specificity, positive and negative predictive values, and accuracy of PNI < 45 to predict platinum resistance were: 0.688, 62.50%, 83.47%, 59.41%, 85.19%, and 77.62%, respectively (fig. 1).
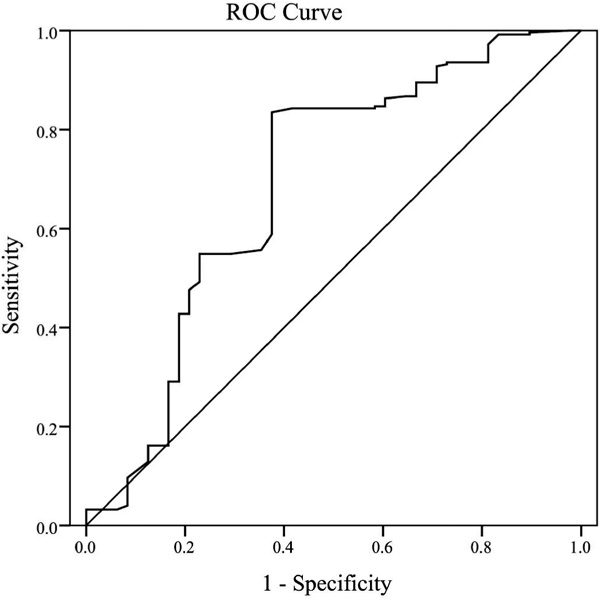
Fig. 1
Receiver operating characteristic curve analysis of the prognostic nutritional index (PNI) for platinum-based chemotherapy outcome prediction in the patients with epithelial ovarian cancer. Area under the curve = 0.688, 95% confidence interval 0.636-0.737; p < 0.001.
Prognostic Factors Influencing Long-Term Survival in EOC Patients with Platinum-Based Chemotherapy
The median PFS in the whole study population was 19 months. The median PFS of patients with a lower PNI (< 45) was 12 months (95% CI 10.62-13.38 months), whereas the median PFS of patients with a higher PNI (≥ 45) was 23 months (95% CI 18.03-27.97 months). The PFS rate was significantly worse in the low-PNI group than in the high-PNI group (p < 0.001) (fig. 2). Moreover, the OS rate was also significantly worse in the low-PNI group (p < 0.001) (fig. 3).
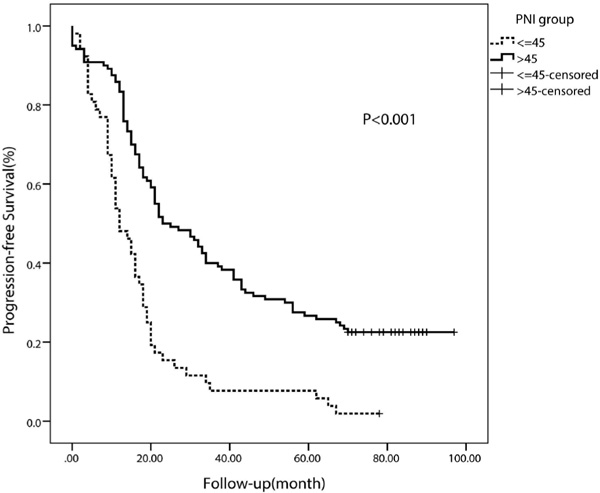
Fig. 2
Kaplan-Meier curve for progression-free survival regarding low versus high prognostic nutritional index (PNI) (p < 0.001).
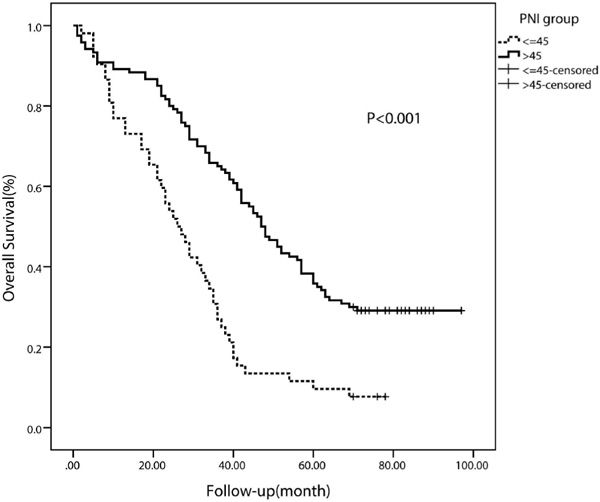
Fig. 3
Kaplan-Meier curve for overall survival regarding low versus high prognostic nutritional index (PNI) (p < 0.001).
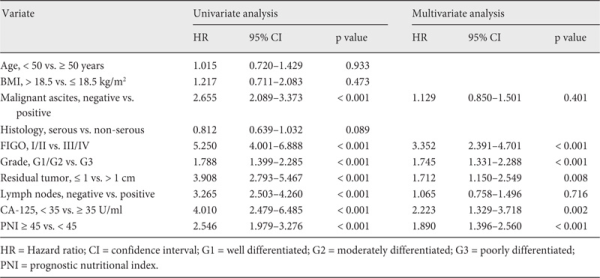
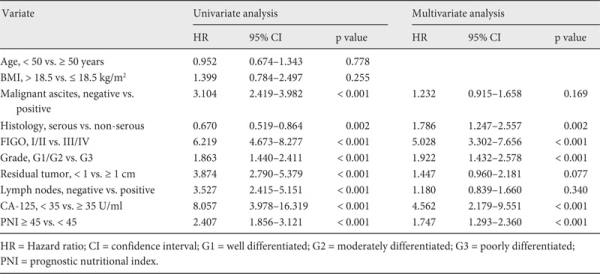
In order to assess the independent prognostic factors, we utilized multivariate Cox proportional hazards analysis as a control for other prognostic values. All patients (n = 344) were evaluated in univariate analysis. Malignant ascites, advanced FIGO stage, high grade, residual tumor, lymph node metastasis, CA-125 ≥ 35 U/ml, and PNI < 45 were found to be associated with PFS (table 3). In addition, a multivariate analysis indicated that advanced FIGO stage (hazard ratio (HR) 3.352, 95% CI 2.391-4.701; p < 0.001), poor differentiation (HR 1.745; 95% CI 1.331-2.288; p < 0.001), CA-125 ≥ 35 U/ml (HR 2.223, 95% CI 1.329-3.718; p = 0.002), residual tumor (HR 1.712, 95% CI 1.150-2.549; p = 0.008), and PNI < 45 (HR 1.890, 95% CI 1.396-2.560; p < 0.001) were poor prognostic factors for PFS. The correlations between OS and various clinicopathological factors are shown in table 4. According to multivariate analysis, preoperative PNI < 45 was an independent risk factor for poor OS (HR 1.747, 95% CI 1.293-2.360; p < 0.001).
Discussion
Calculation of the PNI was based on 2 simple laboratory parameters, albumin and absolute lymphocyte count, which are measured routinely in clinical practice. Albumin is a widely used parameter of nutrition and has been shown to correlate with postoperative complications [,]. In addition, low serum albumin has been reported to be an independent predictor of long-term outcomes in several malignant tumors. It has been detected that serum albumin is a stable predictor of prognosis during initial treatment in patients with diffuse large B cell lymphoma, and the preoperative albumin-to-globulin ratio has been verified to have predictive value for long-term mortality in nasopharyngeal carcinoma [,]. The peripheral lymphocyte count, as a component of the complete blood count, together with the neutrophil-to-lymphocyte ratio and platelets-to-lymphocyte ratio, has been reported to be a critical factor associated with OS in many types of cancers [,,]. It is a parameter of cell-mediated immunity which is important for the host defense against cancer, and a low lymphocyte count may be associated with immune suppression indicating an inadequate immunological response. Thus, we consider the PNI to be a comprehensive indicator for the assessment of the nutritional and immunological status of the patients.
Previous studies illustrated a decreased level of PNI to be an independent adverse prognostic factor in many different kinds of cancer. Mohri et al. [ ]demonstrated that PNI < 45 was an independent prognostic factor for poor survival and significantly correlated with the incidence of postoperative complications and shorter survival in patients with colorectal cancer. Another report pointed out that PNI was significantly associated with age, smoking habits, and weight loss in malignant pleural mesothelioma, and patients with a lower PNI (< 44.6) had a greater risk of death than those with a higher PNI (HR 2.29) []. All of the above studies suggested that the preoperative PNI may be clinically used, not only to identify patients at increased risk of postoperative complications but also to predict long-term survival after surgery. Similarly to the previous reports, in our study we verified that PNI could predict the outcomes of patients with EOC, and patients in the high PNI group had better outcomes than those in the low PNI group. The 3-year PFS and 3-year OS rates of patients were 39.0 and 64.2% in the high PNI group, and 8.2 and 26.5% in the low PNI group, respectively.
Although the prognostic value of PNI has been demonstrated in many malignancies [,,], the underlying mechanisms remain a matter of debate. Malnutrition, as well as compromised immunological status, are believed to be main factors that increase the risk of postoperative complications and tumor spread []. What is more, although PNI was initially proposed as an index of the nutritional status of a patient, albumin and lymphocyte count have a close relationship with the presence of inflammatory responses in cancer patients. It is probable, in view of its prognostic association, that PNI is a reflection of systemic inflammation. Just like other inflammatory markers, including neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, C-reactive protein and the modified Glasgow prognostic score, PNI is also believed to be involved in the systemic inflammatory response and plays an indispensable role in cancer growth and metastasis [,,]. Thus, PNI may serve as an indicator of chronic inflammation, immunity, and nutritional status, all of which are of prognostic significance in cancer.
Furthermore, research is now focusing on the application of PNI as an independent prognostic predictor of clinical benefit in patients receiving chemotherapy. It has been reported that PNI could be a marker for toxicity as well as host status in patients being treated with adjuvant chemotherapy after colorectal cancer surgery []. Similarly, a study of 80 patients with unresectable metastatic colorectal cancer proved that a PNI above an optimal threshold of 44.5 is an independent prognostic factor for OS, and that PNI is a useful marker for predicting the long-term outcome of patients receiving oxaliplatin or irinotecan plus 5-fluorouracil/leucovorin as first-line chemotherapy [].
Nowadays, resistance to platinum-based chemotherapy in women with EOC is a major clinical problem [,]. Despite improvements in median survival and OS using a combination of platinum compounds and paclitaxel, long-term survival rates for patients with advanced EOC remain disappointing. One of the chief obstacles for the use of anti-angiogenic therapy is the lack of predictive markers for treatment efficacy. Hence, it is crucial to identify an effective and simple index to predict chemotherapy response in order to establish optimal medical treatment.
To the best of our knowledge, this is the first study to assess the value of the PNI as a prognostic marker for predicting chemotherapeutic response in patients with EOC who receive platinum-based chemotherapy. A ROC curve was used to calculate the optimal cut-off value for PNI to predict the outcome of chemotherapy in EOC patients, and P-R patients exhibited a significantly lower level of PNI (p < 0.001). A PNI with a cut-off value of 45 is a better tool for differentiating between P-S and P-R patients with EOC. PNI < 45 when used individually had satisfactory specificity (83.47%) but only moderate sensitivity (62.5%).
In conclusion, we demonstrated that the preoperative PNI can be used as a simple and useful marker for predicting chemotherapeutic response and survival prognosis in patients with EOC. It should be noted that this was a retrospective study which may include some confounding factors by nature. For example, the nutritional status in patients with early-stage (I and II) ovarian cancer is better than that in patients with later-stage disease (table 2). Although a multivariate analysis was performed to control for other factors and confirmed PNI to be an independent prognostic factor, more evidence is needed to consolidate our results through larger prospective randomized clinical trials.
Disclosure Statement
None of the authors have any conflict of interest in connection with the submitted article. There were no funding sources supporting the work.
References
- 1. Siegel R, Naishadham D, Jemal A: Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30.
- 2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA: Ovarian cancer. Lancet 2014;384:1376-1388.
- 3. Davis A, Tinker AV, Friedlander M: ‘Platinum resistant' ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol 2014;133:624-631.
- 4. Martin LP, Schilder RJ: Management of recurrent ovarian carcinoma: current status and future directions. Semin Oncol 2009;36:112-125.
- 5. Benizri EI, Bereder JM, Rahili A, Bernard JL, Benchimol D: Ascites and malnutrition are predictive factors for incomplete cytoreductive surgery for peritoneal carcinomatosis from gastric cancer. Am J Surg 2013;205:668-673.
- 6. Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JJ, Clark PE: Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol 2011;59:923-928.
- 7. Onodera T, Goseki N, Kosaki G: (Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients). Nihon Geka Gakkai Zasshi 1984;85:1001-1005.
- 8. Hofbauer SL, Pantuck AJ, de Martino M, Lucca I, Haitel A, Shariat SF, Belldegrun AS, Klatte T: The preoperative prognostic nutritional index is an independent predictor of survival in patients with renal cell carcinoma. Urol Oncol 2015;33:61-68.
- 9. Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, Hirakawa K: Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 2015;141:307-313.
- 10. Pinato DJ, North BV, Sharma R: A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer 2012;106:1439-1445.
- 11. Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H: Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol 2014;20:10537-10544.
- 12. Hubbard TJ, Lawson-McLean A, Fearon KC: Nutritional predictors of postoperative outcome in pancreatic cancer (Br J Surg 2011;98:268-274). Br J Surg 2011;98:1032, 1032-1033.
- 13. Ellis MC, Cassera MA, Vetto JT, Orloff SL, Hansen PD, Billingsley KG: Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 2011;13:59-63.
- 14. Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, Chin CC: Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis 2011;26:473-481.
- 15. Eatrides J, Thompson Z, Lee JH, Bello C, Dalia S: Serum albumin as a stable predictor of prognosis during initial treatment in patients with diffuse large B cell lymphoma. Ann Hematol 2015;94:357-358.
- 16. Du XJ, Tang LL, Mao YP, Sun Y, Zeng MS, Kang TB, Jia WH, Lin AH, Ma J: The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One 2014;9:e94473.
- 17. Schueneman AJ, Sugar EA, Uram J, Bigelow E, Herman JM, Edil BH, Jaffee EM, Zheng L, Laheru DA: Low total lymphocyte count is associated with poor survival in patients with resected pancreatic adenocarcinoma receiving a GM-CSF secreting pancreatic tumor vaccine. Ann Surg Oncol 2013;20(suppl 3): S725-S730.
- 18. Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ, Lee JH: Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216-222.
- 19. Roxburgh CS, McMillan DC: Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-163.
- 20. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M: Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 2013;37:2688-2692.
- 21. Yao ZH, Tian GY, Wan YY, Kang YM, Guo HS, Liu QH, Lin DJ: Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol 2013;139:2117-2123.
- 22. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y: The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol 2013;20:2647-2654.
- 23. Douglas E, McMillan DC: Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat Rev 2014;40:685-691.
- 24. Choi KW, Hong SW, Chang YG, Lee WY, Lee B, Paik IW, Lee H: Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treat Res 2014;86:309-313.
- 25. De Martino M, Pantuck AJ, Hofbauer S, Waldert M, Shariat SF, Belldegrun AS, Klatte T: Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol 2013;190:1999-2004.
- 26. Sagawa M, Yoshimatsu K, Yokomizo H, Osawa G, Matsumoto A, Yano Y, Nakayama M, Yamaguchi K, Shiozawa S, Shimakawa T, Katsube T, Naritaka Y: (Assessment of host status in patients treated with mFOLFOX6 adjuvant chemotherapy after colorectal cancer surgery). Gan To Kagaku Ryoho 2013;40:1587-1589.
- 27. Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, Hirakawa K: Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 2015;141:307-313.
- 28. Jubb AM, Oates AJ, Holden S, Koeppen H: Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer 2006;6:626-635.
- 29. Jubb AM, Harris AL: Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 2010;11:1172-1183.