Introduction
Colorectal cancer (CRC) is one of the most prevalent digestive tract malignancies and is associated with a high mortality rate. It is estimated that approximately 1.8 million new CRC cases and 91,000 fatalities occurred globally in 2020 []. Global cancer statistics have ranked the incidence of CRC third among cancers and second in terms of mortality [], with rankings of third in incidence and mortality in the male population and second and third, respectively, for females []. The incidence and associated mortality rates are high in both China and the USA, and increasing trends have been observed in China [–].
Radiotherapy is a commonly used anti-tumor treatment, used in approximately 50% of cancer patients, and is often effective for controlling the tumor []. According to the radiation dose, radiotherapy is divided into conventional, hyperfractionated, and hypofractionated radiotherapy []. The classical explanation for the effects of radiotherapy is that it acts on the cancer directly or indirectly by inducing oxygen free radicals which subsequently destroy the DNA of the tumor cells [, ]. Although radiotherapy is an effective treatment against CRC, especially rectal cancer, however, it produces resistance in some cases [, ]. Prior investigations have focused largely on the development of radiotherapy resistance by tumor cells, for instance, resulting from alterations in anti-apoptotic genes [–], and there is limited information on the role of the tumor immune microenvironment (TIME) in radiotherapy resistance. The association of resistance with TIME, therefore, requires investigations. The TIME contains various cell types including cancer cells, bone marrow-derived inflammatory cells, fibroblastic cells, and lymphocytes, in addition to an extracellular matrix (ECM) made up of proteoglycans and collagen [, ].
This review describes alterations in the TIME in radiotherapy-resistant CRC patients. This is considered from five perspectives, namely, the tumor ECM, tumor vasculature, cancer-associated fibroblasts (CAFs), tumor-infiltrating immune cells, and cytokines secreted by both tumor and immune cells.
Tumor ECM
The ECM surrounds cells within tissues and organs. It provides physical support and scaffolding for the cells as well as mediating crucial biochemical and biomechanical signals that are important for cellular differentiation, tissue morphogenesis, and homeostasis []. During development, interactions between cells and the ECM regulate cell fate, differentiation, and migration []. The ECM also plays a key role in the TIME in radiotherapy-resistant CRC (Fig. 1a). It was found that radiation-resistant CRC increased the expression of TGF-β and the cell surface glycoprotein podocalyxin-like protein (PODXL) to promote tumor invasion and metastasis []. Furthermore, CRC patients with high TGF-β and PODXL expression have been shown to have worse prognosis [–]. In addition, the expression of the heparan sulfate proteoglycan syndecan-1 (SDC-1) is reduced in radiation-resistant CRC, promoting the expression of genes related to spheroid formation, cell viability, matrix gel invasiveness, and the epithelial-mesenchymal transition, while activating focal adhesion kinase (FAK) and β1-integrin expression and the Wnt signaling pathway, thereby promoting tumor invasion and metastasis [, ]. Some studies have indicated that cell ionizing radiation increases β-galactoside alpha-(2, 6)-sialyltransferase (ST6Gal I) expression and enhances glycoprotein sialylation, particularly of the key cell adhesion molecule integrin β1. Integrin β1 sialylation mediated by ST6Gal I contributes to cell adhesion was found to regulate CRC radiotherapy resistance []. Moreover, radiotherapy can stimulate CRC tumors to secrete placental growth factor (PlGF), which acts on nonirradiated paracrine tumor cells, resulting in radiotherapy resistance []. It was also discovered that patients with high expression of matrix metalloproteinase 1 (MMP1) and matrix metalloproteinase 2 (MMP2) in the ECM of CRC tumor cells developed distant metastases after radiotherapy [].
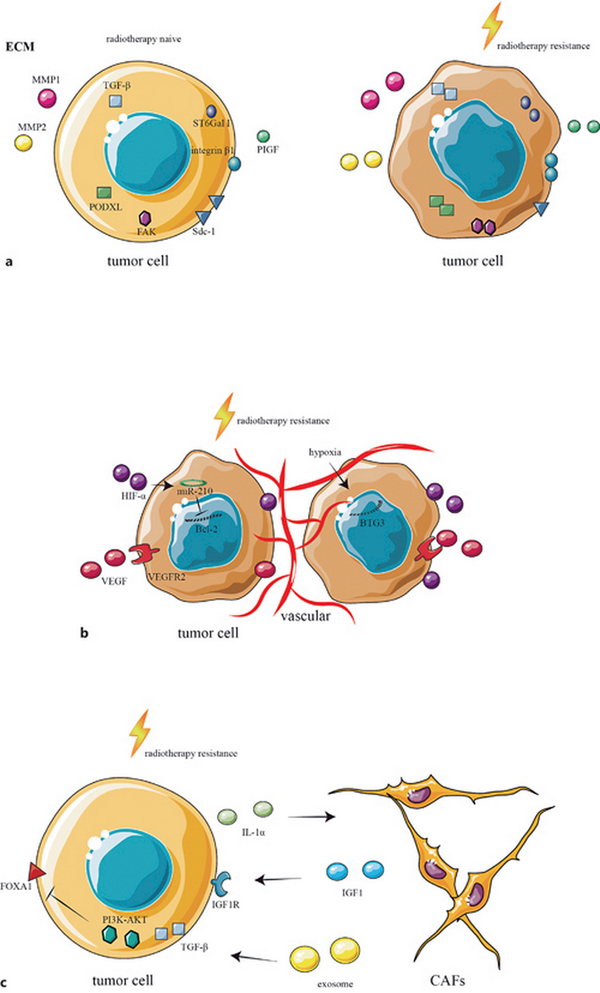
Fig. 1
ECM, vasculature, and CAFs in the TIME of radiotherapy-resistant CRC. a The ECM in the TIME of radiotherapy-resistant CRC, showing increased expression of TGF-β, PDOXL, FAK, integrin β1, PIGF, ST6Gal 1, MMP1, and MMP2, together with reduced expression of SDC-1. b The vasculature in the TIME of radiotherapy-resistant CRC, where HIF-α downregulates the expression of Bcl-2 through miR-210, increasing radiotherapy resistance. Increased expression of VEGFR2 and hypoxia-induced downregulation of BTG3 lead to CRC resistance to radiotherapy. c CAFs in the TIME of radiotherapy-resistant CRC, where the tumor secretes IL-1α to recruit and polarize CAFs, while CAF-secreted IGF1 induce CRC resistance to radiotherapy. CAFs also secrete exosomes leading to the activation of TGF-β and the PI3K-AKT signaling pathway and decreasing the expression of FOXA1, leading to radiotherapy resistance.
Tumor Vasculature
The tumor microenvironment also has an additional important component called tumor vasculature. It arises from two different biological mechanisms: angiogenesis, a process of new blood vessel formation from preexisting ones, and vasculogenesis, which forms new blood vessels by recruiting circulating endothelial progenitor cells []. Hypoxia-inducible factors are principally responsible for signaling the regulation of angiogenesis, which stimulates gene transcription-modulating angiogenesis activation []. Another crucial modulator is vascular endothelial growth factor (VEGF) and its receptor (VEGFR), which also stimulates angiogenesis []. The tumor vasculature changes after radiotherapy (Fig. 1b). Studies suggest that HIF-α and VEGF can promote tumor invasion and metastasis, and radiotherapy-resistant CRC expresses higher HIF-α and VEGF [–]. Furthermore, HIF-α downregulates the expression of Bcl-2 in CRC through mir-210, increasing CRC autophagy and reducing the sensitivity to radiotherapy []. The radiotherapy-resistant CRC highly expresses VEGFR, and transient activation of the VEGF/VEGFR2 pathway in tumor cells causes upregulation of VEGF, VEGFR2, and downstream proteins, promoting radiotherapy resistance by improving the vascular repair []. Hypoxia is one of the important factors of tumor resistance to radiotherapy. Studies have found that hypoxia induces the downregulation of B-cell translocation gene 3 (BTG3), resulting in CRC resistance to radiotherapy [].
Cancer-Associated Fibroblasts
CAFs are essential components of the tumor microenvironment that are activated by adhesion molecules, direct intercellular communication, growth, and other factors. Unlike normal fibroblasts, CAFs are in a state of perpetual activation, cannot transform to a normal phenotype or undergo apoptosis, and enhance tumor progression []. CAFs promote the development of malignancy by stimulating the production of growth factors and cytokines which induce angiogenesis, cell proliferation, metastasis, and invasion []. CAFs also play key roles in inducing radiotherapy-resistant TIMEs (Fig. 1c). Several studies have shown that the proportion of CAFs in the tumor matrices of radiotherapy-resistant CRC tumors was higher than in nonresistant tumors and, furthermore, that CRC patients with higher CAF percentages have a poor prognosis [–]. CRC can secrete IL-1α to recruit CAFs and polarize them to an inflammatory state after radiotherapy, which leads to radiotherapy resistance []. Radiotherapy-activated CAFs stimulate the expression of insulin-like growth factor 1 receptor (IGF1R) on CRC tumor cells through the action of paracrine insulin-like growth factor 1 (IGF1), thus promoting tumor metastasis []. Several studies have found that CAFs secrete exosomes that activate the TGF-β and PI3K-AKT signaling pathways and reduce the expression of Forkhead box protein A1 (FOXA1) in CRC, leading to radiotherapy resistance [–].
Tumor-Infiltrating Immune Cells
To date, most research on the TIME has focused on tumor-infiltrating immune cells. The tumor microenvironment includes almost all immune cell types, such as tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killer (NK) cells, B lymphocytes (B cells), T lymphocytes (T cells), marrow-derived suppressor cells (MDSCs), and neutrophils []. Immune cells play key roles in the TIME in radiotherapy-resistant CRC largely by the secretion of cytokines and activation of signaling pathways (Fig. 2).
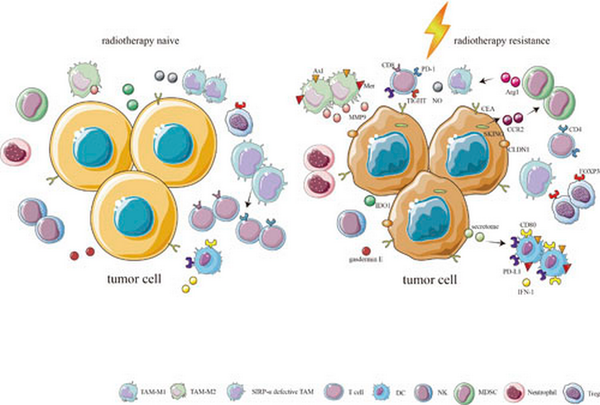
Fig. 2
Immune cells in the TIME of radiotherapy-resistant CRC. The proportions of TAM-M2s, MDSCs, neutrophils, and Tregs in radiotherapy-resistant CRC are higher than in radiotherapy-naive CRC, whereas the proportions of CD4+ T cells, CD8+ T cells, NKs, TAM-M1s, and SIRP-α-deficient TAMs in radiotherapy-resistant CRC are lower than in radiotherapy-naive CRC. CLDN1 expression is positively correlated with the numbers of TAM-M2s, which strongly express Axl, Mer, and MMP9. DCs also strongly express Axl and Mer, as well as showing increased levels of CD80 and PD-L1 through the protein secretome after radiotherapy, inducing radiotherapy resistance. DCs are show decreased expression of IFN-1. Activation of the STING pathway results in increased IFN-I and CCL2 production, recruiting CCR2-positive MDSCs, and reducing the proportions of CD4+ and CD8+ T cells. Increased expression of Arg1 by MDSCs can exhaust type I arginine, which can reduce the ability of macrophages to synthesize NO.
Tumor-Associated Macrophages
Macrophages are produced from progenitor cells in the bone marrow, after which they circulate in the peripheral blood. During homeostatic and inflammatory processes, these cells migrate into tissues and differentiate into mature macrophages after exposure to local proinflammatory cytokines, growth factors, and microbial agents. Macrophages perform various functions in tumors []. The two major macrophage subpopulations are those with a classically activated or inflammatory (M1) phenotype and those with an alternatively activated or anti-inflammatory (M2) type, both of which have different roles. The M1 subtype has anti-tumoral activity, whereas M2 stimulates tumor formation and progression []. It has been reported that higher levels of M2 macrophages in the TIME of CRC tumors were associated with insensitivity of the tumor to radiotherapy [–]. Radiotherapy-resistant CRC tumors express greater amounts of carcinoembryonic antigen (CEA), which induces macrophage polarization to the M2 type, resulting in greater numbers of M2 macrophages in the TIME and leading to a worse prognosis []. In terms of the effects of TAMs on radiotherapy resistance in CRC, it has been observed that autophagy is reduced in TAMs in radiotherapy-resistant CRC, and an increase in TAM autophagy can promote radiotherapy sensitivity to CRC []. It was found that the expression of claudin-1 (CLDN1) promoted radiotherapy resistance to CRC, and TAMs were positively correlated with its expression []. It was also discovered that the macrophage percentage increased in the TIMEs of CRC after radiotherapy, together with increased production of matrix metalloproteinase 9 (MMP9) by the macrophages, inducing tumor proliferation and metastasis and eventually leading to resistance against radiotherapy []. The receptor tyrosine kinases Axl and Mer are expressed in many tumor cells and have carcinogenic effects. Studies have found that Axl and Mer are expressed in macrophages of TIMEs in radiotherapy-resistant CRC and that both proteins are associated with radiotherapy resistance []. CRC tumors where the TIME contain lower proportions of SIRP-α-deficient macrophages were observed to be insensitive to radiotherapy as SIRP-α-deficient macrophages can increase the sensitivity of CRC to radiotherapy by activating tumor antigen-specific cytotoxic T cells []. Thus, it can be stated that M2 macrophages participate in CRC radiotherapy resistance, and the conversion of M2 to M1 macrophages may be key to the reversal of radiotherapy resistance.
Dendritic Cells
DCs are a specific type of immune cell that connects the innate and adaptive immune responses. They are considered the most effective type of antigen-presenting cell, playing an important role in the process of tumor antigen recognition and presentation []. Studies suggest that DCs in CRC express large amounts of CD80 and PD-L1 in the protein secretome and that this increased expression correlated with radiotherapy resistance []. In addition, there is also a significant increase in the proportion of DCs producing large amounts of Ax1 and Mer in radiation-resistant CRC, which can shape the immunosuppressive microenvironment []. Another study found that non-standard NF-κB signaling in CRC-associated DCs after radiotherapy was activated by the STING sensor-dependent DNA sensing pathway, resulting in decreased expression of type I interferon (IFN-I) []. However, there are relatively few studies on the roles of DC in radiotherapy resistance in CRC, and this topic requires further investigation.
Natural Killer Cells
NK cells are innate immune system-related lymphocytes that are responsible for monitoring the surfaces of autologous cells for abnormal expression of major histocompatibility complex class I molecules and cell stress markers and are crucial regulators of the anti-tumor immune response []. CRC patients with high proportions of NK cells in the TIME tend to have good prognosis, while patients with low levels have a poor prognosis []. Moreover, the elimination of NKs from the TIME can increase resistance, and increasing NK numbers in the TIME can increase the sensitivity of CRC to radiotherapy [, ]. Lower proportions of NK cells have been observed in the TIME in radiotherapy-resistant CRC. Combined radiotherapy and immunotherapy treatment was found to lead to significant shrinkage of the tumor together with increased proportions of NK cells in the TIME []. In addition, several studies have found that radiotherapy can kill NKs in the TIME of some CRC patients, and these patients are more prone to distant metastasis after radiotherapy []. Moreover, gasdermin E (GSDME) can increase the radiosensitivity of radiation-resistant CRC by recruiting and activating NKs []. Thus, the percentages of NKs in radiotherapy-resistant CRC are lower, and increasing the percentage of NKs may reverse the radiotherapy resistance.
Myeloid-Derived Suppressor Cells
MDSCs are produced in the bone marrow and stimulate tumor progression by promoting the survival of tumor cells, invasion of healthy tissue, angiogenesis, and metastases []. There are two subgroups of MDSCs, namely, granulocytic or polymorphonuclear MDSCs which resemble neutrophils in phenotype and morphology and monocytic MDSCs which are similar to monocytes in phenotype and morphology []. CRC with a high percentage of MDSCs in the TIME is not sensitive to radiotherapy []. Animal experiments have shown that the proportion of MDSCs in the CRC TIME decreased when the tumor was sensitive to radiotherapy but increased in cases of radiotherapy tolerance []. Radiotherapy can induce the activation of the STING pathway in CRC cells, increase the levels of IFN-I and CCL2, and recruit CCR2-positive MDSCs, while MDSCs can reduce the proportions of CD4+ and CD8+ T cells, shaping the immune suppressive microenvironment, and finally leading to radiotherapy resistance []. Several studies have suggested that arginase 1 (Arg1) which is highly expressed in MDSCs can exhaust type I arginine, significantly inhibiting the classical macrophage activation mode and reducing the ability of macrophages to synthesize nitric oxide (NO), thus reducing the sensitivity of CRC to radiotherapy []. MDSCs thus play an important role in CRC radiotherapy resistance, suggesting that they are a crucial target for the reversal of radiotherapy resistance.
Neutrophils
Neutrophils are the most abundant immune cell population. They belong to the innate immune system and act as the first line of defense in the human body. They are also associated with the immune response against tumors []. Investigations have indicated that CRC patients with high proportions of peripheral blood neutrophils showed a poorer response to radiotherapy than patients with low numbers of neutrophils []. This also applies to CRC tumor tissues [, ]. Other studies have found that CRC tumors with higher neutrophil-lymphocyte ratios are insensitive to radiation [–], and similar interpretations have been drawn for the cell proportions in the peripheral blood [–]. There are relatively few studies on the role played by neutrophils in CRC radiation resistance, which is worthy of further study.
B Lymphocytes and T Lymphocytes
B cells form a crucial part of both adaptive and humoral immune responses in humans. They are also associated with anti-tumor immune responses []. To date, relatively few studies have addressed the role of B cells in CRC radiotherapy resistance, making it an important area for future research. T cells take part in human adaptive and cellular immune responses. They are the most studied TIME-associated immune cells and play important parts in the anti-tumor immune response []. Studies have found that CRC tumors with fewer tumor-infiltrating T cells are not sensitive to radiotherapy [–]. With regard to T cell subtypes, lower proportions of CD4+ T and CD8+ T cells have been observed in the TIME of radiotherapy-resistant CRC tumors [, , –], with a greater proportion of regulatory T cells (Tregs) [, –]. Animal experiments showed that the proportion of Tregs in CRC TIMEs decreased in cases of radiotherapy sensitivity but increased when the tumor was radiotherapy-tolerant []. There are reports that the presence of greater numbers of Treg and PD-1+ T cells in the TIMEs of CRC liver metastases is indicative of poor prognosis, and radiotherapy combined with anti-Treg-antibody treatment can increase the number of CD8+ T cells in the TIME and significantly inhibit tumor growth []. Both single radiotherapy and fractionated radiotherapy lead to increased numbers of Tregs and PD-L1+ and TIGHT+ CD8+ T cells, leading to poor prognosis; however, the prognosis is improved when the radiotherapy is combined with immunotherapy []. CRC patients with higher tumor-infiltrating FOXP3+ Treg and lower CD8+/FOXP3+ T cell ratios had worse prognosis after radiotherapy []. Moreover, when indolamine-2,3-dioxygenase (IDO1) was combined with tumor-infiltrating CD8+ T cells, it was observed that CRC tumors with lower IDO1 expression and fewer CD8+ T cells responded poorly to radiotherapy []. One study investigated the combination of CD8+ T cells and PD-1+ immune cells, finding that CRC tumors with fewer CD8+ T cells and PD-1+ immune cells did not respond well to radiotherapy []. There are many T cell subtypes which play different roles in CRC radiotherapy resistance. Radiotherapy-resistant CRC tends to be associated with the presence of greater numbers of immunosuppressive T cells and fewer immune-active T cells. Reversing this phenomenon may be the key to reversing radiotherapy resistance.
Tumor and Immune Cell-Secreted Cytokines
Cytokines are small proteins that have diverse biological features. They are produced and secreted on stimulation by both immune and nonimmune cells and include the interferons, interleukins, and members of the tumor necrosis factor superfamily, as well as chemokines, colony-stimulating factors, and growth factors []. Cytokines play key roles in the TIME of radiotherapy-resistant CRC (Fig. 3).
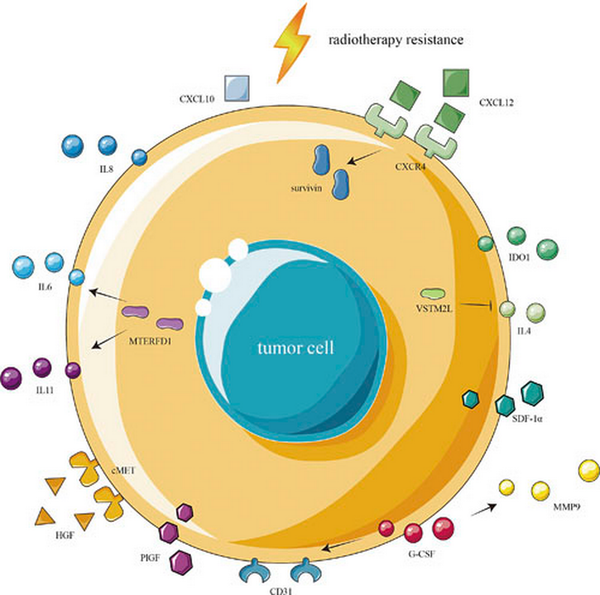
Fig. 3
Cytokines in the TIME of radiotherapy-resistant CRC radiotherapy-resistant CRC shows increased expression of IDO1 induced by activation of the IFN-I signaling pathway, together with increased expression of IL6, IL8, and IL11 and reduced expression of IL4. The activation of the MTERFD1 gene in CRC cells increases the expression of IL6 and IL11, while the activation of VSTM2L downregulates the expression of IL4. Radiotherapy-resistant CRC overexpresses CXCL12 and CXCR4 while showing reduced expression of CXCL10. G-CSF can stimulate the growth of blood vessels by increasing the expression of MMP9 and CD31. Radiotherapy-resistant CRC overexpresses HGF, cMET, SDF-1α, and PlGF.
Radiotherapy promotes IDO1 expression in CRC tumors by activating the IFN-I signaling pathway, and its overexpression leads to radiotherapy resistance []. Interleukin is an important cytokine, and studies have shown that CRC tumors with high expression of IL-8 and IL-6 respond poorly to radiotherapy [, ]. Activation of the mitochondrial transcription termination factor (MTERFD1) gene in CRC cells leads to radiotherapy resistance by increasing the production of IL-6 and IL-11 []. Studies have shown that V-set and transmembrane domain-containing 2-like protein (VSTM2L) is associated with radiotherapy resistance in CRC by down-regulation of the expression of IL-4 [].
Chemokines are also important factors. It was found that radiotherapy-resistant CRC tumors overexpressed both CXCL12 and CXCR4 [], and these increases promoted CRC radiation resistance through the upregulation of survivin []. CRC tumors with low CXCL10 expression are not sensitive to radiotherapy []. Granulocyte colony-stimulating factor (G-CSF) is often used to mitigate the side effects of radiotherapy; however, studies have found that G-CSF can stimulate the growth of blood vessels by increasing the expression of MMP9 and CD31, leading to radiotherapy resistance in CRC []. Hepatocyte growth factor and its receptor cMET play an important role in tumor proliferation, invasion, and metastasis. It has been found that radiotherapy-resistant CRC tumors overexpress both hepatocyte growth factor and cMET, and inhibiting their expression can reduce radiotherapy resistance []. Furthermore, it has been found that radiation-resistant CRC tumors express high levels of stromal cell-derived factor-1α (SDF-1α) and placental growth factor (PlGF) []. The mechanism by which cytokines influence CRC radiation resistance is complex. In summary, it appears that the role of cytokines in the radiation-resistant CRC microenvironment is mainly to stimulate tumor growth and metastasis and induce an immunosuppressive microenvironment.
Role of the TIME in Immunotherapy for CRC
Anti-PD-1 and anti-PD-L1 antibodies have been used as representative immune checkpoint inhibitors in the treatment of advanced melanoma, non-small cell lung cancer, and other solid tumors [–]. CRC patients also benefit from immunotherapy, especially CRC patients with dMMR/MSI-H tumors, who are significantly more sensitive to immune checkpoint inhibitors than CRC patients with microsatellite-stable (MSS)/microsatellite instability-low (MSI-L) tumors [, ]. A recent study found that MSI-H CRC was associated with significantly increased numbers of plasma cells, CD8+ T cells, activated memory CD4+ T cells, follicular T helper cells, NK cells, M1 macrophages, and neutrophils, as well as significantly decreased numbers of Tregs, compared with MSS/MSI-L CRC []. They also found that the expression of stimulatory immune-related genes, such as those encoding chemokines (CX3CL1, CXCL9, and CXCL10), cytokines (such as IFNG and IL1B), and the tumor necrosis factor receptor superfamily (TNFRSF), was significantly upregulated in MSI-H CRC []. Moreover, they found that immune response-related pathways, such as leukocyte migration involved in the inflammatory response, cellular response to IFN-γ, T cell activation, antigen presentation, cytokine- or chemokine-related processes, and macrophage or neutrophil activity, were significantly enriched in MSI-H CRC []. In conclusion, compared with CRC patients with MSS/MSI-L tumors, those with MSI-H tumors benefited significantly from ICI treatment. MSI-H CRC was accompanied by greater immune cell infiltration, higher expression of immune-related genes, and higher immunogenicity than MSS/MSI-L CRC.
Conclusion and Perspective
The TIME in radiotherapy-resistant CRC can be summarized as follows: the proportion of immunosuppressive cells is greater than the numbers of cells associated with immune activation, leading to an overall state of immunosuppression; both the tumor and immunosuppressive cells secrete increased amounts of immunosuppressive regulatory factors, reduce the recognition and presentation of tumor antigens, inhibit immune cell’s anti-tumor effect, and offset the non-targeted anti-tumor effect of radiotherapy. The formation of a radiotherapy-resistant TIME is the result of interactions between immune cells, non-immune cells, and cytokines. Therefore, the reversal of CRC radiation resistance cannot be achieved by altering or targeting only one type of immune cell or cytokine. In contrast, changing the immunosuppressive microenvironment as a whole is key to the reversal of CRC radiation resistance. Therefore, combining radiotherapy with other anti-tumor therapies, including immunotherapy, in a way that can effectively eliminate treatment resistance and achieve synergy under the premise of clinical safety and feasibility should be the focus of future research.
Statement of Ethics
The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. We declare that we have not received support from any organization to submit this work; we have had no financial relationship in the three previous years with any organization that may have an interest in the submitted work; and we have no other relationships or activities that could appear to have influenced the submitted work.
Funding Sources
This article has no funding sources to declare.
Author Contributions
Conception and design, manuscript writing, and final approval of the manuscript: all authors; administrative support: Ye Feng and Defeng Song; provision of study materials or patients: none; collection and assembly of data and data analysis and interpretation: Chao Wang, Meng Yuan, Yongjian Gao, and Ruizhi Hou.
References
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al Cancer statistics in China, 2015 CA Cancer J Clin 2016 66 2 115–32
- 3. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al Cancer statistics in China and United States, 2022: profiles, trends, and determinants Chin Med J 2022 135 5 584–90
- 4. Siegel RL, Miller KD, Fuchs HE, Jemal A Cancer statistics, 2022 CA Cancer J Clin 2022 72 1 7–33
- 5. Harrington KJ, Billingham LJ, Brunner TB, Burnet NG, Chan CS, Hoskin P, et al Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers Br J Cancer 2011 105 5 628–39
- 6. Mortezaee K, Najafi M Immune system in cancer radiotherapy: resistance mechanisms and therapy perspectives Crit Rev Oncol Hematol 2021 157 103180
- 7. Eriksson D, Stigbrand T Radiation-induced cell death mechanisms Tumour Biol 2010 31 4 363–72
- 8. Mukherjee S, Chakraborty A Radiation-induced bystander phenomenon: insight and implications in radiotherapy Int J Radiat Biol 2019 95 3 243–63
- 9. Häfner MF, Debus J Radiotherapy for colorectal cancer: current standards and future perspectives Visc Med 2016 32 3 172–7
- 10. Tam SY, Wu VWC A review on the special radiotherapy techniques of colorectal cancer Front Oncol 2019 9 208
- 11. Wanigasooriya K, Tyler R, Barros-Silva JD, Sinha Y, Ismail T, Beggs AD Radiosensitising cancer using phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT) or mammalian target of rapamycin (mTOR) inhibitors Cancers 2020 12 5 1278
- 12. Park SY, Lee SJ, Cho HJ, Kim JT, Yoon HR, Lee KH, et al Epsilon-globin HBE1 enhances radiotherapy resistance by down-regulating BCL11A in colorectal cancer cells Cancers 2019 11 4 498
- 13. Badawi A, Hehlgans S, Pfeilschifter J, Rödel F, Eberhardt W Silencing of the mRNA-binding protein HuR increases the sensitivity of colorectal cancer cells to ionizing radiation through upregulation of caspase-2 Cancer Lett 2017 393 103–12
- 14. Galofré C, Gönül Geyik Ö, Asensio E, Wangsa D, Hirsch D, Parra C, et al Tetraploidy-associated genetic heterogeneity confers chemo-radiotherapy resistance to colorectal cancer cells Cancers 2020 12 5 1118
- 15. Eder S, Arndt A, Lamkowski A, Daskalaki W, Rump A, Priller M, et al Baseline MAPK signaling activity confers intrinsic radioresistance to KRAS-mutant colorectal carcinoma cells by rapid upregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K) Cancer Lett 2017 385 160–7
- 16. Gao C, Kozlowska A, Nechaev S, Li H, Zhang Q, Hossain DMS, et al TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy Cancer Res 2013 73 24 7211–21
- 17. Hsu YC, Luo CW, Huang WL, Wu CC, Chou CL, Chen CI, et al BMI1-KLF4 axis deficiency improves responses to neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer Radiother Oncol 2020 149 249–58
- 18. Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng J, et al Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway J Exp Clin Cancer Res 2019 38 1 461
- 19. Hanahan D, Coussens LM Accessories to the crime: functions of cells recruited to the tumor microenvironment Cancer Cell 2012 21 3 309–22
- 20. Quail DF, Joyce JA Microenvironmental regulation of tumor progression and metastasis Nat Med 2013 19 11 1423–37
- 21. Frantz C, Stewart KM, Weaver VM The extracellular matrix at a glance J Cell Sci 2010 123 Pt 24 4195–200
- 22. Barthes J, Özçelik H, Hindié M, Ndreu-Halili A, Hasan A, Vrana NE Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances BioMed Res Int 2014 2014 921905
- 23. Lee H, Kong JS, Lee SS, Kim A Radiation-induced overexpression of TGFβ and PODXL contributes to colorectal cancer cell radioresistance through enhanced motility Cells 2021 10 8 2087
- 25. Larsson A, Johansson ME, Wangefjord S, Gaber A, Nodin B, Kucharzewska P, et al Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer Br J Cancer 2011 105 5 666–72
- 26. Angenete E, Langenskiöld M, Palmgren I, Falk P, Oresland T, Ivarsson ML Transforming growth factor beta-1 in rectal tumour, mucosa and plasma in relation to radiotherapy and clinical outcome in rectal cancer patients Int J Colorectal Dis 2007 22 11 1331–8
- 27. Kumar Katakam S, Tria V, Sim WC, Yip GW, Molgora S, Karnavas T, et al The heparan sulfate proteoglycan syndecan-1 regulates colon cancer stem cell function via a focal adhesion kinase-Wnt signaling axis FEBS J 2021 288 2 486–506
- 28. Katakam SK, Pelucchi P, Cocola C, Reinbold R, Vlodavsky I, Greve B, et al Syndecan-1-Dependent regulation of heparanase affects invasiveness, stem cell properties, and therapeutic resistance of Caco2 colon cancer cells Front Oncol 2020 10 774
- 30. Kazimova T, Tschanz F, Sharma A, Telarovic I, Wachtel M, Pedot G, et al Paracrine placental growth factor signaling in response to ionizing radiation is p53-dependent and contributes to radioresistance Mol Cancer Res 2021 19 6 1051–62
- 31. Angenete E, Langenskiöld M, Falk P, Ivarsson ML Matrix metalloproteinases in rectal mucosa, tumour and plasma: response after preoperative irradiation Int J Colorectal Dis 2007 22 6 667–74
- 32. Carmeliet P Angiogenesis in health and disease Nat Med 2003 9 6 653–60
- 33. Pugh CW, Ratcliffe PJ Regulation of angiogenesis by hypoxia: role of the HIF system Nat Med 2003 9 6 677–84
- 34. Ferrara N, Gerber HP, LeCouter J The biology of VEGF and its receptors Nat Med 2003 9 6 669–76
- 35. Feng L, Tao L, Dawei H, Xuliang L, Xiaodong L HIF-1α expression correlates with cellular apoptosis, angiogenesis and clinical prognosis in rectal carcinoma Pathol Oncol Res 2014 20 3 603–10
- 36. Gombodorj N, Yokobori T, Yoshiyama S, Kawabata-Iwakawa R, Rokudai S, Horikoshi I, et al Inhibition of ubiquitin-conjugating enzyme E2 may activate the degradation of hypoxia-inducible factors and, thus, overcome cellular resistance to radiation in colorectal cancer Anticancer Res 2017 37 5 2425–36
- 37. Tachikawa Y, Kawai K, Ozaki K, Nozawa H, Sasaki K, Murono K, et al CD133(+)HIF-1α(-) expression after chemoradiotherapy predicts poor prognosis in rectal cancer Anticancer Res 2022 42 4 2033–43
- 38. Sun Y, Xing X, Liu Q, Wang Z, Xin Y, Zhang P, et al Hypoxia-induced autophagy reduces radiosensitivity by the HIF-1α/miR-210/Bcl-2 pathway in colon cancer cells Int J Oncol 2015 46 2 750–6
- 39. Solberg TD, Nearman J, Mullins J, Li S, Baranowska-Kortylewicz J Correlation between tumor growth delay and expression of cancer and host VEGF, VEGFR2, and osteopontin in response to radiotherapy Int J Radiat Oncol Biol Phys 2008 72 3 918–26
- 40. Ma D, Gao X, Tao J, Yu H, Chai Z Hypoxia-induced downregulation of B-cell translocation gene 3 confers resistance to radiation therapy of colorectal cancer J Cancer Res Clin Oncol 2020 146 10 2509–17
- 41. Kalluri R, Zeisberg M Fibroblasts in cancer Nat Rev Cancer 2006 6 5 392–401
- 42. Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, et al Stromal contribution to the colorectal cancer transcriptome Nat Genet 2015 47 4 312–9
- 43. Verset L, Tommelein J, Moles Lopez X, Decaestecker C, Boterberg T, De Vlieghere E, et al Impact of neoadjuvant therapy on cancer-associated fibroblasts in rectal cancer Radiother Oncol 2015 116 3 449–54
- 44. Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Fujikawa H, et al Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy Int J Oncol 2011 38 3 655–63
- 45. Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, et al Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer Cancer Cell 2022 40 2 168–84.e13
- 46. Tommelein J, De Vlieghere E, Verset L, Melsens E, Leenders J, Descamps B, et al Radiotherapy-activated cancer-associated fibroblasts promote tumor progression through paracrine IGF1R activation Cancer Res 2018 78 3 659–70
- 47. Chen X, Liu Y, Zhang Q, Liu B, Cheng Y, Zhang Y, et al Exosomal miR-590-3p derived from cancer-associated fibroblasts confers radioresistance in colorectal cancer Mol Ther Nucleic Acids 2021 24 113–26
- 48. Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing D, et al Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype Exp Cell Res 2020 391 2 111956
- 49. Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, et al Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3 J Exp Clin Cancer Res 2020 39 1 65
- 50. Mantovani A, Allavena P, Sica A, Balkwill F Cancer-related inflammation Nature 2008 454 7203 436–44
- 51. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al Macrophage plasticity, polarization, and function in health and disease J Cell Physiol 2018 233 9 6425–40
- 52. Qian BZ, Pollard JW Macrophage diversity enhances tumor progression and metastasis Cell 2010 141 1 39–51
- 53. Wilkins A, Fontana E, Nyamundanda G, Ragulan C, Patil Y, Mansfield D, et al Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer J Immunother Cancer 2021 9 3 e001717
- 54. Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, et al Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: immunologic relevance Radiother Oncol 2019 131 52–9
- 55. Yang Y, Tian W, Su L, Li P, Gong X, Shi L, et al Tumor-infiltrating cytotoxic T cells and tumor-associated macrophages correlate with the outcomes of neoadjuvant chemoradiotherapy for locally advanced rectal cancer Front Oncol 2021 11 743540
- 56. Zhu M, Li X, Cheng X, Yi X, Ye F, Li X, et al Association of the tissue infiltrated and peripheral blood immune cell subsets with response to radiotherapy for rectal cancer BMC Med Genomics 2022 15 Suppl 2 107
- 57. Huang EY, Chang JC, Chen HH, Hsu CY, Hsu HC, Wu KL Carcinoembryonic antigen as a marker of radioresistance in colorectal cancer: a potential role of macrophages BMC Cancer 2018 18 1 321
- 58. Shao LN, Zhu BS, Xing CG, Yang XD, Young W, Cao JP Effects of autophagy regulation of tumor-associated macrophages on radiosensitivity of colorectal cancer cells Mol Med Rep 2016 13 3 2661–70
- 59. Chen ZX, Huang HQ, Wen JY, Qin LS, Song YD, Fang YY, et al Active enhancer assessment by H3K27ac ChIP-seq reveals claudin-1 as a biomarker for radiation resistance in colorectal cancer Dose Res 2021 19 4 15593258211058981
- 60. Timaner M, Bril R, Kaidar-Person O, Rachman-Tzemah C, Alishekevitz D, Kotsofruk R, et al Dequalinium blocks macrophage-induced metastasis following local radiation Oncotarget 2015 6 29 27537–54
- 61. Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, et al Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer Proc Natl Acad Sci U S A 2013 110 32 13091–6
- 62. Bian Z, Shi L, Kidder K, Zen K, Garnett-Benson C, Liu Y Intratumoral SIRPα-deficient macrophages activate tumor antigen-specific cytotoxic T cells under radiotherapy Nat Commun 2021 12 1 3229
- 63. Waisman A, Lukas D, Clausen BE, Yogev N Dendritic cells as gatekeepers of tolerance Semin Immunopathol 2017 39 2 153–63
- 64. Heeran AB, Dunne MR, Morrissey ME, Buckley CE, Clarke N, Cannon A, et al The protein secretome is altered in rectal cancer tissue compared to normal rectal tissue, and alterations in the secretome induce enhanced innate immune responses Cancers 2021 13 3 571
- 65. Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, et al Non-canonical NF-κB antagonizes STING sensor-mediated DNA sensing in radiotherapy Immunity 2018 49 3 490–503.e4
- 66. Morvan MG, Lanier LL NK cells and cancer: you can teach innate cells new tricks Nat Rev Cancer 2016 16 1 7–19
- 67. Alderdice M, Dunne PD, Cole AJ, O’Reilly PG, McArt DG, Bingham V, et al Natural killer-like signature observed post therapy in locally advanced rectal cancer is a determinant of pathological response and improved survival Mod Pathol 2017 30 9 1287–98.
- 68. Chi CH, Wang YS, Yang CH, Chi KH Neoadjuvant immunotherapy enhances radiosensitivity through natural killer cell activation Cancer Biother Radiopharm 2010 25 1 39–45
- 69. Sia J, Hagekyriakou J, Chindris I, Albarakati H, Leong T, Schlenker R, et al Regulatory T cells shape the differential impact of radiation dose-fractionation schedules on host innate and adaptive antitumor immune defenses Int J Radiat Oncol Biol Phys 2021 111 2 502–14
- 70. Olivo Pimentel V, Marcus D, van der Wiel AM, Lieuwes NG, Biemans R, Lieverse RI, et al Releasing the brakes of tumor immunity with anti-PD-L1 and pushing its accelerator with L19-IL2 cures poorly immunogenic tumors when combined with radiotherapy J Immunother Cancer 2021 9 3 e001764
- 71. Koda K, Saito N, Oda K, Seike K, Kondo E, Ishizuka M, et al Natural killer cell activity and distant metastasis in rectal cancers treated surgically with and without neoadjuvant chemoradiotherapy J Am Coll Surg 2003 197 2 254–60
- 72. Tan G, Lin C, Huang C, Chen B, Chen J, Shi Y, et al Radiosensitivity of colorectal cancer and radiation-induced gut damages are regulated by gasdermin E Cancer Lett 2022 529 1–10
- 73. Kumar V, Patel S, Tcyganov E, Gabrilovich DI The nature of myeloid-derived suppressor cells in the tumor microenvironment Trends Immunol 2016 37 3 208–20
- 74. Gabrilovich DI Myeloid-derived suppressor cells Cancer Immunol Res 2017 5 1 3–8
- 75. Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, et al Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer Transl Res 2015 166 6 721–32.e1.
- 76. Frey B, Rückert M, Weber J, Mayr X, Derer A, Lotter M, et al Hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors Front Immunol 2017 8 231
- 77. Liang H, Deng L, Hou Y, Meng X, Huang X, Rao E, et al Host STING-dependent MDSC mobilization drives extrinsic radiation resistance Nat Commun 2017 8 1 1736
- 78. Leonard W, Dufait I, Schwarze JK, Law K, Engels B, Jiang H, et al Myeloid-derived suppressor cells reveal radioprotective properties through arginase-induced l-arginine depletion Radiother Oncol 2016 119 2 291–9
- 79. Liew PX, Kubes P The neutrophil’s role during health and disease Physiol Rev 2019 99 2 1223–48
- 81. Lv QY, Zou HZ, Xu YY, Shao ZY, Wu RQ, Li KJ, et al Expression levels of chemokine (C-X-C motif) ligands CXCL1 and CXCL3 as prognostic biomarkers in rectal adenocarcinoma: evidence from Gene Expression Omnibus (GEO) analyses Bioengineered 2021 12 1 3711–25
- 82. Lee YJ, Lee SB, Beak SK, Han YD, Cho MS, Hur H, et al Temporal changes in immune cell composition and cytokines in response to chemoradiation in rectal cancer Sci Rep 2018 8 1 7565
- 83. Ren DL, Li J, Yu HC, Peng SY, Lin WD, Wang XL, et al Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer World J Gastroenterol 2019 25 1 118–37
- 84. Kim TG, Park W, Kim H, Choi DH, Park HC, Kim SH, et al Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy Tumori 2019 105 5 434–40
- 85. Yoon S, Oh Y, Oh SY Clinical implications of combined lymphocyte and neutrophil count in locally advanced rectal cancer after preoperative chemoradiotherapy World J Surg 2021 45 8 2591–600
- 86. Yang G, Chang JS, Choi JE, Baek ES, Kim SS, Byun HK, et al Association of neutrophil-to-lymphocyte ratio, radiotherapy fractionation/technique, and risk of development of distant metastasis among patients with locally advanced rectal cancer Radiat Oncol 2022 17 1 100
- 87. Ishikawa D, Nishi M, Takasu C, Kashihara H, Tokunaga T, Higashijima J, et al The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer In Vivo 2020 34 2 863–8
- 88. Ke TM, Lin LC, Huang CC, Chien YW, Ting WC, Yang CC High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict poor survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy Medicine 2020 99 17 e19877
- 89. Dudani S, Marginean H, Tang PA, Monzon JG, Raissouni S, Asmis TR, et al Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation BMC Cancer 2019 19 1 664
- 90. Cheong C, Shin JS, Suh KW Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer World J Gastroenterol 2020 26 44 7022–35
- 91. Zhang X, Li J, Peng Q, Huang Y, Tang L, Zhuang Q, et al Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy Cancer Manag Res 2019 11 191–9
- 92. Ward WH, Goel N, Ruth KJ, Esposito AC, Lambreton F, Sigurdson ER, et al Predictive value of leukocyte- and platelet-derived ratios in rectal adenocarcinoma J Surg Res 2018 232 275–82
- 93. Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, et al Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy Dig Surg 2015 32 6 496–503
- 94. Shen L, Zhang H, Liang L, Li G, Fan M, Wu Y, et al Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation Radiat Oncol 2014 9 295
- 95. De Felice F, Rubini FL, Romano L, Bulzonetti N, Caiazzo R, Musio D, et al Prognostic significance of inflammatory-related parameters in patients with anal canal cancer Int J Colorectal Dis 2019 34 3 519–25
- 96. Franchina DG, Grusdat M, Brenner D B-cell metabolic remodeling and cancer Trends Cancer 2018 4 2 138–50
- 97. Kumar BV, Connors TJ, Farber DL Human T cell development, localization, and function throughout life Immunity 2018 48 2 202–13
- 98. Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegård J, et al Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor Mod Pathol 2011 24 5 671–82
- 99. Randrian V, Desette A, Emambux S, Derangere V, Roussille P, Frouin E, et al New artificial intelligence score and immune infiltrates as prognostic factors in colorectal cancer with brain metastases Front Immunol 2021 12 750407
- 100. Zhai Z, Wang Z, Jin M, Zhang K Peripheral blood CD45RO+T cells is a predictor of the effectiveness of neoadjuvant chemoradiotherapy in locally advanced rectal cancer Medicine 2021 100 25 e26214
- 101. Hong SW, Lee S, Kim YJ, Ahn S, Park IJ, Hong SM, et al Immune profile by multiplexed immunohistochemistry associated with recurrence after chemoradiation in rectal cancer J Gastroenterol Hepatol 2022 37 3 542–50
- 102. Kamran SC, Lennerz JK, Margolis CA, Liu D, Reardon B, Wankowicz SA, et al Integrative molecular characterization of resistance to neoadjuvant chemoradiation in rectal cancer Clin Cancer Res 2019 25 18 5561–71
- 103. Zou Q, Hu B, Yu HC, Ren DL Characteristics of CD8+ T cell infiltration in colorectal cancer and their correlation with prognosis Zhonghua Wei Chang Wai Ke Za Zhi 2021 24 12 1086–92
- 104. Akiyoshi T, Gotoh O, Tanaka N, Kiyotani K, Yamamoto N, Ueno M, et al T-cell complexity and density are associated with sensitivity to neoadjuvant chemoradiotherapy in patients with rectal cancer Cancer Immunol Immunother 2021 70 2 509–18
- 105. Imaizumi K, Suzuki T, Kojima M, Shimomura M, Sakuyama N, Tsukada Y, et al Ki67 expression and localization of T cells after neoadjuvant therapies as reliable predictive markers in rectal cancer Cancer Sci 2020 111 1 23–35
- 106. Matsutani S, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, et al Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer Cancer Sci 2018 109 4 966–79
- 107. Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer Radiat Oncol 2011 6 49
- 108. Gerber SA, Lim JYH, Connolly KA, Sedlacek AL, Barlow ML, Murphy SP, et al Radio-responsive tumors exhibit greater intratumoral immune activity than nonresponsive tumors Int J Cancer 2014 134 10 2383–92
- 109. Billiard F, Buard V, Benderitter M, Linard C Abdominal γ-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine Int J Radiat Oncol Biol Phys 2011 80 3 869–76
- 110. McCoy MJ, Hemmings C, Miller TJ, Austin SJ, Bulsara MK, Zeps N, et al Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer Br J Cancer 2015 113 12 1677–86
- 111. Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, Rega D, et al Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients Oncotarget 2015 6 10 8261–70
- 112. Ji D, Song C, Li Y, Xia J, Wu Y, Jia J, et al Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer J Immunother Cancer 2020 8 2 e000826
- 113. Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, et al Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination J Immunother Cancer 2019 7 1 160
- 114. Zaghloul H, Abbas A, Abdulah D Tumor microenvironment mediators CD8(+)- and FOXP3(+)-labeled T lymphocytes are prospective prognosticators in curatively treated rectal cancer patients J Gastrointest Cancer 2021 52 1 177–86
- 115. Schollbach J, Kircher S, Wiegering A, Seyfried F, Klein I, Rosenwald A, et al Prognostic value of tumour-infiltrating CD8+ lymphocytes in rectal cancer after neoadjuvant chemoradiation: is indoleamine-2,3-dioxygenase (Ido1) a friend or foe? Cancer immunology, immunotherapy Cancer Immunol Immunother 2019 68 4 563–75
- 116. Kitagawa Y, Akiyoshi T, Yamamoto N, Mukai T, Hiyoshi Y, Yamaguchi T, et al Tumor-infiltrating PD-1+ immune cell density is associated with response to neoadjuvant chemoradiotherapy in rectal cancer Clin Colorectal Cancer 2022 21 1 e1–11
- 117. Waldmann TA Cytokines in cancer immunotherapy Cold Spring Harb Perspect Biol 2018 10 12 a028472
- 118. Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, et al Interferon-induced Ido1 mediates radiation resistance and is a therapeutic target in colorectal cancer Cancer Immunol Res 2020 8 4 451–64
- 119. Tada N, Tsuno NH, Kawai K, Murono K, Nirei T, Ishihara S, et al Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients Oncol Rep 2014 31 1 463–71
- 120. Gordon MA, Gil J, Lu B, Zhang W, Yang D, Yun J, et al Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation Pharmacogenomics 2006 7 1 67–88
- 121. Liu X, Cao X, Liu C, Cao Y, Zhao Q, Tan X, et al MTERFD1 promotes cell growth and irradiation resistance in colorectal cancer by upregulating interleukin-6 and interleukin-11 Int J Biol Sci 2019 15 12 2750–62
- 122. Liu H, Zhang Z, Zhen P, Zhou M High expression of VSTM2L induced resistance to chemoradiotherapy in rectal cancer through downstream IL-4 signaling J Immunol Res 2021 2021 6657012
- 123. Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Kawamoto A, et al Stromal CXCR4 and CXCL12 expression is associated with distant recurrence and poor prognosis in rectal cancer after chemoradiotherapy Ann Surg Oncol 2010 17 8 2051–8
- 124. Wang D, Jiao C, Zhu Y, Liang D, Zao M, Meng X, et al Activation of CXCL12/CXCR4 renders colorectal cancer cells less sensitive to radiotherapy via up-regulating the expression of survivin Exp Biol Med 2017 242 4 429–35
- 125. Li C, Wang Z, Liu F, Zhu J, Yang L, Cai G, et al CXCL10 mRNA expression predicts response to neoadjuvant chemoradiotherapy in rectal cancer patients Tumour Biol 2014 35 10 9683–91
- 126. Kim JS, Son Y, Bae MJ, Lee M, Lee CG, Jo WS, et al Administration of granulocyte colony-stimulating factor with radiotherapy promotes tumor growth by stimulating vascularization in tumor-bearing mice Oncol Rep 2015 34 1 147–54
- 127. Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Fujikawa H, Matsushita K, et al Inhibition of HGF/cMET expression prevents distant recurrence of rectal cancer after preoperative chemoradiotherapy Int J Oncol 2012 40 2 583–91
- 128. Kim HJ, Bae SB, Jeong D, Kim ES, Kim CN, Park DG, et al Upregulation of stromal cell-derived factor 1α expression is associated with the resistance to neoadjuvant chemoradiotherapy of locally advanced rectal cancer: angiogenic markers of neoadjuvant chemoradiation Oncol Rep 2014 32 6 2493–500
- 129. Goodman A, Patel SP, Kurzrock R PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas Nat Rev Clin Oncol 2017 14 4 203–20
- 130. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma N Engl J Med 2013 369 2 134–44
- 131. Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, et al Safety and activity of anti-PD-L1 antibody in patients with advanced cancer N Engl J Med 2012 366 26 2455–65
- 132. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al PD-1 blockade in tumors with mismatch-repair deficiency N Engl J Med 2015 372 26 2509–20
- 133. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer J Clin Oncol 2018 36 8 773–9
- 134. Lin A, Zhang J, Luo P Crosstalk between the MSI status and tumor microenvironment in colorectal cancer Front Immunol 2020 11 2039
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 2021 71 3 209–49.
- 24. Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research Cancer Epidemiol Biomarkers Prev 1995 4 5 549–54.
- 29. Lee M, Park JJ, Lee YS Adhesion of ST6Gal I-mediated human colon cancer cells to fibronectin contributes to cell survival by integrin beta1-mediated paxillin and AKT activation Oncol Rep 2010 23 3 757–61.
- 80. Sia J, Mou W, Agas RA, Xie J, Burns M, Varghayee N, et al Long-term patterns of failure and the value of blood prognostic markers in anal cancers treated with intensity-modulated radiation therapy Clinical Colorectal Cancer 2021 21 2 e102–12.
Chao Wang, Meng Yuan, Yongjian Gao, and Ruizhi Hou contributed equally to this work.
