Introduction
The hepatitis E virus (HEV) is a RNA virus causing a high global disease burden []. In the developing world, where HEV is most prevalent, infections are mostly due to poor sanitary conditions, and severe or fatal HEV infections have been reported during late pregnancy, in very young children (<2 years) and in patients with preexisting liver diseases. In developed countries, food-borne transmission (e.g., consumption of undercooked meat) or unscreened blood and tissue donation are the major routes of infection []. The HEV antibody prevalence exceeds 30–50% in some areas of developed countries, with some decrease recently reported from the USA and Germany []. Chronic infections are observed in immunocompromised patients such as those with solid organ transplantation []. The diagnosis of HEV infection in immunocompromised patients may be challenging, as elevation of liver function tests (LFTs) may only be moderate and attributable to various causes such as drug toxicity or chronic graft-versus-host disease []. It has been reported that persistent HEV replication in immunocompromised hosts may result in liver failure [, ].
Ribavirin is currently the only widely accepted treatment option for HEV infection, whereas other therapies display less or no success []. However, ribavirin is not approved for this indication and recommendations on its use are less well defined []. Data on HEV infections in immunocompromised patients have predominantly been gained from organ transplant recipients, and reports on HEV infections among patients with hematologic malignancies (HMs) are limited [, , ]. We retrospectively evaluated patients with HM or other states of immunosuppression and HEV infections treated at our tertiary care cancer center and sought to identify potential hallmarks associated with outcomes.
Materials and Methods
Patients were retrospectively identified from the laboratory database without any predefined diagnostic algorithm. This study was approved by the Ethics Committee of the Charité University Hospital of Berlin. Due to the retrospective, anonymized data analysis, waiver for consent from patients was granted.
Anti-HEV IgM and IgG were analyzed by ELISA and immunoblot (recomWell and recomBlot, Microgen GmbH, Neuried, Germany). Real-time RT-PCR for the detection of HEV-RNA targeting the viral ORF3 was used as previously described [].
Decisions about the use of ribavirin were made by the treating physicians. The duration of the infection was estimated from the first positive PCR (or positive serology in patients without an initial PCR) until the first negative PCR (patients with virological responses) or until the last follow-up PCR (patients without responses). Virological responses (VR) were classified as sustained VR (SVR) in patients with consistently negative HEV-PCR results for ≥3 months, or as VR in patients with negative results during an available follow-up of <3 months or return of LFTs to a normal range (or to pre-HEV levels attributed to known causes such as graft-versus-host disease).
Quantification of lymphocyte subsets was performed using routine flow cytometry. For comparisons of T-cell subsets, we identified patients with available lymphocyte subset quantifications close to the onset of the HEV infection (within 3 months prior) and a confirmed SVR, or in patients with a relapse after VR, close to an interval of 3 month after the first negative HEV-PCR test.
Statistical comparisons for continuous variables were performed by using the Mann-Whitney test. Differences were regarded as significant at a p value ≤0.05. Calculations were performed with a commercially available software package (GraphPad Software Inc., San Diego, USA).
Results
Twenty-two patients, with one exception, were diagnosed during March 2015–November 2021 (median age: 59 years, range: 18–84; 15 male). A single patient, whose early course has already been reported, was diagnosed in 2007 []. The number of samples analyzed per patient by PCR widely ranged (2–26; median 8). Ten of 22 patients received ribavirin, and we found no indication toward a more frequent use of ribavirin in recently diagnosed patients (Table 1). One of 12 patients without and 3 of 10 patients with ribavirin therapy did not experience a viral clearance. There was a non-significant trend toward a longer median duration of the HEV infection in patients who received ribavirin treatment compared to those without antiviral therapy (3 vs. 1.8 months, p = 0.07). Overall, 20 of the 22 patients suffered from HMs, and 9 had received a hematopoietic stem cell transplantation (HSCT). Patient 22 (Table 1) had several underlying diseases, including a combined immunodeficiency associated with a heterozygous KAT2A mutation. Another patient had a combined immunodeficiency due to heterozygous IKBKB mutation (patient 21) []. The HEV infection was confirmed by detection of HEV-RNA except for 2 patients, who were anti-HEV IgM positive.
Twelve patients did not receive any antiviral therapy. Of these, 11 cleared the infection (Table 1), including 3 patients post allogeneic HSCT (alloHSCT). Another alloHSCT patient (patient 12; Table 1) without ribavirin therapy, whose early course has already been reported [], had repeatedly detectable HEV-RNA during further follow-up. This patient acquired the HEV infection prior to alloHSCT, whereas the intervals between alloHSCT and known onset of the HEV infection in the 3 other patients were >6 months. Ten patients received ribavirin treatment. Daily ribavirin doses were ≥800 mg in 9 patients and the minimum treatment duration was 2.9 months, except for a single patient (patient 17). Overall, 7 of 10 patients responded to ribavirin treatment (SVR 6, VR 1). The remaining 3 patients experienced a relapse despite adequate ribavirin doses and a treatment duration ≥3 months. Patient 20 required intensive care treatment due to severe infections, which precluded a second course of ribavirin. This patient deceased due to liver failure after prolonged duration (>27 months) of the HEV infection. Patient 21 received two subsequent courses of ribavirin (one combined with peginterferon alfa-2a) for 6 months, which again resulted only in a transient response. This patient died due to liver failure after more than 4 years with refractory HEV infection. Patient 22 suffering from combined immunodeficiency and three distinct malignancies relapsed after a transient VR.
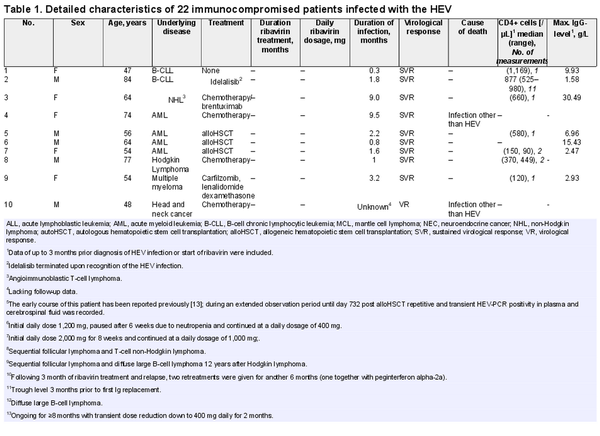
The numbers of circulating CD4+ cells were available in 7 patients without ribavirin therapy and were >200 µL in 5 patients with spontaneous viral clearance (Table 1). Three patients disclosed remarkable findings. Patient 13 showed elevated LFTs and persistently low numbers of CD4+ cells following autologous HSCT (autoHSCT). The HEV serology on day 287 post autoHSCT was negative, but retrospective analysis of a stored plasma sample demonstrated presence of 2,870,000 HEV-RNA copies/mL already on day 295 (Fig. 1a). Ribavirin was given for 3 months, which resulted in an SVR. Of note, the numbers of circulating CD4+ cells consistently increased to >200/µL in parallel to the virological response (Fig. 1a). Patient 14 developed elevated LFTs after autoHSCT. In this patient, the initial numbers of circulating CD4+ cells were low and showed a recovery in parallel to a response to ribavirin (Fig. 1b).
Fig. 1
Detailed responses and numbers of CD4+ T-cells in 3 patients with HEV infections: a Patient No. 13 with mantle cell lymphoma, autologous hematopoietic stem cell transplantation (autoHSCT), and ribavirin therapy. b Patient No. 14 with composite lymphoma, autoHSCT, and ribavirin therapy. c Patient No. 2 with B-CLL, profound immunoglobulin deficiency (IgG <1.6 g/L), but persistently normal CD4+ lymphocyte counts, and spontaneous termination of the HEV infection. Upper limit of normal for ASAT <50 U/L. B-CLL = B-cell chronic lymphocytic leukemia.
Patient 2 (Table 1), an 84-year old male with B-cell chronic lymphocytic leukemia experienced a spontaneous SVR despite a profound hypogammaglobulinemia (IgG <1.6 g/L, previous anaphylaxis precluded immunoglobulin replacement). The baseline numbers of CD4+ cells in this patient were normal and further increased with waning HEV-RNA load (Fig. 1c).
Sequential numbers of circulating CD4+ T cells were available in 9 patients. Of these, 6 patients experienced an SVR and 3 patients relapsed after a transient response to ribavirin. We observed an increase of the numbers of CD4+ circulating T cells in 5 of 6 patients with an SVR, whereas patients with a relapse showed a decrease of CD4+ T-cell counts (2 patients) or a slight increase (1 patient) only (Fig. 2). The median ratio of the CD4+ T-cell counts (follow-up/prior) was 2.8 in patients with an SVR compared to only 1.0 in patients with a relapse, but this did not reach statistical significance (p = 0.17).
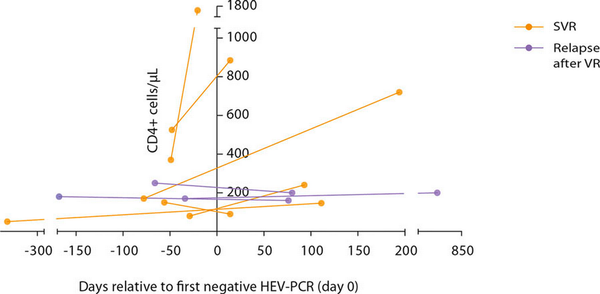
Fig. 2
Serial numbers of CD4+ T cells in patients with a sustained virological response (SVR) or relapse after initial virological response (VR).
Discussion
The number of identified patients at our tertiary care cancer center is relatively low, which is in line with previous reports [, , ]. However, patients were not systematically tested (no diagnostic algorithm), and HEV infections might have been missed. In a recently published, retrospective study, eight (3.4%) of 236 alloHSCT patients were positive for HEV-RNA []. Five of these patients were negative for anti-HEV antibodies. Patient 13 from the present study was also negative for anti-HEV antibodies despite a high viral load, which corroborates recommendations to use molecular testing for reliable recognition of HEV infections in immunocompromised patients [, ].
It is noteworthy that 3 alloHSCT patients experienced a spontaneous SVR, which is possibly linked to a late onset of the HEV infection after transplantation. A reduction of current immunosuppressive treatment is recommended, but this is largely based on observations in solid organ transplant recipients [, ]. In a series of 16 patients with chronic HEV infections post solid organ transplantation, patients with low tacrolimus trough levels were more likely to clear the infection []. A reduction of the immunosuppression was reported to be successful in HSCT patients with HEV infection [, ] but was associated with mortality in 2 patients with alloHSCT in another study [], so that this approach must be carefully balanced against potentially life-threatening deterioration of autoimmune reactions.
The importance of adequate B-cell responses has been well acknowledged for infections with the hepatitis B and C virus. Various B-cell-directed therapies increase the risk of hepatitis B reactivation [, ]. Also, reactivation and flares have been observed in frequent association with rituximab-based and high-dose steroid therapies in patients infected with the hepatitis C virus []. It is less clear, whether B-cell responses are of major importance in HEV infections. In a recently published series of 5 patients with chronic HEV infection following therapy with the B-cell-targeting antibody rituximab, only 1 patient responded to ribavirin with an SVR []. In another retrospective patient series, CD20-directed therapies correlated with a prolonged time to viral clearance and the development of chronic hepatitis []. However, the spontaneous SVR in our patient with B-cell chronic lymphocytic leukemia, who had profound hypogammaglobulinemia but normal CD4+ cells, suggests that a B-cell response might not be essentially required for HEV clearance.
Lymphopenia and low CD4+ T-cell numbers have been associated with chronic HEV infections in patients with solid organ transplantation []. Similarly, baseline lymphopenia was significantly associated with treatment failures in patients with solid organ transplantation and chronic HEV infection []. It has been demonstrated that specific T-cell responses were significantly lower and absent in patients with solid organ transplantation and chronic HEV infection compared to patients with resolved HEV infection []. Similar data in patients with HM are scarce. In the aforementioned series of patients with chronic HEV infection following therapy with rituximab, all 5 patients presented with CD4-lymphopenia (<200/µL) []. The remarkable recovery from CD4-lymphopenia in 2 patients from our study and increasing numbers of CD4+ T-cells in the majority of patients with VRs likely reflects recovery from T-cell exhaustion. This is in line with observations in HIV/HEV-coinfected patients, which indicated that HEV clearance is associated with higher CD4+ cell counts [].
Our study expands the limited knowledge about HEV infections in patients with HMs. The observation of CD4+ T-cell recoveries in association with viral responses may indicate an early response marker. However, this was a retrospective study in a limited number of patients without standardized diagnostic assessments and individual therapeutic decisions. Thus, more detailed studies with standardized protocols are needed.
Conclusions
Early ribavirin treatment in immunocompromised patients infected with HEV and CD4-lymphopenia appears meaningful to prevent chronic HEV infections. Patients infected with HEV and normal numbers of circulating CD4+ T cells might be observed without treatment awaiting spontaneous termination of HEV replication, but efforts to avoid chronic HEV infections appear mandatory.
Acknowledgments
Mrs. Ingwersen is a MD candidate at the Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany. This work is submitted in partial fulfillment of the requirement for the MD. The authors would like to thank Thomas Bauer for graphic design.
Statement of Ethics
This study was approved by the Ethics Committee of the Charité – Universitätsmedizin Berlin (ref. No. EA4/016/19, 27th, February 2019) and the principles set forth in the Declaration of Helsinki were followed. Patient consent was waived due to the retrospective nature of this study and anonymized data analysis, according to national regulations.
Conflict of Interest Statement
P. L. C. has received consulting fees from Novartis, Incyte, BMS, honoraria from Novartis, BMS, Pfizer, Incyte; G. M. has received honoraria from Amgen, Gilead, Merck Serono, Janssen-Cilag, payments from the German Cancer Society and German Society for Hematology and Medical Oncology as a member of Guidelines Writing Committees; and S. S. has received consulting fees from Amgen, Gilead, Pfizer, SERB SAS, honoraria from the Akademie für Infektionsmedizin e.V., Amgen, AVIR Pharma, CSi Hamburg GmbH, Gilead, Labor28, Novartis, Persberg Group GmbH/DGIM e.V., Pfizer, Vivantes GmbH, financial support for research projects from Protherics Medicines Development Ltd, and travel grants from Gilead and Novartis, all of which unrelated to the topic of this paper. All other authors have no conflicts of interest to declare.
Funding Sources
This research was funded by a grant to J.H. from the German Federal Ministry of Health with regard to a decision of the German Bundestag by the Federal Government, CHED-project grant No: ZMVI1-2516-AUK-701/BMG: 321-4471-02/157. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, and in the writing of the manuscript.
Author Contributions
VI, TS has made substantial contributions to the conception and design of the work, acquired, analyzed, and interpreted data, and has drafted the work. JH and SS has made substantial contributions to the conception and design of the work, acquired, analyzed, and interpreted data, and was a major contributor in writing the manuscript. MM, CL, and TB acquired, analyzed, and interpreted data, and has drafted the work. PLC acquired, analyzed, and interpreted data, and has drafted the work. GM and UK has made substantial contributions to the conception and design of the work, analyzed, and interpreted data, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Nimgaonkar I, Ding Q, Schwartz RE, Ploss A Hepatitis E virus: advances and challenges Nat Rev Gastroenterol Hepatol 2018 15 2 96–110
- 2. Li P, Liu J, Li Y, Su J, Ma Z, Bramer WM, et al The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis Liver Int 2020 40 7 1516–28
- 3. Sooryanarain H, Meng XJ Hepatitis E virus: reasons for emergence in humans Curr Opin Virol 2019 34 10–7
- 4. Kamar N, Mansuy JM, Esposito L, Legrand-Abravanel F, Peron JM, Durand D, et al Acute hepatitis and renal function impairment related to infection by hepatitis E virus in a renal allograft recipient Am J Kidney Dis 2005 45 1 193–6
- 6. Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al Hepatitis E virus and chronic hepatitis in organ-transplant recipients N Engl J Med 2008 358 8 811–7
- 7. von Felden J, Alric L, Pischke S, Aitken C, Schlabe S, Spengler U, et al The burden of hepatitis E among patients with haematological malignancies: a retrospective European cohort study J Hepatol 2019 71 3 465–72
- 8. Schulz M, Papp CP, Bock CT, Hofmann J, Gerlach UA, Maurer MM, et al Combination therapy of sofosbuvir and ribavirin fails to clear chronic hepatitis E infection in a multivisceral transplanted patient J Hepatol 2019 71 1 225–7
- 9. European Association for the Study of the Liver Electronic address easloffice@easlofficeeuEuropean Association for the Study of the Liver EASL clinical practice Guidelines on hepatitis E virus infection J Hepatol 2018 68 6 1256–71
- 10. Versluis J, Pas SD, Agteresch HJ, de Man RA, Maaskant J, Schipper ME, et al Hepatitis E virus: an underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation Blood 2013 122 6 1079–86
- 11. Ghandili S, Lindhauer C, Pischke S, Zur Wiesch JS, Von Kroge PH, Polywka S, et al Clinical features of hepatitis E infections in patients with hematologic disorders Haematologica 2022 107 12 2870–83
- 12. Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus J Virol Methods 2006 131 1 65–71
- 13. le Coutre P, Meisel H, Hofmann J, Rocken C, Vuong GL, Neuburger S, et al Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation Gut 2009 58 5 699–702
- 14. Cardinez C, Miraghazadeh B, Tanita K, da Silva E, Hoshino A, Okada S, et al Gain-of-function IKBKB mutation causes human combined immune deficiency J Exp Med 2018 215 11 2715–24
- 15. Swartling L, Norden R, Samuelsson E, Boriskina K, Valentini D, Westin J, et al Hepatitis E virus is an infrequent but potentially serious infection in allogeneic hematopoietic stem cell transplant recipients Bone Marrow Transplant 2020 55 7 1255–63
- 16. Mallet V, van Bommel F, Doerig C, Pischke S, Hermine O, Locasciulli A, et al Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5) Lancet Infect Dis 2016 16 5 606–17
- 17. Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssiere L, et al Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation Transplantation 2010 89 3 353–60
- 18. Mikulska M, Penack O, Wendel L, Knelange N, Cornelissen JJ, Blijlevens N, et al HEV infection in stem cell transplant recipients-retrospective study of EBMT Infectious Diseases Working Party Bone Marrow Transplant 2022 57 2 167–75
- 19. Herishanu Y, Katchman H, Polliack A Severe hepatitis B virus reactivation related to ibrutinib monotherapy Ann Hematol 2017 96 4 689–90
- 20. Ogawa E, Wei MT, Nguyen MH Hepatitis B virus reactivation potentiated by biologics Infect Dis Clin North Am 2020 34 2 341–58
- 21. Torres HA, Hosry J, Mahale P, Economides MP, Jiang Y, Lok AS Hepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational study Hepatology 2018 67 1 36–47
- 22. Schulz M, Biedermann P, Bock CT, Hofmann J, Choi M, Tacke F, et al Rituximab-containing treatment regimens may imply a long-term risk for difficult-to-treat chronic hepatitis E Int J Environ Res Public Health 2020 17 1 341
- 23. Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, et al Ribavirin for chronic hepatitis E virus infection in transplant recipients N Engl J Med 2014 370 12 1111–20
- 24. Suneetha PV, Pischke S, Schlaphoff V, Grabowski J, Fytili P, Gronert A, et al Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection Hepatology 2012 55 3 695–708
- 25. Debes JD, Pisano MB, Lotto M, Re V Hepatitis E virus infection in the HIV-positive patient J Clin Virol 2016 80 102–6
- 5. Ruutu T, Carreras E Hepatic complications 7th ed. In: Carreras E, Dufour C, Mohty M, Kroger N, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapiesChamCH)2019. p. 373–9.