A 24-year-old woman was diagnosed with von Hippel-Lindau (VHL) syndrome, which manifested as cerebral, spinal, and retinal hemangioblastomas (RHB). Although she underwent multiple surgeries to save her vision, her right eye's condition worsened to no light perception (NLP). As a result, it was decided to observe her without additional ophthalmic treatments. Treatment was started on belzutifan (Welireg, Merck) on October 12, 2023, due to neurologic indications. Remarkably, within just 2 months, there was a significant reduction in the size of the RHB in her right eye. Figure 1A–C displays fundus photographs of the patient's eye, taken 12, 8, and 4 months before starting treatment with belzutifan, showing the stable condition of the RHB. Figure 1D shows the fundus photograph of the same eye, captured just 2 months following the initiation of therapy. A dramatic response to the treatment is evident, characterized by fibrosis and shrinkage of the RHB.
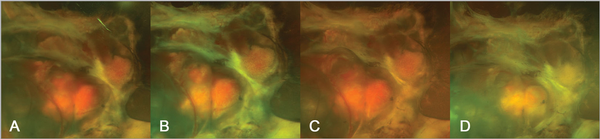
Figure 1
Advanced proliferative vitreoretinopathy with large central retinal hemangioblastoma in detached retina demonstrating progressive activity of the lesion (A) 12 months, (B) 8 months, and (C) 4 months prior to belzutifan. (D) Two months following initiation of belzutifan, the lesion has demonstrated complete regression.
Belzutifan, an oral hypoxia-inducible factor-2α (HIF-2α) inhibitor, was approved on August 13, 2021, by the United States Food and Drug Administration (US FDA) for adult VHL patients who need treatment for associated renal cell carcinoma, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors, when immediate surgery is not indicated. Recent findings have also highlighted its efficacy in reducing the size, vascularity, and exudation in certain types of RHB and in treating giant RHB with extrascleral extension. Additionally, it has shown promise in treatment of RHB in a pediatric patient.
References
- 1. Fallah J, Brave MH, Weinstock C , , et al. FDA approval summary: belzutifan for von Hippel-Lindau disease-associated tumors. Clin Cancer Res. 2022;28(22):4843–4848–. PMID:
- 2. Fairbanks AM, Hoyek S, Patel NA. Systemic treatment reduces von-Hippel-Lindau-associated retinal capillary hemangioblastoma. Ophthalmology. 2023;130(5):524. PMID:
- 3. Wiley H, Coleman HR, Jonasch E , , et al. Oral HIF-2α inhibitor belzutifan for ocular von Hippel-Lindau (VHL) disease. Invest Ophthalmol Vis Sci. 2021;62(8):32.
- 4. Mashayekhi , A. Retinal hemangioblastoma may be partially reduced with belzutifan treatment. American Academy of Ophthalmology. Published October 27, 2022. Accessed January 13, 2024. https://www.aao.org/education/editors-choice/retinal-hemangioblastoma-may-be-partially-reduced-
- 5. Grimes JM, Gershkovich A, Bogomolny D, Marr BP. Two cases of von Hippel-Lindau syndrome-associated retinal hemangioblastoma treated with belzutifan. Retin Cases Brief Rep. 2024;18(3):319–322–. https://journals.lww.com/retinalcases/abstract/2024/05000/two_cases_of_von_hippel_lindau_syndrome_associated.11.aspx
- 6. Cotton CC, Chandrabhatla AS, Andrews PH, Purrow BW, Shildkrot YE. Belzutifan for treatment of giant retinal hemangioblastoma with extrascleral extension associated with von Hippel-Lindau syndrome. Retin Cases Brief Rep. 2023. PMID:
- 7. Jones AA, Schloemer NJ, Wirostko WJ. Successful treatment of von Hippel-Lindau (VHL) disease-associated retinal capillary hemangioblastoma (RCH) with belzutifan in a pediatric patient. Retin Cases Brief Rep. 2023. PMID:
