Vestibular schwannomas (VSs) are rare and benign tumors arising in the cerebellopontine angle, typically causing hearing loss, tinnitus, and balance disorders. In addition, facial numbness or pain, headache, and facial paresis may occur. A substantial minority of the tumors (22%‐48%) are progressive and may eventually lead to brainstem compression or increased intracranial pressure. Management options comprise active surveillance, surgery, or radiotherapy and aim to prevent severe sequelae while maintaining patients’ quality of life (QoL). None of these modalities will improve symptoms, and all yield the risk of deterioration of hearing, balance, and facial nerve function. Active surveillance is the management option for indolent tumors, whereas radiotherapy or surgical removal is indicated for progressive or large tumors, both resulting in >90% tumor control., Furthermore, the choice for a specific treatment option depends on additional tumor characteristics (eg, localization and size) and patient‐related factors such as the burden and type of symptoms and patient preference.
Regardless of the treatment modality, a VS affects a patient's QoL., , , , Ongoing dizziness and headache seem the most important determinants of poor QoL, as is large tumor size., QoL seems most affected directly after diagnosis., The effect of other determinants on general QoL, such as sex and education, is not widely reported in VS research.
In 2010, Shaffer et al developed the Penn Acoustic Neuroma Quality of Life (PANQOL) questionnaire. This disease‐specific questionnaire enabled more accurate QoL measurement than the generic QoL questionnaires.7,, , Several studies have published the results on the QoL of different treatment modalities., , , , Kerezoudis et al have defined the minimal clinically important differences (MCIDs) of PANQOL total and subdomain scores, which advanced the interpretation of changes in PANQOL beyond the level of statistical significance.
Little is known about the longitudinal impact of VS on QoL in the long term and whether patients regret the initial treatment decision. Longitudinal studies show changes within individuals over time, which cannot be detected in cross‐sectional studies. This provides essential information since patients grow old with their tumor and the side effects of the chosen treatment. Previous studies on long‐term QoL in patients with VS were cross‐sectional in design and showed no clinically relevant differences between treatment modalities., , Longitudinal studies lack long‐term follow‐up (2‐3 years),, do not use a disease‐specific questionnaire,, , or focus only on 1 treatment modality., , This study aims to find the long‐term longitudinal QoL outcomes and evaluates decisional regret in patients with VS.
Methods
This longitudinal study was performed at the Leiden University Medical Center (LUMC), an expert center for VS in the Netherlands. Data were first collected in 2014 for a cross‐sectional study on QoL and second in 2020. The Medical Ethical Committee LDD waived the necessity for medical ethical approval under Dutch law and approved the study regarding data handling and privacy regulations (N19.112).
Patients who had participated in 2014 were reapproached for participation by mail or email. Inclusion criteria were age ≥18 years and a unilateral VS. Patients with other skull base pathologies were excluded. All patients were referred to the LUMC between 2004 and 2014.
Patients completed the PANQOL, a validated questionnaire measuring VS‐related QoL and consisting of 26 items divided over 7 subdomains (hearing, balance, face, pain, energy, anxiety, and general health)., The Likert scale answers were summed per subdomain and recoded to range from 0 to 100, with higher scores indicating better QoL. A total score was calculated as the arithmetic mean of the subdomain scores. Incomplete answers were excluded on a subdomain level. In addition, patients completed the Decision Regret Scale (DRS), a 4‐item validated questionnaire. A total score was calculated from 0 to 100, with higher scores indicating high regret. Scores of 0 were defined as no regret and >50 as considerable regret. Incomplete questionnaires were excluded from the analysis. Furthermore, demographic parameters about sex, age, occupation, and education level were collected. Statistics Netherlands (CBS) definition for low, middle, and high education level was used.
Tumor size, treatment modality, time since diagnosis, and treatment were retrospectively acquired from patient records. Treatment modality was categorized as active surveillance, surgery, radiotherapy, or both surgery and radiotherapy. At our center, multimodal therapy is performed only if the first modality (either surgery or radiotherapy) fails (ie, as salvage treatment). Patients in whom radiotherapy failed underwent salvage surgery and vice versa. Tumor size was scored at the start of treatment or in the case of active surveillance at the time of the first questionnaire in 2014 using the reporting system proposed by Kanzaki et al. Categories large and giant were merged because of the small number of patients in these categories.
Statistical analyses were performed in R version 4.0.5 using Rstudio 1.3.959 (Rstudio; PBC). Means and standard deviations were calculated for normally distributed variables and medians and interquartile ranges (IQRs) for nonnormally distributed continuous variables. For categorical variables, counts and frequencies were calculated. A nonresponder analysis was performed using unpaired t tests.
Differences in PANQOL subdomains between 2014 and 2020 were tested per treatment modality with a paired t test and Bonferroni correction to prevent type I error. In addition, the differences were compared to the median anchored subdomain and total specific MCID reported by Kerezoudis et al, who defined the MICD for the total PANQOL score as 12.5 points and for the subdomain “balance” as 14.0 points. A difference smaller than the MCID was defined as stable and larger differences as either deterioration or improvement of QoL. The MCIDs were used to assess the clinical relevance of QoL changes on group and individual level., Differences in decisional regret between treatment modalities were tested pairwise using the Wilcoxon rank test with Bonferroni correction.
Long‐term effects of time since treatment and treatment modality on QoL were analyzed using a linear mixed model (R‐package nlme) to account for repeated measurement data. Model assumptions were visually checked. The total PANQOL score was the dependent variable, with 2 measurements per patient (2014 and 2020). Covariates such as age, education level, sex, and tumor size were stepwise included in the model, and model selection was based on the Akaike information criterion, as were interactions between time since treatment and other covariates. In the final model, random intercepts were used with fixed variables. All tests were 2‐sided and P values <.05 were considered statistically significant. Patients treated (by surgery, radiotherapy, or both) after 2014 (ie, between the 2 measurements) were analyzed separately. Because of the small sample size, no further statistical analysis was performed on these data.
Results
In 2014, 913 patients completed 1 or more PANQOL subdomains. In 2020, 867 of 913 patients were still alive and were reapproached, of whom 536 responded (62% response rate), as shown in Figure 1. After the first measurement in 2014, 36 patients were actively treated with either surgery or radiotherapy. These patients were analyzed separately. In total, 487 patients completed 1 or more subdomains of the PANQOL.
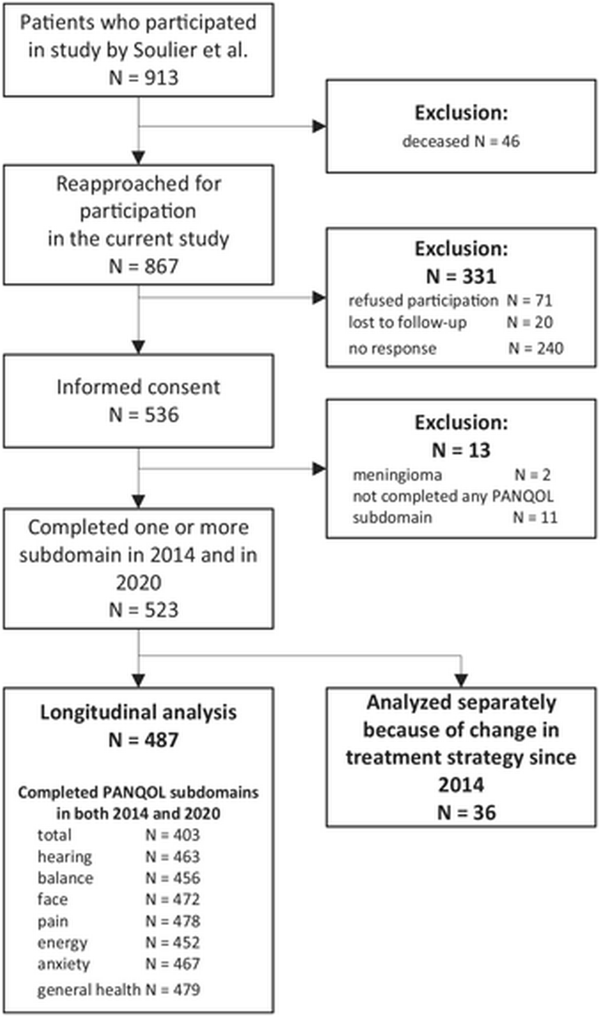
Figure 1
Flowchart of study participants. The number of patients who completed the Penn Acoustic Neuroma Quality of Life (PANQOL) questionnaire both in 2014 and 2020 are shown in the last box per subdomain.
In the nonresponder group (n = 331), 240 (62%) did not respond for unknown reasons, 20 (6%) were lost to follow‐up, and 71 (24%) declined participation. The most frequent reason for declining participation was lack of time (31%), followed by health problems other than VS (14%); 28% did not provide a reason. Responders were on average 9 months younger, had higher education levels (high‐level education 21% vs 14%), and had received surgery more often (22% vs 10%) than nonresponders.
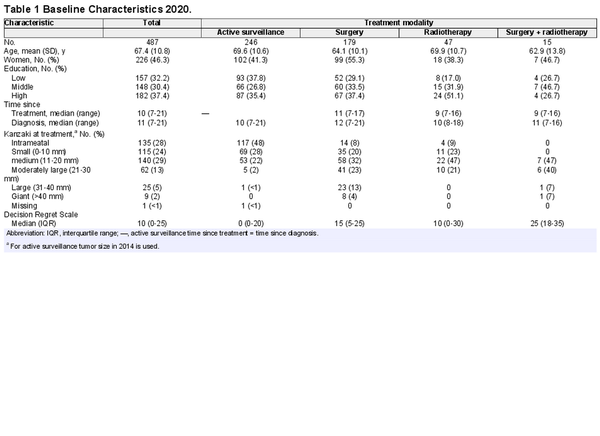
The median time since treatment was 10 years and since diagnosis 11 years. Patients who underwent surgery were on average younger, were more often female, and had larger tumors at the start of the treatment (Table 1).
The paired PANQOL scores of 2014 and 2020 are shown in Figure 2. Only balance scores showed deterioration over time in both the active surveillance (–6.3; 95% CI, –3.9 to –9.3) and surgery groups (–4.8; 95% CI, –1.9 to 7.7). Both differences did not exceed the MCID of 14 points. The group receiving both surgery and radiotherapy showed a nonsignificant trend of deteriorating scores at all subdomains.
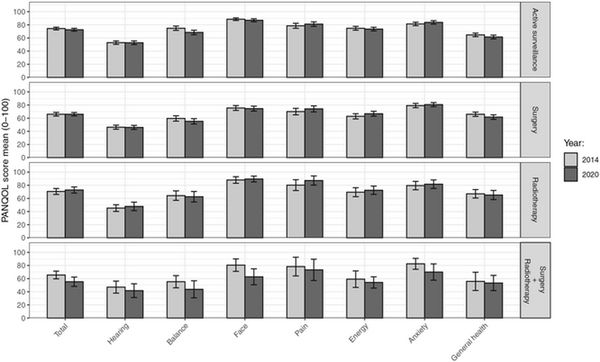
Figure 2
Paired unadjusted mean Penn Acoustic Neuroma Quality of Life (PANQOL) scores 2014 and 2020 per treatment modality. Error bars indicate 95% CIs of the means. Higher scores indicate better quality of life. The total score is the arithmetic mean of the subdomains.
When the changes over time in PANQOL scores were compared with the MCID (12.5 points), most patients (n = 278, 69%) were stable (ie, showed a difference of less than 12.5 points). In the active surveillance group, 36 patients (17.9%) had a deterioration in the overall PANQOL score, whereas 27 (13.4%) patients reported an improvement in PANQOL. Most (135 patients; 67.2%) had a stable PANQOL score. In the surgery group, 23 patients (15.7%) deteriorated, 18 (11.9%) improved, and 108 (71.5%) were stable. In the radiotherapy group, 4 patients (11.1%) deteriorated, 4 (11.1%) improved, and 27 (75.0%) remained stable. These differences between treatment strategy groups are small and not statistically significant (χ, P = .8). In addition, we found no baseline differences between patients with a stable long‐term QoL and those with decreasing or increasing QoL scores over time.
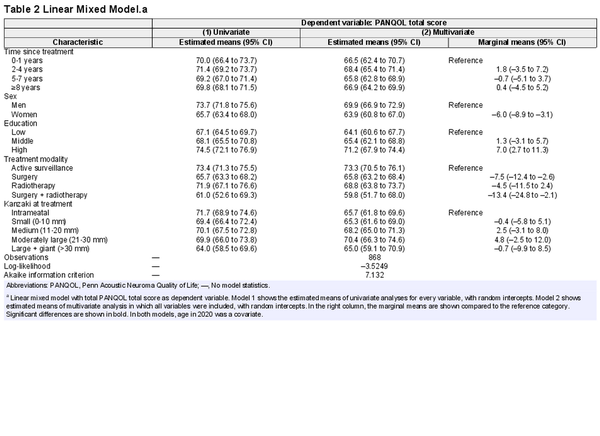
The effects of sociodemographic and clinical characteristics on the total PANQOL score were assessed using a linear mixed model (Table 2 and Figure 3). There was no statistically significant association between time since treatment and total score, and associations between other covariates and PANQOL score were not dependent on the time since treatment. Therefore, the interaction terms were omitted from the final model.
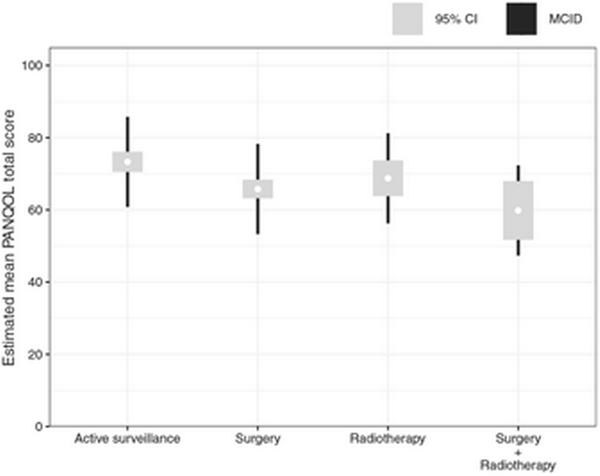
Figure 3
Estimated means of the Penn Acoustic Neuroma Quality of Life (PANQOL). Results of the linear mixed model of the total PANQOL score per treatment modality corrected for confounding factors (age, sex, education level, time since treatment, tumor size at treatment) per treatment.
A statistically significant correlation was found between the level of education and QoL (7.2‐point difference on the PANQOL; 95% CI, 3.4‐10.6), although the difference did not reach the MCID. Also, women had a statistically significant lower QoL than men (–6.0; 95% CI, –8.9 to –3.1), although not exceeding the MCID. This sex‐related difference occurred across all treatment modalities. The lower total scores of women for active surveillance (–6.1; 95% CI, –10.8 to –1.3) and surgery (–9.3; 95% CI, –14.6 to –4.1) were smaller than the MCID of 12.5. For radiotherapy (–12.0; 95% CI, –23.3 to –0.6) and radiotherapy + surgery (–14.8; 95% CI, –29.3 to –0.3), the differences were close to or above the MCID. Although individual sociodemographic characteristics did not exceed PANQOL MCIDs, a combination of different sociodemographic factors might. For example, males with a high level of education tended to have higher total scores (+13.2) than females with a low level of education.
In addition, receiving a disability pension (n = 37) was associated with lower PANQOL total scores. The mean difference in total scores of full‐time employed patients (n = 205) and patients with a disability pension were 25.3 (95% CI, 20.5‐30.1), exceeding the 12.5 MCID. Furthermore, differences of total scores of unemployed (n = 7; –13.0; 95% CI, 1.6 to –27.6) and voluntary unemployed (n = 36; –13.3; 95% CI, –8.0 to –18.7) exceeded the MCID.
Total PANQOL scores in the surgery (–7.5; 95% CI, –11.2 to –3.8) and surgery + radiotherapy (–13.5; 95% CI, –22.1 to –4.8) groups were lower than the active surveillance group. The difference with the group receiving both surgery and radiotherapy exceeded the 12.5‐point MCID. When analyzing the separate subdomains, differences were found for balance after surgery (–14.8) and radiotherapy (–15.4), exceeding the MCID of 14. Differences in anxiety in the radiotherapy group (–18) exceeded the MCID (13).
Decision regret was analyzed per treatment modality (Table 1 and Supplemental Table A in the online version of the article). In the active surveillance group, 52% scored 0, indicating no regret at all, and 2% scored >50, indicating considerable regret. After surgery, the median score was 15 (IQR, 5‐25), while 23% had no regret and 7% had considerable regret. After radiotherapy, the median score was 5 (IQR, 0‐20), 49% had no regret, and 6% had considerable regret. Patients in the surgery + radiotherapy group had the highest DRS scores, with a median of 25 (IQR, 18‐35). Only 7% had no regret in this patient group, while 20% had considerable regret. Compared to active surveillance, surgery (P < .0001) and surgery + radiotherapy (P = .002) scored significantly worse. The difference between surgery and radiotherapy was not statistically significant.
Between the 2 measurements, 36 patients were actively treated, of whom 28 completed all PANQOL questions in 2014 and 2020. Of the patients receiving surgery (n = 9), the QoL of 1 patient deteriorated (11%), and the other patients remained stable (ie, within the MCID limits) over time. In the radiotherapy group, 7 (36.8%) deteriorated, 3 (15.8%) improved, and 9 (47.4%) remained stable. Median DRSs in the groups were 10 and 20 for surgery and radiotherapy, respectively.
Discussion
This longitudinal study showed that although the individual variation is considerable, on average, the QoL of patients with VS remains stable over time and is comparable for all treatment modalities, except for patients requiring salvage therapy after initial therapy failure. These patients seemed to have a lower QoL that declined over time. This group also has the highest decision regret, while in other groups, decision regret is low.
Although the disease‐specific QoL remained stable on average, a minority of the patients with VS reported changing PANQOL scores over time. There were no significant differences in the proportion of patients experiencing decreased or increased QoL between treatment modalities. In addition, we could not find reliable predictors for either improvement or deterioration of QoL over time.
Since the development of the disease‐specific PANQOL, several large cross‐sectional studies have been performed assessing QoL in patients with VS., , , , , None have shown clinically relevant differences between treatment modalities. In agreement with the current study, McLaughlin et al and Carlson et al reported a slightly lower QoL after surgery that did not exceed the MCID. Previous short‐term longitudinal studies that used the PANQOL showed no differences in QoL outcomes per treatment modality and no differences over time, as was observed in this study.,
A clinically relevant lower QoL was found in patients requiring multiple treatments (ie, salvage therapy by radiotherapy or surgery after initial therapy failure). Although the group size was limited, the differences were statistically significant. In addition, this group seemed to have a declining trend over time in all PANQOL subdomains. This finding is in agreement with the study by Carlson et al, who reported a statistically significant difference between active surveillance and multimodality treatment.
Importantly, as in previous studies, treatment groups were not similar at baseline in this study. For example, patients undergoing surgery tended to be younger and had larger tumors, and patients requiring both radiotherapy and surgery had failed initial treatment. These differences reflect the indications for specific treatment modalities at our center. Although preservation of QoL is an important goal of VS management, other factors (eg, tumor progression) usually determine the necessity for active intervention. The choice of treatment modality is also not determined by its intrinsic contribution to a patient's QoL. Moreover, the finding that long‐term QoL is comparable for all 3 management strategies does not mean that treatments are interchangeable from a QoL perspective or that the choice or timing of treatment is of little relevance. Rather, the comparability of long‐term quality life after different VS treatment strategies in retrospect can be viewed as the result of personalized treatment decisions, deploying a specific therapy in a specific patient at a specific moment in the course of the disease.
The current study shows that even >10 years after treatment, QoL is stable across modalities, which supports the results of 2 cross‐sectional studies on long‐term QoL., Patients in the active surveillance and surgery group had a minor deterioration of the balance subdomain, however not exceeding the MCID. This minor decline was not observed in 2 short‐term longitudinal studies., The deterioration could be due to an aging effect, which might cause increased balance problems combined with the VS. Other longitudinal studies have reported contradicting changes in anxiety, especially in patients undergoing surgery., In the current study, no changes in anxiety were observed. It might be possible that anxiety is affected shortly after diagnosis and/or treatment and remains stable over time afterward.
The current study identified several factors associated with worse long‐term QoL in patients with VS besides the requirement of salvage treatment: female sex and disability pension. Sex‐related differences in QoL were observed, with lower QoL in women specifically for the balance and anxiety subdomains. To our knowledge, the difference in QoL between male and female patients has not been described before in VS. The PANQOL validation study identified no sex‐related differences. Many studies have corrected for sex but did not report the effect of sex on the QoL.4,6‐8,14,15 However, this sex difference has been reported in other diseases and in the general population too, which can only be partly explained by differences in social‐economic status., , Other possible explanations might be sex‐related differences in reporting symptoms or an actual difference in the disease‐related QoL.
Decision regret was low in the active surveillance group and slightly higher in radiotherapy and surgery groups. One previous study in patients with VS supported the findings in this study, albeit using nonvalidated questions, reporting 97%, 96%, and 85% satisfaction for active surveillance, radiotherapy, and surgery, respectively. A systematic review in various diseases (mainly oncology) showed a mean DRS score of 16.5. In the current study, only patients requiring salvage therapy, and thus receiving both surgery and radiotherapy during the course of the disease, had a higher decisional regret. This is not surprising as in these patients, initial treatment had failed.
This study has some limitations. The retrospective design carries an inherent risk of selection bias, although this design was inevitable for gathering long‐term longitudinal results of patients diagnosed before the development of the disease‐specific questionnaire. It is possible that a certain selection of patients was more likely to participate, for example, patients with sequelae. The first survey was performed after diagnosis and treatment in most patients, and the results therefore represent the longitudinal QoL after different treatment strategies, rather than the effect of treatment on QoL. Furthermore, the group of nonresponders might introduce selection bias since this group had different demographic characteristics. In addition, the patient group requiring salvage therapy was small because recurrences are relatively rare.
Conclusion
This longitudinal study shows that QoL in patients with VS is stable over time and that different management strategies (surgery, radiotherapy, and active surveillance) result in comparable long‐term QoL outcomes. There is, however, considerable individual variation. Factors associated with a decreased long‐term QoL in patients with VS are female sex, receiving a disability pension, and the need for salvage treatment after initial therapy failure.
Author Contributions
Olaf M. Neve, study concept and design, data collection and analysis, interpretation and implications of results, drafting manuscript, final approval of the manuscript; Jeroen C. Jansen, interpretation and implications of results, critical revision of the manuscript and final approval; Radboud W. Koot, implications of results, critical revision of the manuscript and final approval; Mischa de Ridder, implications of results, critical revision of the manuscript and final approval; Peter Paul G. van Benthem, implications of results, critical revision of the manuscript and final approval; Anne M. Stiggelbout, study concept and design, implications of results, critical revision of the manuscript and final approval; Erik F. Hensen, study concept and design, implications of results, critical revision of the manuscript and final approval.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: Leiden University Medical Center, strategic fund.
Acknowledgments
We thank professor Hein Putter of the LUMC statistics department for his advice and help in choosing the right statistical approach.
References
- 1. Carlson ML, Link MJ. Vestibular schwannomas. N Engl J Med. 2021;384(14):1335–1348.
- 2. Møller MN, Hansen S, Miyazaki H, Stangerup S, Caye‐Thomasen P. Active treatment is not indicated in the majority of patients diagnosed with a vestibular schwannoma: a review on the natural history of hearing and tumor growth. Curr Otorhinolaryngol Rep. 2014;2:242–247.
- 3. Neve OM, Soulier G, Hendriksma M, et al. Patient‐reported factors that influence the vestibular schwannoma treatment decision: a qualitative study. Eur Arch Otorhinolaryngol. 2021;278(9):3237–3244.
- 4. Soulier G, Van Leeuwen BM, Putter H, et al. Quality of life in 807 patients with vestibular schwannoma: comparing treatment modalities. Otolaryngol Head Neck Surg. 2017;157(1):92–98.
- 5. Carlson ML, Tveiten OV, Driscoll CL, et al. Long‐term quality of life in patients with vestibular schwannoma: an international multicenter cross‐sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015;122:833–842.
- 6. Carlson ML, Tombers NM, Kerezoudis P, Celda MP, Lohse CM, Link MJ. Quality of life within the first 6 months of vestibular schwannoma diagnosis with implications for patient counseling. Otol Neurotol. 2018;39(10):e1129–e1136.
- 7. Lodder WL, Van Der Laan BFAM, Lesser TH, Leong SC. The impact of acoustic neuroma on long‐term quality‐of‐life outcomes in the United Kingdom. Eur Arch Otorhinolaryngol. 2018;275(3):709–717.
- 8. Robinett ZN, Walz PC, Miles‐Markley B, Moberly AC, Welling DB. Comparison of long‐term quality‐of‐life outcomes in vestibular schwannoma patients. Otolaryngol Head Neck Surg. 2014;150(6):1024–1032.
- 9. Carlson ML, Tveiten ØV, Driscoll CL, et al. What drives quality of life in patients with sporadic vestibular schwannoma? Laryngoscope. 2015;125(7):1697–1702.
- 10. Shaffer BT, Cohen MS, Bigelow DC, Ruckenstein MJ. Validation of a disease‐specific quality‐of‐life instrument for acoustic neuroma. Laryngoscope. 2010;120(8):1646–1654.
- 11. van Leeuwen BM, Herruer JM, Putter H, Jansen JC, van der Mey AG, Kaptein AA. Validating the Penn Acoustic Neuroma Quality of Life Scale in a sample of Dutch patients recently diagnosed with vestibular schwannoma. Otol Neurotol. 2013;34(5):952–957.
- 12. Kristin J, Glaas MF, Schipper J, et al. Patient quality of life after vestibular schwannoma removal: possibilities and limits to measuring different domains of patients’ wellbeing. Eur Arch Otorhinolaryngol. 2019;276(9):2441–2447.
- 13. Kerezoudis P, Yost KJ, Tombers NM, Celda MP, Carlson ML, Link MJ. Defining the minimal clinically important difference for patients with vestibular schwannoma: are all quality‐of‐life scores significant? Neurosurgery. 2019;85(6):779–785.
- 14. Miller LE, Brant JA, Naples JG, Bigelow DC, Lee JYK, Ruckenstein MJ. Quality of life in vestibular schwannoma patients: a longitudinal study. Otol Neurotol. 2020;41(2):e256–e261.
- 15. Carlson ML, Barnes JH, Nassiri A, et al. Prospective study of disease‐specific quality‐of‐life in sporadic vestibular schwannoma comparing observation, radiosurgery, and microsurgery. Otol Neurotol. 2021;42(2):e199–e208.
- 16. Windisch P, Tonn J‐C, Fürweger C, et al. Longitudinal changes of quality of life and hearing following radiosurgery for vestibular schwannoma. Cancers (Basel). 2021;13(6):1315.
- 17. Breivik CN, Varughese JK, Wentzel‐Larsen T, Vassbotn F, Lund‐Johansen M. Conservative management of vestibular schwannoma—a prospective cohort study: treatment, symptoms, and quality of life. Neurosurgery. 2012;70(5):1072–1080.
- 18. Park SS, Grills IS, Bojrab D, et al. Longitudinal assessment of quality of life and audiometric test outcomes in vestibular schwannoma patients treated with gamma knife surgery. Otol Neurotol. 2011;32(4):676–679.
- 19. Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–292.
- 20. Statistics Netherlands . Standaard onderwijsindeling 2016. Den Haag. Accessed March 8, 2021. https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/onderwijs-en-beroepen/standaard-onderwijsindeling--soi--/standaard-onderwijsindeling-2016
- 21. Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–649.
- 22. McGlothlin AE, Lewis RJ. Minimal clinically important difference. JAMA. 2014;312(13):1342.
- 23. Sedaghat AR. Understanding the minimal clinically important difference (MCID) of patient‐reported outcome measures. Otolaryngol Head Neck Surg. 2019;161(4):551–560.
- 24. McLaughlin EJ, Bigelow DC, Lee JY, Ruckenstein MJ. Quality of life in acoustic neuroma patients. Otol Neurotol. 2015;36(4):653–656.
- 25. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926.
- 26. Pettersen KI, Reikvam A, Rollag A, Stavem K. Understanding sex differences in health‐related quality of life following myocardial infarction. Int J Cardiol. 2008;130(3):449–456.
- 27. Cherepanov D, Palta M, Fryback DG, Robert SA. Gender differences in health‐related quality‐of‐life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010;19(8):1115–1124.
- 28. Gijsbers van Wijk CMT, Kolk AM. Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med. 1997;45(2):231–246.
- 29. Carlson ML, Tveiten ØV, Lund‐Johansen M, Tombers NM, Lohse CM, Link MJ. Patient motivation and long‐term satisfaction with treatment choice in vestibular schwannoma. World Neurosurg. 2018;114:e1245–e1252.
- 30. Becerra Pérez MM, Menear M, Brehaut JC, Légaré F. Extent and predictors of decision regret about health care decisions. Med Decis Making. 2016;36(6):777–790.
- 31. Johnson TP. Response rates and nonresponse errors in surveys. JAMA. 2012;307(17):1805.