Traditionally, staging of head and neck squamous cell carcinoma (HNSCC) includes conducting an examination under anesthesia and establishing a tissue diagnosis via direct laryngoscopy (DL) with biopsy. This process requires a trip to the operating room, costing thousands of dollars. Patients who are frail or have tenuous airway anatomy may incur risks while undergoing general anesthesia. Some patients may require awake tracheostomy in order to obtain a biopsy or may suffer from anesthetic complications. In addition, increased duration of time from diagnosis to initiation of treatment has been associated with increased mortality. Time from presentation to tissue diagnosis adds a median of 14 days to the overall time from presentation to initiation of treatment, which is influenced by choice of biopsy technique.
Prior work has shown that head and neck cancers involving the base of tongue (BOT), larynx, and hypopharynx can be accurately staged by analysis of awake endoscopy and high‐resolution imaging studies, provided tissue from the primary or regional site can be obtained. Ultrasound (US) imaging has been widely used for thyroid imaging as well as in the evaluation of cancer in the head and neck region, most often during initial assessment of suspicious neck masses, suspected lymph node involvement after primary tumor localization, and monitoring for recurrent nodal disease. The accuracy of ultrasonographic detection of nodal metastasis is similar to values seen for computed tomography (CT), which is further increased by combining the results of US and CT, , or by using US‐guided fine needle aspiration (FNA)., , , There is promise for expanded use of this US‐guided tissue sampling in obtaining tissue diagnosis of primary HNSCC, as demonstrated in previous studies., , ,
Only a few studies, with small sample sizes, have assessed the ability of US imaging to visually identify and delineate the extent of primary tumors to diagnose, T‐stage, and guide management., , , , , , This accuracy has been assessed when compared with or used in conjunction with other techniques including CT, positron emission tomography (PET) scan, CT‐PET, flexible endoscopy, examination under anesthesia (EUA)‐DL, and excisional biopsy. Our experience demonstrates that the primary sites of supraglottic larynx, BOT, and hypopharynx are easily visualized by US, especially when advanced in size. This study investigates a less costly, potentially less risky, and more expedient diagnostic approach for those patients with select upper aerodigestive (UAD) tract cancer via upfront US imaging and US‐guided needle biopsy rather than the traditional EUA‐DL with biopsy.
Methods
The records of patients at the Veterans Affairs Medical Center (VAMC) Memphis and Regional One Health (ROH) hospitals with suspected supraglottic, oropharyngeal, and hypopharyngeal cancer were reviewed from 2011 to 2016. Patients who received US‐guided needle biopsy of their primary tumor were selected. The patient age, gender, ethnicity, site, stage, US results, operative biopsy results, days until DL biopsy from first clinic visit (potential delay in diagnosis since US can be performed at the first clinic visit), and treatment results were abstracted. This information was placed into a deidentified database. Needle biopsy (18 gauge) was compared with operative tissue diagnosis (either DL biopsy results or definitive surgical excision) via Fisher exact test due to the small sample size. Statistics were performed in Microsoft Excel (Redmond, Washington). Institutional review board approval was obtained from the University of Tennessee and the VAMC Memphis.
Results
Seventeen patients who underwent US for needle biopsy of a primary tumor were included (Table1); 6 (38%) underwent only US biopsy due to frailty or airway risk. Their average age was 63 years (±7 years). Ten (59%) were African American and 7 (41%) Caucasian. Of the 17 patients, 2 were not proven to have cancer via biopsy, 4 were staged as T2/3, and 11 were staged as T4. Three patients were staged as N0/1, and 12 were N2a‐c. Two patients were found to have multiple lung metastases at diagnosis.
US‐obtained needle biopsy yielded malignant cells in 76% of cases (13/17). Overall sensitivity was 86% and specificity was 100%, while positive predictive value (PPV) was 100% and negative predictive value (NPV) was 50%. Eleven of these patients also received operative intervention (either DL or definitive surgical excision). Of these patients, 73% (8/11) were found to have squamous cell carcinoma (SCC) via US‐guided needle biopsy. A median 10‐day presentation to diagnosis interval (possible delay to DL diagnosis vs US biopsy at first clinic visit) was observed for those who underwent additional DL. Of the 3 patients remaining, 18% (2/11) had a negative or insufficient result by US biopsy who received a tissue diagnosis of HNSCC on DL, and 9% (1/11) had an insufficient result on both US and DL biopsy. Using the Fisher exact test on patients who underwent both US and operation (either by DL or resection), we found no difference between US‐obtained and operatively obtained tissue (P =. 27). Three of these patients required awake tracheostomy to perform DL with biopsy.
The overall nonsurgical treatment group was 11 of 16 (67%). Of the positive operative patients (n = 10), 2 went on to primary chemoradiation treatment, 5 patients had surgical excisions including total laryngectomy, and 2 went on to palliative treatment. Our patient with 2 negative biopsies (US needle biopsy and DL with biopsy, Patient 10) did not receive further treatments but agreed to close observation. One patient who was a poor anesthetic candidate had an insufficient needle biopsy and died shortly thereafter (Patient 3).
Six patients did not undergo DL due to underlying comorbidities or airway risk. Five (83%) of these patients had positive US needle biopsies of the primary tumor. Four of them underwent subsequent primary chemoradiation therapy, and 1 had induction chemotherapy followed by resection. The remaining patient (insufficient per US‐guided needle biopsy) received no subsequent treatments.
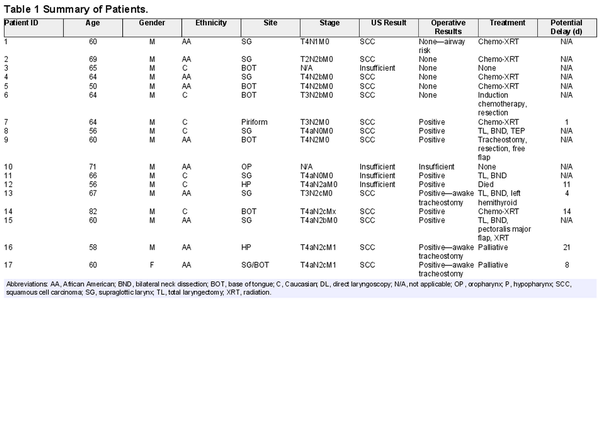
Overall, there were no complications secondary to US‐guided needle biopsy. Comparison of high‐resolution CT imaging and US imaging demonstrated excellent parity in patients with hypopharynx (Figure1), BOT (Figure2), and supraglottic larynx (Figures3 and 4) sites of disease. Select indicators of more advanced tumor stage were readily visualized on US images, such as involvement of strap muscles (Figure1), invasion of extrinsic tongue muscles (Figure2), and invasion of the thyroid cartilage (Figure4).
Discussion
The gold standard for diagnosis of HNSCC is direct visualization with biopsy during DL. This provides a tissue diagnosis, tumor visualization for staging and surgical consideration, and an opportunity to obtain a more extensive excisional biopsy for uncertain lesions. DL requires an additional trip to the operating room prior to definitive treatment, which is associated with anesthesia risks, procedure risks (including risk of requiring tracheostomy, which may preclude opportunity for surgical biopsy), cost, and potential delay in treatment. The package time between presentation and treatment of advanced‐stage HNSCC should proceed as quickly as possible to avoid risk of disease advancement and subsequent poorer outcomes. When comparing risk of progression, same day US‐guided biopsy can eliminate a median 14‐day delay in treatment associated with DL. Our study found a similar 10‐day median delay.
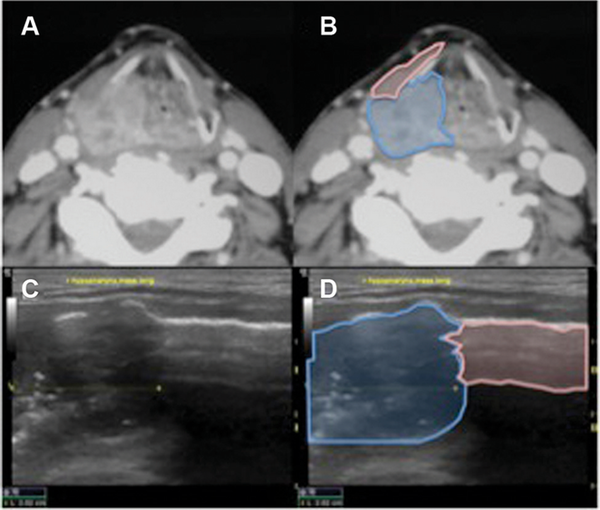
Figure 1
Hypopharynx tumor, patient 12. (A) Axial computed tomography (CT). (B) Enhanced axial CT showing tumor (blue) extending to strap muscle (pink). (C) Sagittal 10‐mHz ultrasound (US). (D) Enhanced US showing tumor (blue) involving strap muscle (pink).
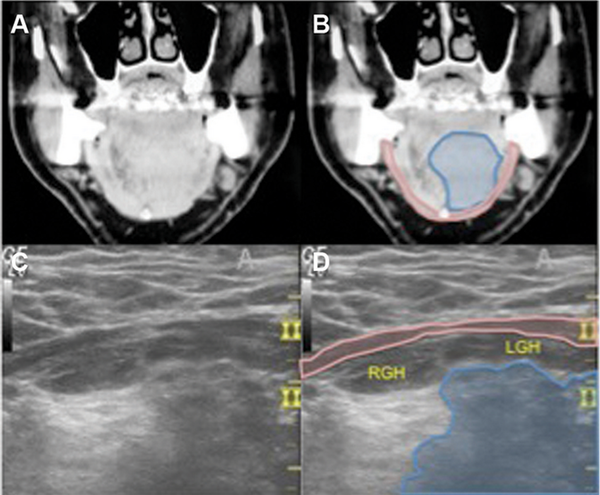
Figure 2
Base of tongue, patient 14. (A) Coronal computed tomography (CT). (B) CT showing tumor (blue) approaching mylohyoid (pink). (C) Axial 10‐mHz ultrasound (US). (D) US showing left geniohyoid muscle (LGH) effacement; note detail on US vs CT.
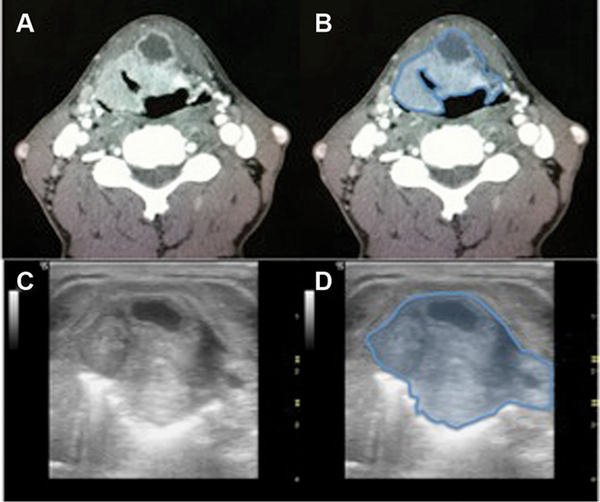
Figure 3
Supraglottic larynx, patient 4. (A) Axial computed tomography (CT). (B) Axial CT at level of thyrohyoid membrane showing tumor (blue). (C) Axial 10‐mHz ultrasound (US). (D) US showing tumor (blue), hyperechoic signal posterior to tumor consistent with secretions.
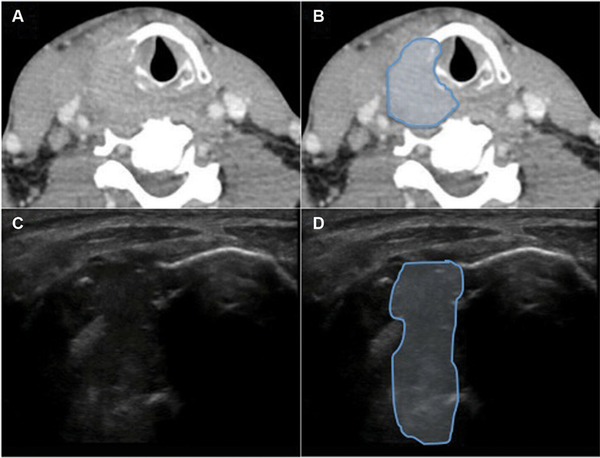
Figure 4
Hypopharynx‐larynx, patient 16. (A) Axial computed tomography (CT). (B) Axial CT showing tumor (blue) completely through thyroid cartilage. (C) Axial 10‐mHz ultrasound (US). (D) US showing tumor (blue) invading completely through thyroid cartilage.
Point of care US provides an opportunity to obtain an immediate low‐cost, well‐tolerated, real‐time imaging examination while simultaneously facilitating low‐risk biopsy, altering management decisions during the initial office visit. Combining US imaging and high‐resolution CT scan (with or without PET imaging) can give accurate details regarding the extent of further structural involvement and may prove to be adequate for staging purposes. US offers unique information, such as ability to assess laryngeal mobility, having a sensitivity and specificity of detecting hemilarynx fixation at 77% and 100%, respectively. US may be superior for visualizing some staging parameters such as extralaryngeal spread including cartilage involvement (Figure5) and extrinsic tongue muscle involvement, but generally it appears best used in combination with CT‐PET, as each modality contributes unique information. Additionally, DL may not be required for anatomic analysis or may be replaced with awake, office‐based flexible laryngoscopy. In cases where an accurate US‐guided biopsy is obtained, extent is often clearly delineated by imaging, and mucosal surface is viewed in office via laryngoscopy, there may be no additional benefit from operative DL. This approach can especially benefit those patients at highest risk for complications of anesthesia.
Within the past few years, US guidance has been used for obtaining tissue from primary HNSCC tumors involving the BOT, larynx, and hypopharynx. Three studies have evaluated US‐guided BOT tissue sampling with good preliminary results although small sample sizes. Our study correlates with the 100% accuracy for identifying and diagnosing BOT cancers., , The reported sensitivity for detecting hypopharynx and larynx tumors ranges from 92% to 100% and was 86% in our study. There is a very high specificity and PPV for this tumor type, as our study correlated with the literature as 100%., , , ,
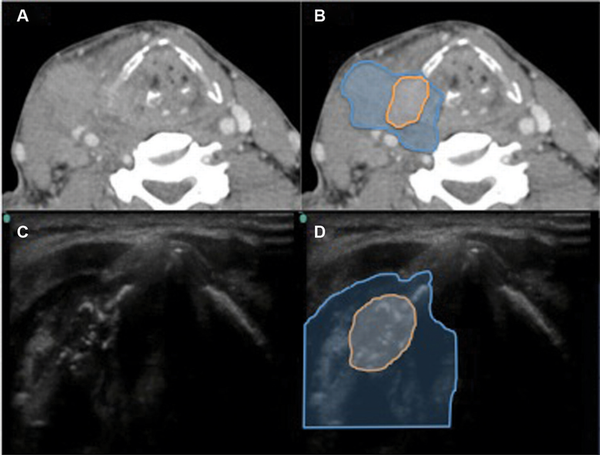
Figure 5
Hypopharynx‐larynx, patient 16. (A) Axial computed tomography (CT). (B) Axial CT showing tumor (blue) with cartilage involvement (orange). (C) Axial 10‐mHz ultrasound (US). (D) US view of similar area with cartilage invasion hallmarked by scattered hyperechoic signals (orange).
Future management options should also be considered, as many patients with advanced malignancies undergo primary treatment via chemoradiation due to poor surgical candidacy, patient preference, or site amenability to nonsurgical management. Chemoradiation is especially useful for advanced‐stage UAD malignancies where long‐term speech and swallowing function could be preserved. Further, this subgroup of cancer sites (oropharynx, hypopharynx, and supraglottis) often presents at a higher local stage than other sites, making these tumors more amenable to US evaluation. If a patient is likely to undergo chemoradiation as primary intervention, this further solidifies the decision to forego a trip to the operating room under general anesthesia. In our study, only 31% of patient with positive biopsies went on to surgical intervention.
No major complications were reported in any of the studies analyzed herein using US‐guided tissue sampling of primary HNSCC tumors., , , , , It has been suggested that needle‐tract seeding is a potential complication of this technique, reported at less than 1% for FNA. Additional diagnostic procedures that may circumvent a trip to the operating room are also available. Awake, in‐office flexible fiberoptic biopsy can be difficult to tolerate by some patients, is more cost‐effective than a trip to the operating room, but is diagnostic only about two‐thirds of the time., , Core needle biopsy yields similar results to FNA and has been proposed as a second‐line diagnostic method if multiple FNAs have been negative., This method may be most useful for deep neck lesions with CT guidance. These methods were not assessed. Last, US is user dependent. This imaging modality has great potential, but a learning curve is associated with its use. Although the Fisher test showed statistically insignificant differences, this small sample size may not show that US is as effective as direct laryngoscopy in assessing these lesions with need for additional comparison, especially those at high risk for general anesthesia.
Conclusion
Ultrasound‐guided needle biopsy can be used effectively to obtain a tissue diagnosis of advanced‐stage UAD tract malignancies. As part of the head and neck cancer surgeon’s armamentarium, US offers unique benefits, simultaneously giving staging information in real‐time and providing ease of accessibility for biopsy. Given the advanced stage at presentation of these tumors and the use of chemoradiation as primary treatment in many cases, this method may obviate the need for operative biopsy.
Author Contributions
Aaron Smith, conception and design, acquisition, analysis and interpretation of data, physically performing the biopsies, drafting the work, revising the work, final approval of work, accountable for all aspects of the work; Anthony Grady, design of work, drafting work, revising work, final approval, accountable for all aspects of work; Francisco Vieira, acquisition, analysis, and interpretation of data, physically performing the biopsies, revising work, final approval, accountable for all aspects of work; Merry Sebelik, conception and design, acquisition of data, physically performing the biopsies analysis and interpretation, revising the work, final approval of work, accountable for all aspects of the work.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
References
- 1. van Harten MC, Hoebers FJ, Kross KW, van Werkhoven ED, van den Brekel MW, van Dijk BA. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51:272–278.
- 2. Patel UA, Brennan TE. Disparities in head and neck cancer: assessing delay in treatment initiation. Laryngoscope. 2012;122:1756–1760.
- 3. Challis M, Tomeh C, Sebelik ME. Initial staging of head and neck cancer: does a trip to the operating room add meaningful data? Paper presented at: Proceedings of the 8th International Conference on Head and Neck Cancer; July 21‐25, 2012; Toronto, ON. http://ahns.jnabstracts.com/2012/Detail?ID=0718.
- 4. Shetty D, Jayade BV, Joshi SK, Gopalkrishnan K. Accuracy of palpation, ultrasonography, and computed tomography in the evaluation of metastatic cervical lymph nodes in head and neck cancer. Indian J Dent. 2015;6:121–124.
- 5. Haberal I, Celik H, Gocmen H, Akmansu H, Yoruk M, Ozeri C. Which is important in the evaluation of metastatic lymph nodes in head and neck cancer: palpation, ultrasonography, or computed tomography? Otolaryngol Head Neck Surg. 2004;130:197–201.
- 6. Dobros W, Pacura B, Malczak J. [The value of cervical lymph node ultrasound examination in patients suffering from laryngeal cancer]. Przegl Lek. 2006;63(suppl 7):45–48.
- 7. Beppu T, Sasaki T, Kawabata K, et al. [Usefulness and limitations in ultrasonography for diagnosing neck lymph node metastases in patients with hypopharyngeal squamous cell carcinoma: comparison with pathological findings following neck dissection]. Nihon Jibiinkoka Gakkai Kaiho. 2005;108:794–800.
- 8. Borgemeester MC, van den Brekel MW, van Tinteren H, et al. Ultrasound‐guided aspiration cytology for the assessment of the clinically N0 neck: factors influencing its accuracy. Head Neck. 2008;30:1505–1513.
- 9. Saha S, Woodhouse NR, Gok G, Ramesar K, Moody A, Howlett DC. Ultrasound guided core biopsy, fine needle aspiration cytology and surgical excision biopsy in the diagnosis of metastatic squamous cell carcinoma in the head and neck: an eleven year experience. Eur J Radiol. 2011;80:792–795.
- 10. Stoeckli SJ, Haerle SK, Strobel K, Haile SR, Hany TF, Schuknecht B. Initial staging of the neck in head and neck squamous cell carcinoma: a comparison of CT, PET/CT, and ultrasound‐guided fine‐needle aspiration cytology. Head Neck. 2012;34:469–476.
- 11. Kraft M, Laeng H, Schmuziger N, Arnoux A, Gurtler N. Comparison of ultrasound‐guided core‐needle biopsy and fine‐needle aspiration in the assessment of head and neck lesions. Head Neck. 2008;30:1457–1463.
- 12. Meacham RK, Boughter JD Jr, Sebelik ME. Ultrasound‐guided fine‐needle aspiration of the tongue base: a cadaver feasibility study. Otolaryngol Head Neck Surg. 2012;147:864–869.
- 13. Lopchinsky RA, Amog‐Jones GF, Pathi R. Ultrasound‐guided fine needle aspiration diagnosis of supraglottic laryngeal cancer. Head Neck. 2013;35:E31–E35.
- 14. Wagner JM, Conrad RD, Cannon TY, Alleman AM. Ultrasound‐guided transcutaneous needle biopsy of the base of the tongue and floor of the mouth from a submental approach. J Ultrasound Med. 2016;35:1009–1013.
- 15. Zhang Y, Huang Y, Wu Y, Kong L, Huang S, Hu C. [Nasal endoscope, MRI, and ultrasound‐guided fine needle aspiration in the diagnosis of primary head and neck tumor]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:223–226.
- 16. Kaida H, Ishibashi M, Kurata S, et al. The utility of FDG‐PET for detecting multiple primary cancers in hypopharyngeal cancer patients. Nuklearmedizin. 2009;48:179–184.
- 17. Dhoot NM, Singh S, Choudhury B, et al. Evaluation of hypopharyngeal carcinoma using high‐resolution ultrasound: comparison with CT. J Clin Ultrasound. 2014;42:143–149.
- 18. Hu Q, Zhu SY, Zhang Z, Luo F, Mao YP, Guan XH. Assessment of glottic squamous cell carcinoma: comparison of sonography and non‐contrast‐enhanced magnetic resonance imaging. J Ultrasound Med. 2011;30:1467–1474.
- 19. Xia CX, Zhu Q, Cheng Y, Zhao HX, Jin ZZ. Sonographic assessment of hypopharyngeal carcinoma: preliminary study. J Ultrasound Med. 2011;30:217–225.
- 20. Braun U, Stellamor K, Seelmann O, Mosser H, Hruby W, Glaninger J. [Laryngeal and hypopharyngeal carcinoma—the limits and advantages of sonography]. Rofo. 1989;151:23–26.
- 21. Jecker P, Schuon R, Hlawatsch A. [Ultrasound of hypopharyngeal and oesophageal cancer: possibilities and limitations to staging and planning of therapy]. Ultraschall Med. 2005;26:312–317.
- 22. Blanco RG, Califano J, Messing B, et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PloS One. 2014;9:e87565.
- 23. Chen CN, Lin CY, Ko JY, et al. Application of ultrasound‐guided core biopsy as a novel diagnostic tool for base of tongue cancer: our experiences with ten patients. Clin Otolaryngol. 2016;41:86–90.
- 24. Ansarin M, De Fiori E, Preda L, et al. Ultrasound‐guided transcutaneous tru‐cut biopsy to diagnose laryngopharyngeal masses: a pilot study. Cancer. 2007;109:2268–2272.
- 25. Preda L, De Fiori E, Rampinelli C, et al. US‐guided transcutaneous tru‐cut biopsy of laryngo‐hypopharyngeal lesions. Eur Radiol. 2010;20:1450–1455.
- 26. De Fiori E, Conte G, Ansarin M, et al. The role of ultrasound‐guided transcutaneous tru‐cut biopsy in diagnosing untreated and recurrent laryngo‐hypopharyngeal masses. Eur J Radiol. 2016;85:158–163.
- 27. Chen CN, Hsiao TY, Ko JY, et al. Ultrasound‐guided core biopsy for hypopharyngeal cancer with difficult endoscopic approaches: our experience in eleven patients. Clin Otolaryngol. 2014;39:45–49.
- 28. Ahuja A, Evans R. Practical Head and Neck Ultrasound. London, UK: Greenwich Medical Media; 2000.
- 29. Lippert D, Hoffman MR, Dang P, McCulloch TM, Hartig GK, Dailey SH. In‐office biopsy of upper airway lesions: safety, tolerance, and effect on time to treatment. Laryngoscope. 2015;125:919–923.
- 30. Naidu H, Noordzij JP, Samim A, Jalisi S, Grillone GA. Comparison of efficacy, safety, and cost‐effectiveness of in‐office cup forcep biopsies versus operating room biopsies for laryngopharyngeal tumors. J Voice. 2012;26:604–606.
- 31. Richards AL, Sugumaran M, Aviv JE, Woo P, Altman KW. The utility of office‐based biopsy for laryngopharyngeal lesions: comparison with surgical evaluation. Laryngoscope. 2015;125:909–912.
- 32. Bowyer DJ, Smillie I, Ganly I. Diagnostic utility of freehand core‐needle biopsy in head and neck masses. J Laryngol Otol. 2013;127:175–180.
- 33. Wu EH, Chen YL, Wu YM, Huang YT, Wong HF, Ng SH. CT‐guided core needle biopsy of deep suprahyoid head and neck lesions. Korean J Radiol. 2013;14:299–306.