Thyroid nodules are common, and while the majority of thyroid nodules are ultimately benign,, determination of which nodules represent malignancy is generally sought. Based on clinical and ultrasonography findings, patients may undergo fine‐needle aspiration (FNA) biopsy for cytological diagnosis using The Bethesda System for Reporting Thyroid Cytopathology, which further stratifies malignancy risk. Classification groups for cytologic specimens include nondiagnostic, benign, or malignant. Two classification groups are considered indeterminate: atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS; Bethesda III nodules) and follicular neoplasm/suspicious for follicular neoplasm (FN/SFN; Bethesda IV nodules). Importantly, these diagnoses come with intermediate risks of malignancy, with published data considering AUS/FLUS to have a 5% to 15% risk of malignancy and FN/SFN to have 15% to 30% risk. Therefore, a large proportion of these intermediate FNA results may represent benign disease.
Owing to the nonnegligible risk of malignancy in AUS/FN/SFN, the American Thyroid Association (ATA) guidelines have recommended 3 possible approaches to management: surveillance with repeated ultrasonography ± repeat biopsy, diagnostic hemithyroidectomy, or molecular testing.
Despite being a common procedure, diagnostic hemithyroidectomy is not without risk. Patients are subjected to a risk of bleeding, infection, and injury to essential nerves for laryngeal function. In addition, up to 10% of patients undergoing a hemithyroidectomy alone will still require lifelong thyroid hormone supplementation. In lieu of surgery, continued surveillance can help mitigate these risks but may be associated with distress and anxiety. For these reasons, molecular tests have been developed as a means to improve prediction of which indeterminate nodules represent cancer and obviate the need for diagnostic hemithyroidectomy. Unfortunately, the costs of molecular diagnostic tests are not eligible for provincial health insurance for most patients in Canada and can be prohibitively high.
Therefore, physicians and patients are faced with a difficult decision regarding management of indeterminate thyroid nodules. There may be a variety of factors that affect patient decision making in this scenario. Our objective was to identify which of these factors were associated with decision making in a cohort of Bethesda III/IV thyroid nodules.
Methods
This was a multi‐institutional retrospective cross‐sectional study of patients with Bethesda III and Bethesda IV thyroid nodules diagnosed via FNA biopsy at 2 academic centers. Institutional research ethics board approvals were obtained from the Nova Scotia Health Authority Research Ethics Board and The Ottawa Hospital Research Ethics Board.
Patient Selection
All patients presenting with Bethesda III or Bethesda IV FNA results from January 2014 to April 2019 were eligible to participate. This is the approximate time span that the Bethesda classification has been used in the participating centers. These patients were identified through a review of all FNA biopsies completed at each institution during the specified time span via institutionally held cytopathology databases. Cytopathologists receive referrals from within the institutions themselves as well as from nearby community hospitals. Exclusion criteria included patients younger than 18 years, patients with health record information that was unavailable, patients with a prior or concurrent FNA diagnosis of Bethesda V or Bethesda VI, and patients who were not seen by a head and neck surgeon.
Variables of Interest
Patient charts were reviewed for personal health information. Demographic information collected included age, sex, postal code, and medical history. Patient clinic notes were reviewed for attending surgeon, thyroid cancer risk factors, and patient outcome. Thyroid ultrasound reports were reviewed to determine nodule size. Nodules were considered small if they were <3 cm in greatest diameter and large if ≥3 cm. Patient surgeon data were used to identify payment models (salary vs fee for service (FFS)) and practice types (tertiary vs community).
Socioeconomic status (SES) was operationalized as income quintiles. Patient postal codes were used in combination with the 2016 Canadian Census of population and the Postal Code Conversion File (PCCF+6C) to render dissemination areas (DAs). The PCCF+6C assigns standard census geographies based on postal code. The DA is the smallest level of disaggregation at which census information is available and allowed average income per single‐person equivalent in a DA to be examined.
Outcome
Our outcome of interest was the initial management decision made by patients. For the purposes of this study, we considered the initial decision to be the one made at the first consultation with all required information.
Statistical Analysis
Descriptive variables were summarized using absolute (n) and relative (%) frequencies for categorical variables and mean and SD for continuous variables.
Both unadjusted and adjusted analyses were completed. Normality of continuous variables was assessed using the Kolmogrov‐Smirnov test and visualization of quantile‐quantile plots. For the former, univariate analysis was performed on categorical variables using the Fisher exact test. Continuous variables were analyzed using the Student t test. A P value of ≤.05 of standardized difference >0.1 was considered statistically significant.
We performed a multilevel mixed logistic regression with 2 levels (level 1: patients; level 2: surgeons) and report a random‐intercept, fixed‐slope model (see Supplemental Appendix A1 in the online version of the article). Multilevel models allow estimation of the effects of all variables included in the model, thus allowing the odds of patients to undergo surgery to vary between surgeons so that we could determine both the average odds of a patient undergoing surgery and the variation of the odds from one surgeon to another. To quantify the amount of total variation that is accounted for by variation among the surgeons, we calculated an intraclass correlation (ICC). Thus, the ICC provides the percentage chance of undergoing surgery explained by between‐surgeon differences.
Statistical analysis was performed on SPSS Statistics v21 (SPSS, Inc) and SAS University Edition 9.4 (SAS Institute).
Results
A total of 956 patients met study inclusion criteria. A total of 538 (56%) patients underwent diagnostic hemithyroidectomy, 413 (43%) chose surveillance, and the remaining 5 (1%) patients chose molecular testing. Given how few patients elected to proceed with molecular testing (n = 5, 0.5%), the remainder of the analysis includes the surveillance and surgery cohorts only.
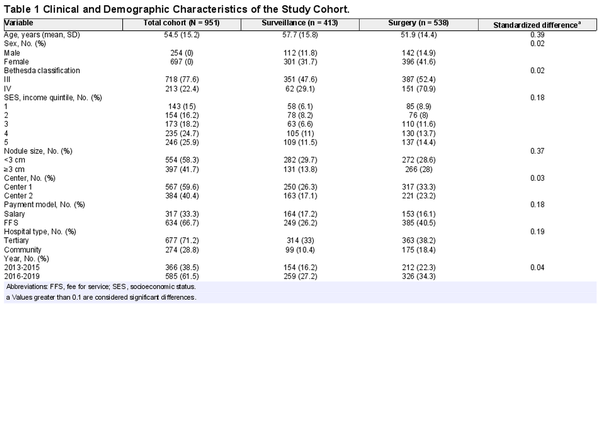
Most patients had Bethesda III nodules (n = 738, 77%). The mean (SD) age was 54.4 (15.2) years, and the population was predominately female (n = 697, 73%). Baseline characteristics are found in Table1. Patient symptoms and thyroid cancer risk factors were not consistently documented in dictated clinic notes and therefore were not included in this analysis.
There was no significant difference between the proportion of males (55.9%) and females (56.8%) who chose to proceed with surgery (P =. 492). There was also no significant difference in management decision between the 2 centers (center 1: 55.9% surgery; center 2: 57.6% surgery; P =. 492) or after the 2015 ATA guidelines were published (before update: 57.9% surgery; after update: 55.7% surgery; P =. 55). Finally, there was no change in the proportion of patients who opted for surgery according to patient SES (quintile 1, 59.4%; quintile 2, 49.4%; quintile 3, 63.6%; quintile 4, 55.3%; quintile 5, 55.7%; P =. 55).
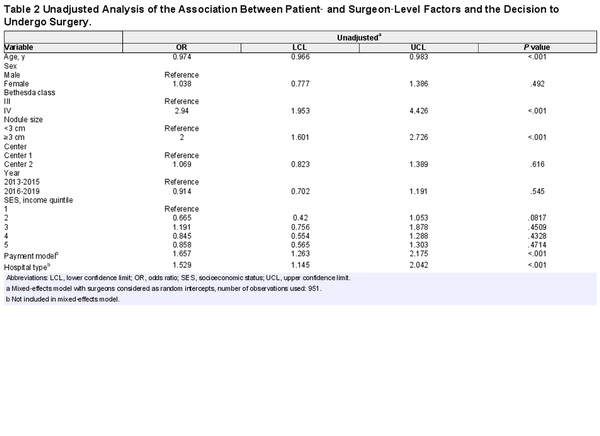
Larger nodule size (odds ratio [OR], 2.0; 95% CI, 1.601‐2.726; P <. 001) was associated with the decision for surgery. In contrast, older patients were less likely to choose surgery (OR, 0.974 per 1‐year increase in age; 95% CI, 0.966‐0.983; P <. 001). Patients with Bethesda IV nodules were more likely to undergo surgery (OR, 2.94; 95% CI, 1.953‐4.426; P <. 0001).
There was a significant variation in management decision based on the patient’s attending surgeon (range of patients undergoing surgery per surgeon: 15%‐83%; P≤. 0001; Figure1). Patients who saw a physician remunerated with an FFS payment model had significantly higher odds of undergoing surgery than patients with a salaried surgeon (OR, 1.657; 95% CI, 1.263‐2.175; P <. 001). In addition, patients in tertiary care hospitals were more likely to proceed with surveillance than those in community hospitals (OR, 1.529; 95% CI, 1.145‐2.042; P <. 001; Table2). When stratified by practice setting or remuneration plan, substantial variation in the utilization of surgery remained between surgeons (see Suppl. Figures S1 and S2 in the online version of the article).
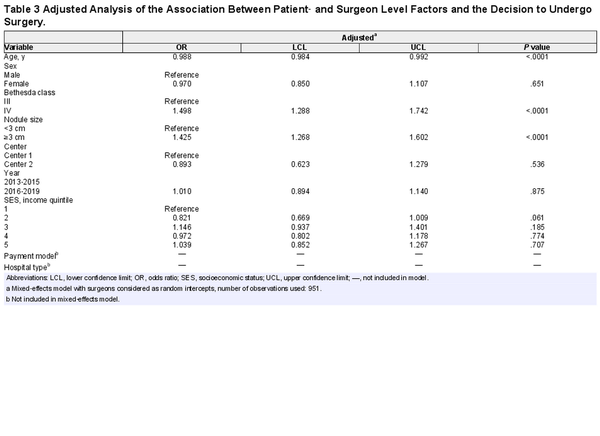
In adjusted analysis, age, Bethesda classification, and nodule size remained associated with the decision to undergo surgery (Table3). Older patients had 1.2% lower odds of undergoing surgery for each 1‐year increase in age (OR, 0.988; 95% CI, 0.984‐0.991; P <. 001). Patients with larger nodules (OR, 1.425; 95% CI, 1.268‐1.602; P <. 001) and Bethesda IV nodules (OR, 1.498; 95% CI, 1.288‐1.742; P <. 001) were more likely to undergo surgery. As seen in unadjusted analysis, there was substantial variation between the surgeons in the odds of patients undergoing surgery. In total, 11.5% of the variance in patient decisions were explained by between‐surgeon differences (ICC, 0.115).
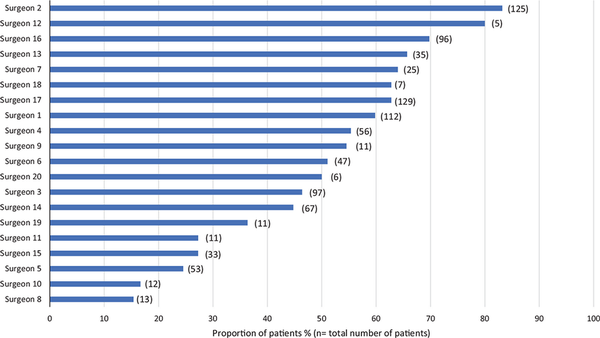
Figure 1
Surgeons and the proportion of their patients with indeterminate thyroid nodules who underwent surgery.
Discussion
Management decisions for indeterminate thyroid nodules are complex and represent scenarios of clinical uncertainty. The choice to undergo diagnostic surgery, perform molecular diagnostic testing, or observe with serial imaging is dependent on many patient‐ and surgeon‐level factors. In this study, we demonstrated that there is substantial between‐surgeon variation in the decision to pursue surgery, and additional patient‐level and surgeon‐level considerations are important. To our knowledge, this is the first multi‐institutional study to investigate the association of these factors with decision making in the management of indeterminant thyroid nodules.
The management of indeterminate thyroid nodules is an evolving field. Traditional guidelines were recently replaced with more conservative treatment recommendations in the 2015 ATA Management Guidelines., For patients with Bethesda III nodules, contemporary guidelines recommend a repeat FNA in lieu of proceeding directly with either surveillance or surgery. In addition, the newer guidelines allow molecular testing to supplement malignancy risk stratification for Bethesda IV nodules rather than proceeding directly to surgery. Although these changes were introduced midway through the study period, no significant difference in management decision was found after their implementation.
Molecular testing has emerged as a cytologic testing adjunct to allow for more precise estimations of the malignancy risk. Several mutations associated with thyroid cancer have been identified, and multiple options now exist for molecular testing. Tests vary in their sensitivity and specificity, but they all offer a higher degree of certainty than FNA biopsy alone. How molecular testing can be best integrated into routine clinical practice is an active area of interest. A recent meta‐analysis comparing various molecular tests estimated that up to 85% of surgeries for indeterminate nodules could be avoided with negative molecular testing results., For example, a multicenter cross‐sectional cohort survey found a decline in surgical resection rates from 74% to 7.6% for indeterminate nodules after implementing molecular testing for 6 months. Despite these possible advantages, molecular testing is costly and not covered by universal health care insurance in many jurisdictions. Financial burden is the most likely deterrent for molecular testing in our patient population, as only 5 patients chose this management option.
Previous studies have postulated that patients presenting with indeterminate thyroid nodules may experience significant decisional conflict. This conflict has been linked to emotional distress, canceled surgeries, and nonadherence to treatment plans. Taylor et al found that a third of patients with indeterminate thyroid nodules experienced significant decisional conflict around management options.
Since Glover’s seminal work on 1938, variations in clinical management have been widely documented., Geographical differences in management decisions may be explained by uncertainty regarding best practices, overuse of unnecessary procedures, or variations in the prevalence of enthusiasts for a particular approach. Recently, Hall et al demonstrated significant variations in the management of well‐differentiated thyroid cancer. A population‐based study of thyroidectomies in Ontario, Canada, found that high‐volume surgeons performed total thyroidectomy in 77% of cases, compared to 46% for lower volume. Although the current study found no overall variation between institutions, we did identify a significant difference based on surgeon, measuring nearly a 6 times difference between some surgeons in the proportion of patients undergoing surgery. This variation may be driven by practice setting and remuneration, as community‐based surgeons and those with an FFS payment plan were more likely to perform surgery after adjusting for nodule size and Bethesda class.
Shared decision making integrates patients’ values and preference with the scientific expertise of their treating teams. Through this process, shared decision making has been shown to improve quality of care and reduce variation in both care and costs across regions., We found a significant variation in treatment decisions due to surgeon‐level factors, and shared decision making may be a promising avenue to promote consistency of care. It is also important to recognize patient‐specific factors that may influence decisional conflict, including patient personality, emotions, and current life circumstance., Unfortunately, these factors cannot be accurately captured in a retrospective design.
The findings of this study must be interpreted in context of its design. Unmeasured confounders may influence patient management decisions, including known risk factors such as family history and radiation exposure, as well as physician‐specific factors, such as waitlist time. In addition, we are not able to describe differences in how management options were presented to patients by their providers. The choice of wording may have an important impact on patient decision making. Dixon et al conducted a study on the role of disease label in patient perceptions of low‐risk malignant thyroid neoplasms and demonstrated that disease label plays a significant role in how patients think about these low‐risk lesions. They suggested that removing the word cancer from the consultation vocabulary may reduce aggressive treatment of low‐risk lesions. Additional factors not examined in our analysis, such as ultrasound characteristics of included nodules, may play a role in surgical decision making. However, given the lack of consensus guidelines on how to incorporate these findings with the cytopathological diagnosis of an indeterminate nodule, decision making is likely individual to each surgeon. As such, the multilevel mixed model assists in controlling for these additional factors. Last, this study took place at 2 institutions in a universal health care system where molecular diagnostic testing was not covered. Generalizability to other health care systems or areas in which molecular diagnostic tests are more affordable to patients may be limited. Despite these limitations, our results still provide important insight into the decision‐making process for thyroid surgery in Canada.
Future analysis could examine further patient‐, surgeon‐, and system‐level factors (including wait times) to evaluate their effect on decision making in thyroid surgery.
Conclusion
Patient‐centered decision making is a desirable component of medical care. This study identified factors that may influence patient decision making for indeterminate thyroid nodules. While it seems clinically appropriate that larger nodules, higher Bethesda class, and younger age were associated with the decision for surgery, we also identified attending surgeon, surgeon payment model, and hospital type as important factors. Given the significant variation of treatment choice by physician, standardizing the discussion around the management of Bethesda III and Bethesda IV nodules may help promote consistency and improve patient‐centered care.
Author Contributions
Victoria Kuta, data collection, data analysis, manuscript preparation; David Forner, ethics, data analysis, manuscript preparation; Jason Azzi, ethics, data collection; Dennis Curry, data collection; Christopher W. Noel, statistics consultations; Kelti Munroe, data collection; Martin Bullock, pathology review, manuscript preparation; Ted McDonald, generation of income quintiles; S. Mark Taylor, manuscript preparation; Matthew H. Rigby, manuscript preparation; Jonathan Trites, manuscript preparation; Stephanie Johnson‐Obaseki, cosenior author, ethics, manuscript preparation; Martin J. Corsten, cosenior author, ethics, manuscript preparation.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Supplemental Material
Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X211015937
References
- 1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- 2. Nam‐Goong IS, Kim HY, Gong G, et al. Ultrasonography‐guided fine‐needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004;60(1):21–28.
- 3. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta‐analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243–2255.
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
- 5. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat. 2009;38(6):1228–1234.
- 6. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–142.
- 7. Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: review and current state. Cancer. 2018;124(5):888–898.
- 8. Trimboli P, Treglia G, Guidobaldi L, et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: a meta‐analysis. Endocrine. 2014; 46(1):52–59.
- 9. Mitchell J, Yip L. Decision making in indeterminate thyroid nodules and the role of molecular testing. Surg Clin North Am. 2019;99(4):587–598.
- 10. Duick DS, Klopper JP, Diggans JC, et al. The impact of benign gene expression classifier test results on the endocrinologist‐patient decision to operate on patients with thyroid nodules with indeterminate fine‐needle aspiration cytopathology. Thyroid. 2012;22(10):996–1001.
- 11. Graham ME, Haworth R, Chorney J, Bance M, Hong P. Decisional conflict in parents considering bone‐anchored hearing devices in children with unilateral aural atresia. Ann Otol Rhinol Laryngol. 2015;124(12):925–930.
- 12. Taylor BA, Hart RD, Rigby MH, Trites J, Taylor SM, Hong P. Decisional conflict in patients considering diagnostic thyroidectomy with indeterminate fine needle aspirate cytopathology. J Otolaryngol Head Neck Surg. 2016;45:16.
- 13. Ogink PT, van Wulfften Palthe O, Teunis T, et al. Practice variation among surgeons treating lumbar spinal stenosis in a single institution. Spine (Phila Pa 1976). 2019;44(7):510–516.
- 14. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–1129.
- 15. Hall SF, Irish JC, Griffiths RJ, Whitehead M. Explaining the variation in surgical practice for differentiated thyroid cancer in Ontario, Canada. JAMA Otolaryngol Head Neck Surg. 2019;145(10):949–954.
- 16. Forner D, Noel CW, Shuman AG, et al. Shared decision‐making in head and neck surgery: a review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):839–844.
- 17. Boss EF, Mehta N, Nagarajan N, et al. Shared decision making and choice for elective surgical care: a systematic review. Otolaryngol Head Neck Surg. 2016;154(3):405–420.
- 18. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- 19. Ritchie KC, Chorney J, Hong P. Parents’ decisional conflict, self‐determination and emotional experiences in pediatric otolaryngology: a prospective descriptive‐comparative study. Int J Pediatr Otorhinolaryngol. 2016;86:114–117.
- 20. Sawka AM, Ghai S, Yoannidis T, et al. A prospective mixed‐methods study of decision‐making on surgery or active surveillance for low‐risk papillary thyroid cancer. Thyroid. 2020;30(7):999–1007.
- 21. Dixon PR, Tomlinson G, Pasternak JD, et al. The role of disease label in patient perceptions and treatment decisions in the setting of low‐risk malignant neoplasms. JAMA Oncol. 2019;5(6):817–823.