Introduction
Vitiligo is a chronic cutaneous disease, known to mankind since time immemorial. It presents with depigmented or sometimes hypopigmented, asymptomatic, macular lesions without any signs of inflammation. It affects about 0.5–2% of dermatology patients worldwide across all age groups. In India, the prevalence of vitiligo has been reported between 0.25% and 4% of dermatology outpatients, going up to 8.8% in Gujarat and Rajasthan.
The Vitiligo Consensus Conference of 2023 broadly classified into segmental vitiligo (SV) and non-segmental vitiligo (NSV) [Table 1]. The segmental form is less common and presents unilaterally along a segment, usually noticed earlier in life during childhood. In contrast, non-segmental vitiligo is bilaterally symmetrical and may be generalized (vitiligo vulgaris), acrofacial, localized or mucosal. It has also been observed that nearly one-fifth of vitiligo patients can have concurrent autoimmune disorders such as thyroid disorders, alopecia areata, psoriasis, rheumatoid arthritis, type 1 diabetes, and pernicious anemia, among others, and this should be kept in mind while managing a vitiligo patient.
Table 1
Classification of vitiligo
| Type | Vitiligo subtype | Remarks |
|---|---|---|
| Segmental Vitiligo (SV) | UnisegmentalBisegmentalPlurisegmental | Presence of one or more depigmented macules distributed on one side of the body |
| Non-Segmental vitiligo (NSV) | Acrofacial | Vitiligo lesions limited to face, head, hands, and feet |
| Generalized | Can include other body areas | |
| Universal | Most extensive form of vitiligo. This term is used when depigmentation covers >80% of total body surface area | |
| Mucosal | More than one mucosal site involved. Involvement of oral and/or genital mucosae | |
| Rare variants | ||
| - Follicular | Depigmentation of hair follicle without initial involvement of surrounding skin | |
| - Vitiligo minor | incomplete defect in pigmentation with a pale skin color compared with healthy skin | |
| - Vitiligo punctata | 1–1.5 mm sharply demarcated macules | |
| Mixed | Segmental + Non-Segmental Vitiligo | Concomitant occurrence of NSV and SV |
| Unclassified | Focal | Small, isolated patch, which has not evolved into NSV after a period of at least 2 years and does not fit into a segmental distribution |
| Mucosal | One mucosal site only |
The diagnosis of vitiligo is mainly clinical, but it should be differentiated from other hypopigmented or depigmented conditions such as post-inflammatory hypopigmentation, nevus depigmentosus, nevus anemicus, and idiopathic guttate hypomelanosis. An important differential diagnosis in a background of inadvertent intralesional steroid injections is steroid-induced depigmentation, which can clinically mimic vitiligo. Dermoscopy offers an exciting non-invasive tool to confirm as well as understand the stage of disease and can guide future management as well.
Managing vitiligo at all ages is important as it has a considerable effect on the quality of life, and the color contrast, especially in patients with skin of color, can be psychologically distressing for the patient. It is important to stabilize the progressive disease and further plan for repigmentation of the affected area, or in some cases, depigmentation of the remaining normally pigmented skin. A multimodal approach targeting the autoimmune nature of the disease, activation of melanocyte regeneration, and reducing oxidative stress often has a favorable response in most patients.
Outline of Vitiligo Management
Treatment modalities include topical and oral immunosuppressives and phototherapy, which are useful for stabilization and repigmentation of vitiligo, and surgical procedures, which help in repigmentation of stable vitiligo. Treatment modalities are selected based on disease activity (stable versus progressive disease), disease severity, patient preference (including accessibility and cost), and response evaluation. Patients should be counseled about the disease, the treatment goals, and expectations. The prognosis should be explained beforehand along with cost considerations. Treatment is usually based on the clinical type of vitiligo. The treatments and their mechanisms of action have been mentioned in Table 2.
Table 2
Mechanism of action of various drugs used in vitiligo
| Drug | Mechanism of action | |
|---|---|---|
| Topical treatments | ||
| 1. | Topical corticosteroids | -Modulation of immune response -Reduction of CD4+ and CD8+ T lymphocytic cells -Reduction of macrophages. -Inhibit complement mediated melanocytic destruction -Reduce autoantibodies |
| 2. | Topical calcineurin inhibitors | - Downregulation of pro-inflammatory cytokines (like TNF-α, IL-1,2,3,4,5 and GM-CSF) -Induction of anti-inflammatory cytokines (like interleukin-10) -Migration and proliferation of melanocytes and melanoblasts -Increasing tyrosinase expression & dopa-oxidase activity -Anti-oxidative effect |
| 3. | Topical JAK inhibitors | -Reduces CD8+ T cells -Reduces CXCL9 and CXCL10 chemokines |
| 4. | Vitamin D3 Analogs | -Promote melanocyte formation -Upregulates melanogenesis -Immunomodulatory action -Inhibit TNF-α and IFN-gamma |
| 5. | Decapeptides | -Stimulates growth and migration of melanocytes |
| 6. | 5-Fluorouracil | -Stimulates follicular melanocytes -Selectively destroys keratinocytes, and makes melanocytes multiply -Immunomodulator action |
| Oral treatments | ||
| 1. | Oral Corticosteroids | -Modulation of immune response -Reduction of CD4+ and CD8+ T lymphocytic cells -Reduction of macrophages. -Inhibit complement mediated melanocytic destruction -Reduce autoantibodies |
| 2. | Cyclosporine | -Inhibits IL-2, and therefore CD8+ and CD4+cells. |
| 3. | Methotrexate | -Inhibits T cell and TNF-α activation -Downregulates B cells -Inhibits intracellular expression of adhesion molecules. |
| 4. | Azathioprine | -Reduces T cells and associated cytokines (IL-2) and IFN-γ) -Immunomodulatory action |
| 5. | Minocycline | -Immunomodulatory and anti-inflammatory properties -Free-radical destruction -Salvages melanocytes from oxidative damage |
| 6. | Simvastatin | -Inhibit IFNγ-induced STAT1 activation -Reduces CD81 T-cells in skin |
| 7. | Afamelanotide | -Acts on melanocortin 1 receptor (MC1R), and stimulates transfer of eumelanin into melanosomes |
| 8. | JAK Inhibitors | -Reduces CD8+ T cells -Reduces CXCL9 and CXCL10 chemokines |
| 9. | IL-23 inhibitors | -Reduces IL- 23 levels |
| Depigmenting therapies | ||
| 1. | MBEH | -Structural analog of tyrosine -Cytotoxic toward melanocytes -Contact sensitizer inducing delayed type hypersensitivity |
Types of Vitiligo
Segmental Vitiligo (SV)
This type of vitiligo has a unilateral distribution and may conform to a dermatomal segment. This subtype is often encountered in younger age groups (15–30%), with an average age of 18 years.
Segmental vitiligo is generally less responsive to medical treatments such as topical steroids, topical calcineurin inhibitors, and phototherapy. This reduced response is attributed to leucotrichia, a common feature of SV, thereby causing an absence of melanocyte reservoir in hair follicles. However, if treated early, lesions seem to be more responsive, and medical treatment may help in stopping further disease progression. Topical treatment (corticosteroids, calcineurin inhibitors, or JAK inhibitors) and targeted UVB therapy are therefore recommended in patients with active disease. A combination of treatments such as topical tacrolimus and excimer laser with or without systemic corticosteroids was also found to be beneficial.
However, in patients with stable disease, surgical methods offer a better alternative. Different surgical techniques include tissue grafts (full-thickness punch grafts, split-thickness grafts, and suction blister grafts) and cellular grafts (cultured melanocytes, cultured epithelial sheet grafts, and non-cultured epidermal cellular grafts). Surgical techniques themselves are most successful in cases of SV, and repigmentation seems to be maintained during follow-up in most patients. Figure 1 provides a brief outline of the management of SV.
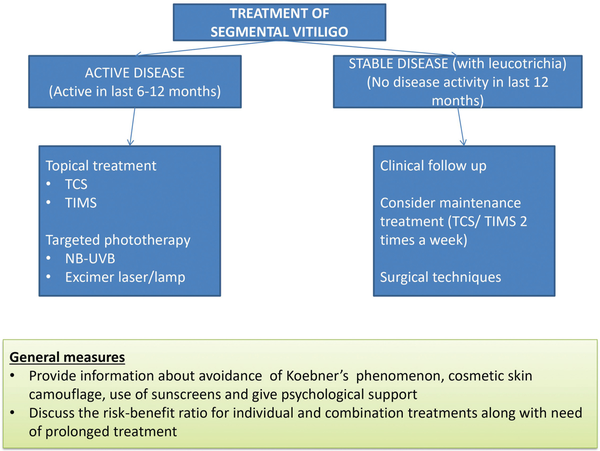
Figure 1
Treatment of segmental vitiligo. Disease activity is analysed based on the development of new lesions, or expansion of already present lesions. This is based on the history of patient, serial photographs, and visible disease activity signs like the presence of confetti-like depigmentation, hypochromic borders, and Koebner’s phenomenon. A stable lesion is defined as no new lesion and no expansion of previously developed lesions in the last 12 months. NB-UVB = narrowband ultraviolet B, TCS = topical corticosteroids, TIMS = topical immunomodulators.
Non-segmental Vitiligo (NSV)
Patients with NSV form the majority of cases of vitiligo and based on the disease activity, it can either be a stable or unstable disease. The management varies for the 2 types; and for refractory stable vitiligo, surgery may often be required. Figure 2 provides an overview of the management of NSV based on disease activity.
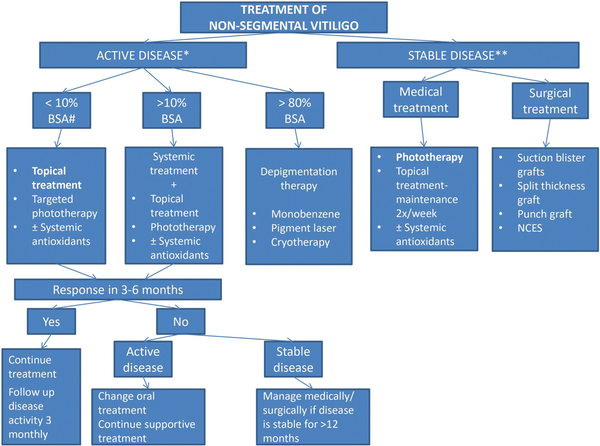
Figure 2
Treatment of non-segmental vitiligo. Active disease is analysed based on development of new lesions, or expansion of already present lesions. This is based on the history of patient, serial photographs and visible disease activity signs like the presence of confetti-like depigmentation, hypochromic borders, and Koebner’s phenomenon. ** Stable lesion-no new active lesion and no expansion of previously developed lesions since the past 12 months. # For Body surface area (BSA) evaluation, the palm is considered to represent 1% BSA. The general measures are similar to those for segmental vitiligo. Topical treatment includes topical corticosteroids (TCS) like mometasone furoate, topical calcineurin inhibitors (TCIs) like tacrolimus and pimecrolimus and topical JAK inhibitors like Ruxolitinib. Targeted phototherapy can be in the form of NB-UVB or excimer laser/lamp. Surgical treatment is only undertaken if vitiligo is stable for >12 months resistant to medical treatment and phototherapy and has limited extent.
Activity of the disease can be assessed by the presence of Koebner’s or Micro-Koebner’s phenomenon, hypochromic lesions or borders, and confetti-like depigmentation. It is considered to be rapidly progressive if new lesions appear or there is enlargement of pre-existing lesions. In contrast, if there is no increase in the lesions for 12 months, the disease is considered stable, and the patient can be planned for surgery if medical management has failed.
Treatment of Vitiligo
Topical Modalities
Topical treatment is the first line of management with limited involvement. This includes topical corticosteroids (TCS) as well as topical immunomodulators (TIM) including topical calcineurin inhibitors (TCIs) and topical JAK inhibitors. For better results, TCS and TIM can be combined with phototherapy.
Topical corticosteroids (TCS)
TCS are considered to be the first line of management in both children and adults, especially at extra-facial sites. The proposed mechanism of action in vitiligo is by modulation of immune response by reduction of CD4+ and CD8+ T lymphocytic cells, as well as reduction of macrophages. Further, they reduce autoantibodies implicated in vitiligo, as well as inhibit complement mediated melanocytic destruction. It has been observed that these agents are better at stabilizing the disease than at repigmentation. They can generally be applied for 3–6 months (2–4 months in children). Side effects like telangiectasia, atrophy, acneiform eruption, hypertrichosis, striae, and glaucoma with periorbital usage as well as the suppression of hypothalamic-pituitary-adrenal axis due to systemic absorption can be minimized by intermittent use. Maintenance therapy with twice-a-week TCS can maintain remission and prevent recurrences.
Topical calcineurin inhibitors (TCI)
TCIs Tacrolimus and pimecrolimus are the treatments of choice in facial sites as well as sensitive areas such as flexures, where side effects of TCS may be predominant. Multiple mechanisms of action have been identified in vitiligo, including downregulation of pro-inflammatory cytokines (like tumor necrosis factor) and induction of anti-inflammatory cytokines (like interleukin-10), thus acting as an immunosuppressive agent. It also induces melanogenesis by causing migration and proliferation of melanocytes and melanoblasts, induction of melanogenesis by increasing tyrosinase expression and dopa-oxidase activity, and additionally by their anti-oxidative effect. A twice-daily application of TCI can be recommended for facial vitiligo, and it can be used under occlusion in non-facial vitiligo, as an alternative to TCS. The treatment should be prescribed initially for 6 months and can be prolonged for 12 months or more when effective. Their effect is also significantly enhanced when combined with NB-UVB therapy. TCI may be similar or slightly inferior to TCS in terms of efficacy in extra facial skin. They, however, have an excellent safety profile.
Tacrolimus is preferred over pimecrolimus, because pimecrolimus has a larger molecular size, and therefore has less cutaneous absorption. Pimecrolimus, however, can be used in patients who cannot tolerate tacrolimus and in infants. Common adverse effects encountered include pruritus, erythema, and burning sensation, which can be seen more with tacrolimus, but generally subsides over time. A concern that TCI can increase lymphomas and skin cancer has been refuted epidemiologically, and TCI were not seen to enhance the risk when used for long durations.
A proactive approach has also been suggested for vitiligo, similar to atopic eczema. A maintenance dose of twice weekly TCI can further promote repigmentation, as well as prevent recurrence of vitiligo, which is estimated to be as high as 40% in vitiligo patients. The guiding principle behind this approach is the low level of continuing autoimmunity mechanisms against melanocytes, which are counteracted with twice weekly use of TCIs.
Topical JAK inhibitors
The Janus kinase family, consisting of JAK1, JAK2, JAK3, and TYK2, exhibits pleiotropic effects on transducing multiple extracellular signals involved in regulating signaling, differentiation, migration, and apoptosis. Inhibition of this pathway offers an exciting modality to inhibit multiple cytokines at once.
Recently, ruxolitinib cream (Opzelura 1.5% cream), a JAK inhibitor, has been approved for NSV after two clinical trials proved its effectiveness in NSV patients. It is the first FDA-approved pharmacologic treatment to address repigmentation in vitiligo and is approved for patients >12 years of age. Satisfactory patient response may require treatment for more than 24 weeks. Common adverse effects noted are application site acne, pruritus, erythema, headache, urinary tract infection, and fever. However, its use in combination with biologics, other JAK inhibitors, or potent immunosuppressants like cyclosporine or azathioprine is not recommended.
Topical tofacitinib (2% cream) has also been tried, and variable success was achieved. Other congeners such as ritlecitinib (JAK3 inhibitor), brepocitinib (JAK1 and TYK2 inhibitor), and JAK1/3 inhibitor (ATI-50002), combined with phototherapy are also under trial. The efficacy has been observed to be better in sun-exposed areas, facial lesions, in Fitzpatrick skin types IV–VI, with concomitant NBUVB therapy.
Vitamin D3 Analogs
Topical vitamin D3 analogs have been used for vitiligo because they promote melanocyte formation, thereby inducing and promoting melanogenesis. Additionally, they have immunomodulatory properties. However, practically they are not useful as monotherapy in vitiligo, and are at best supportive of other treatments such as TCS and phototherapy. The optimum dosage for 4 weeks (ointment) and 8 weeks (cream) is 100 g weekly or 50 µg/g of topical calcipotriol twice daily. Beyond the suggested dose, there is a risk of hypercalcemia, besides local irritation.
Decapeptides
Basic fibroblast growth factor (bFGF)-related decapeptide solution has been used recently for vitiligo patches. It is a sequence of amino acids derived from bFGF, a mitogen, stimulating the growth and migration of melanocytes, and thus helps in repigmentation. It is prescribed topically once a day at night, followed by sun exposure for 5–10 minutes in the morning sunlight. Better repigmentation rates have been observed when used in combination with tacrolimus 0.1% ointment. In a study, repigmentation rates of a combination of bFGF and tacrolimus versus tacrolimus alone in 120 patients of stable vitiligo were assessed, and the combination group showed >50% repigmentation in 66.7% patients in comparison to 39.2% in the tacrolimus group after a period of 1 year. Hence, a combination of decapeptides and tacrolimus is a safer alternative to steroids for sensitive and facial regions.
5-Fluorouracil
5-Fluorouracil (5FU-1%, 5%) is another topical drug that shows differential and selective cytotoxicity toward epidermal cells. Melanocytes are less vulnerable to 5-fluorouracil compared to keratinocytes. 5-FU stimulates follicular melanocytes, which migrate during epithelialization and induce pigmentation. In an in vitro study, it was found that low concentration of 5-FU selectively destroys keratinocytes within 3 weeks, whereas melanocytes multiply, leading to repigmentation. 5-FU has also been combined with other surgical modalities such as fractional CO2 laser, dermabrasion, and microneedling in cases of stable vitiligo present on difficult-to-treat areas such as acral lesions. Microneedling works with 5-FU due to the concept of therapeutic wounding, and better drug penetration, leading to a faster and more homogenous repigmentation in vitiligo lesions.
Phototherapy
This treatment modality remains an essential tool for the management of all kinds of vitiligo. Table 3 highlights the salient features of different kinds of phototherapy.
Table 3
Various types of phototherapy and their uses in vitiligo
| S. No. | Type of photo-therapy | Dosing | Uses | Adverse effects | Remarks |
|---|---|---|---|---|---|
| 1. | NB-UVB (Total body) (311 nm) | -For Fitzpatrick skin type I–III: 200 mJ/cm2 to avoid phototoxic reactions -For Fitzpatrick skin type IV–VI: consider higher starting doses 400–500 mJ/cm2. Dose is increased by 10–20% or held depending on the severity of erythema, up to a maximum dose of 1500 mJ/cm2 for the face and 3000 mJ/cm2 for the body. | Early localized lesions or having signs of rapid progressionActive or stable vitiligo (non-segmental) | UVB-induced phototoxicity to eyes and a theoretical (but undocumented) increased risk of photocarcinogenicity to genitals. Therefore, patients are instructed to close their eyes and cover genitals during therapy | -Repigmentation may begin in as soon as 1–2 months -Repigmentation should begin within 30 treatments -If no response is seen in 3–6 months, it should be discontinued. |
| 2. | NB-UVB (Targeted)(311 nm) | Segmental vitiligoStable localized non-segmental vitiligo | Cover eyes (if treating face). | ||
| 3. | Oral PUVA(320–400 nm after psoralen treatment) | Initial dose is 2–3 J/cm2 and is increased by 0.5 J/cm2 (provided patient has not developed erythema or burning sensation over apparently normal skin)8MOP in dose of 0.6–0.8 mg/kg is given orally followed by whole body irradiation after 1–3 hours | -Early localized lesions or having signs of rapid progression -Active or stable vitiligo (non-segmental) | -Ocular and systemic toxicity. -Increases risk of melanoma and non-melanoma skin cancers. -Commonly causes nausea and headache. -Uneven/ cosmetically unacceptable repigmentation | -Darker skin types respond better (compared to fair skinned). -Prolonged treatment lasting for 150–200 sessions -Oral 8MOP or TMP are commonly used -Contraindicated in children and pregnant females |
| 4. | Topical PUVA(320–400 nm after applying psoralen) | A 0.005–0.01% ointment/gel/lotion of 8-MOP is applied to the affected skin, which is exposed to UVA 30 minutes later. Initial dose of UVA is 0.05 J/cm2 (face) and 0.1 J/cm2 (other sites). The dose is increased by 0.05 J/cm2 at each session until a maximum of 1 J/cm2 is reached. If marked erythema develops following first session, treatment should be omitted until the erythema settles, and restarted using a 1:4 diluted solution | -Segmental vitiligo -Localized non-segmental with smaller lesions involving less than 5% BSA | -Perilesional hyperpigmentation -Greater phototoxic risk | -Topical PUVA may be delivered in different forms: as whole-body bath PUVA (widespread disease) or as gel, cream, or lotion PUVA (localized vitiligo). -TMP and 5MOP are more phototoxic topically. -Smaller cumulative dose of UVA are safe for children -Application of sunscreen to the surrounding uninvolved skin can prevent undue tanning. |
| 5. | Oral PUVASOL (320–400 nm after psoralen treatment) | 0.6 mg/kg of 8MOP is given after breakfast. After 1.5–2 hours, sun exposure is advised for 5 minutes. Done 2–3 times/week (minimum 1 day gap in between); time of exposure is increased by 5 minutes weekly till maximum of 30–45 minutes. | -Early localized lesions -Rapidly progressive vitiligo -Active or stable vitiligo (non-segmental) | Ocular and systemic toxicityIncreases the risk of both melanoma and non-melanoma skin cancer;Commonly causes nausea and headacheUneven/cosmetically unacceptable repigmentation | -Preferred in patients who cannot do institutional visits for NB-UVB or targeted phototherapy -Biggest disadvantage (compared to PUVA) is the absence of precise UVA dosimetry. -Ideal time for solar irradiation is 9.30 am–11 am or 2 pm–3.30 pm in tropical countries. -Oral TMP is preferred over 8MOP due to its’ weaker phototoxic effects. -If there is no improvement even after 30–40 sittings, it should be discontinued. |
| 6. | Topical PUVAsol (320–400 nm after applying psoralen) | 0.03–0.1% 8MOP in a cream/lotion base is applied to the affected area and given sun exposure after 30 minutes beginning with 0.5–1 minute. Treatment is done 2–3 times/week (with at least 1 day gap).Duration of sun exposure is increased by 0.5–1 minute every week till slight erythema appears, after which the time is kept constant. | -Segmental vitiligo -Localized non-segmental with smaller lesions involving <5% BSA who cannot do institutional visits for targeted phototherapy | -Painful blistering -Perilesional hyperpigmentation-Greater phototoxic risk | -Inexpensive -Lacks systemic effects of oral psoralens -Different modalities: paint PUVA-sol, soak PUVA-sol, and bath PUVA-sol (not practical). -Fewer treatments needed. -Smaller cumulative dose of UVA. -Safe for children. -Less effective at arresting disease activity -Application of sunscreen to the surrounding uninvolved skin can prevent undue tanning. |
| 7. | Excimer Laser/Lamp (308 nm) | Dosing may start at 100 mJ/cm2 and is gradually increased weekly by 10–25% Three session/week causes faster repigmentation than two sessions/week, but the final response depends on the total number of treatments and not frequency | -Segmental vitiligo -Stable localized non-segmental | Similar to NB-UVB therapy | Safer and equally effective for smaller vitiligo lesions |
Narrow Band UVB Phototherapy (NB-UVB)
It is the most preferred phototherapy modality, especially in widespread or rapidly progressive unstable vitiligo. Early initiation of NB-UVB phototherapy is beneficial in halting the disease progression and repigmenting the skin especially in difficult-to-treat conditions such as segmental and acral vitiligo. The pigmentation can be perifollicular or marginal and results in a good color match.
The most commonly used source is Philips TL-01 (100 W), a fluorescent bulb coated with phosphor. It emits at 310–315 nm with a peak emission at 311 nm. The upright booth is the commonly employed unit consisting of 24–48 bulbs, best suited for multiple, widespread lesions of vitiligo.
The limitations include resistant anatomical sites such as fingers, toes, and bony prominences and areas lacking a melanocyte reservoir, such as segmental lesions with leucotrichia. The recommendations for NB-UVB phototherapy are highlighted in Table 4.
Table 4
NB-UVB Phototherapy recommendations
| Treatment parameter | Recommendations |
|---|---|
| Frequency of administration | Optimal: three times/week; Acceptable: two times/week |
| Dosing protocol | Initiate dose at 200 mJ/cm2 (regardless of skin type); Increase dose by 10–20% per treatment |
| Maximum acceptable dose | Face: 1500 mJ/cm2; Body: 3000 mJ/cm2 |
| Maximum number of exposures | Skin type IV–VI: No limit; Skin type I–III: No clear consensus |
| Course of NBUVB | Treatment response assessed after 18–36 exposures; - Minimum number of doses needed to determine lack of response: 48 exposures; - Maximum number of sessions needed to determine lack of response − 72 (due to late responders) |
| Dose adjustment (based on degree of erythema) | - No erythema: increase next dose by 10–20% - Pink asymptomatic erythema: hold at current dose till erythema disappears; then increase by 10–20% - Bright red asymptomatic erythema: phototherapy should be stopped until affected areas become light pink, thereafter, resume treatment at last tolerated dose - Symptomatic erythema (including pain and blistering): stop phototherapy until the skin heals and erythema fades to a light pink, then resume at last tolerated dose |
| Dose adjustment following missed doses | - 4–7 days − hold dose constant - 8–14 days − decrease dose by 25% - 15–21 days between treatments- by 50% - >3 weeks − restart at initial dose |
| Outcome measures to evaluate response | - Serial photography (to establish baseline severity, disease stability, response to treatment) - Use validated scoring systems (like VASI or VETF) to quantify degree of response |
| Post-treatment recommendations | Sunlight avoidance and sunscreen application. |
| Topical products before phototherapy | Avoid all topical products for 4 hours except mineral oil (Mineral oil enhances light penetration in areas of dry, thickened skin, like elbows and knees) |
| Tapering NBUVB after complete repigmentation has been achieved | First month: phototherapy twice weekly Second month: phototherapy once weekly Third and fourth months: phototherapy every other week After 4 months: discontinue phototherapy |
| Follow-up | Skin type I–III: yearly follow-up for total body skin examination to monitor for adverse effects of phototherapy, including cutaneous malignancy Skin type IV–VI: no need to return for safety monitoring as no reports of malignancy exist with this group All patients: return upon relapse for treatment |
| Minimum age for NBUVB in children | Age when a child can reliably stand in the phototherapy unit with their eyes closed or wearing goggles (Generally around 7–10 years of age) |
| Treatment of eyelid lesions | Keep eyes closed during treatment; can use adhesive tape. |
| Special sites | -Cover face during phototherapy if uninvolved -Shield male genitalia -Protect female areola with sunscreen prior to treatment, (especially skin types I–III) |
| Combination treatments for stabilization | Oral antioxidants, topical treatments, oral pulse corticosteroids |
| Pretreatment monitoring | Generally not advised; Antinuclear antibody (ANA) screening may be done for non-photoadaptors |
| Treatment of NBUVB induced skin changes | Xerosis: emollient or mineral oil Skin thickening: topical corticosteroids or keratolytics |
| Response predictors | Favorable response predictors: pediatric age, face and neck location, recent disease onset. During treatment: Presence of perifollicular pigmentation on dermoscopy is predictive of a positive response to NB-UVB Poor response predictors: areas with leucotrichia, large, longstanding-lesions and acral areas do not repigment easily |
Though NB-UVB therapy can itself stabilize and repigment a vitiligo patch, combination treatments are preferable for faster and better results. A favorable response has been noted with calcineurin inhibitors, oral corticosteroids, oral polypodium leucotomas, and afamelanotide whereas inconsistent results were found with vitamin D3 analogs and topical pseudocatalase.
Most commonly encountered acute side effects of NB-UVB therapy are erythema and xerosis. No significant association between NB-UVB therapy and skin cancers has been noted. A Korean study reported that among 60,321 cases, more than 200 sessions of NB-UVB increased the risk of actinic keratosis, while even up to 500 sessions did not increase the risks of melanoma or non-melanoma skin cancer. Lichenoid papules may also develop, especially in individuals who undergo more than 300 treatment sessions.
Photochemotherapy
PUVA: Photochemotherapy employs the use of a photosensitizer chemical drug that is combined with a source of irradiation to produce clinical effects. The most commonly used treatment modality is psoralen-induced PUVA therapy. Psoralen, a furocoumarin obtained from Psoralea corylifolia and Ammi majus, is either ingested or applied topically and given UVA irradiation either in a standardized unit or solar radiation (PUVAsol). PUVA can act via the stimulation of melanocytes, particularly hair melanocytes, which migrate to adjacent vitiliginous skin. Additionally, localized and systemic immunosuppression is also induced.
Before starting the treatment, counseling the patient regarding nature of disease and of therapy, requiring prolonged course is essential. Eye protection using UV protective glasses and covering genitalia, especially for males is essential. For oral treatment, psoralen is ingested, and irradiation is done 2 hours later, whereas for topical therapy, treatment is done after 30 minutes. The dosing schedule has been described in Table 2.
Oral PUVA is now no longer preferred due to various adverse effects associated with oral psoralens, such as gastralgia, nausea, risk of severe generalized phototoxicity, and photocarcinogenesis. Topical PUVA or topical PUVASOL therapy is useful for localized lesions without systemic complications. Common adverse effects of topical PUVA or topical PUVASOL therapy are erythema and risk of cutaneous phototoxicity. Sometimes blistering may also be seen. However, the possible association of topical PUVA with an increased risk of cutaneous cancers is unclear.
Khellin UVA/KUVA: Another photochemotherapy regimen, KUVA, consists of khellin as the photosensitizer, which can be combined with UVA. It is a furanochromone extracted from the plant Amni visnaga. Unlike psoralens, KUVA’s lack of phototoxicity makes it safer to use as a treatment with natural sunlight daily or on alternate days. Mutagenic potential is also lower as compared to psoralens, and it causes less darkening of normal skin, making it a safer regime. Khellin penetrates inside the hair follicles and is activated in the presence of UVA light, which leads to the stimulation of melanocytes present in the hair follicles.
Orally, 100 mg khellin is given at 2 hours before treatment, and UVA irradiation is done thereafter. The efficacy has been comparable with PUVA. However, systemic KUVA has been largely abandoned due to significant liver toxicity in up to 30%patients and other adverse effects such as nausea, vomiting, and liver tenderness.
Topical khellin can also be applied in a moisturizing cream at a concentration of 3–5%. Topical ‘KUVA–sun’ can be used in tropical countries effectively as it has no side effects when used topically and is a much safer alternative to topical PUVASOL. However, its use is limited owing to the difficulties in obtaining khellin.
Excimer Devices
Traditional phototherapy devices used incoherent light source to target the lesion and even exposure to normal skin. However, targeted phototherapy employs the use of coherent light and radiation of only affected area. Practically, they seem to be equally or more efficacious that NB-UVB due to delivery of supra-erythemogenic doses at higher irradiance.
Monochromatic Excimer Light (MEL) is one of the most commonly used targeted therapies these days. Xenon chloride (XeCl) devices are used, which emit a single 308 nm wavelength coherently (laser) or incoherently (lamp). A unidirectional, coherent emission by excimer laser is typically preferred for smaller area (targeted therapy), while a multidirectional, incoherent emission by excimer lamp benefits the treatment of a larger surface area.
The treatment protocol for excimer is similar to NB-UVB in terms of initial dose, dose escalation, and treatment frequency. Another alternative is to use an anatomical-based initial dosing, known as the Friedman protocol (e.g., 100 mJ/cm2 for periorbital area, 300 mJ/cm2 for elbows, 600 mJ/cm2 for fingers/toes, etc.) Similar to other treatments for vitiligo, anatomic location is the best guide for treatment response to excimer. Lesions located on face, neck, genitals, and trunk achieve better repigmentation than those on bony prominences, such as hands, feet, knees, and elbows.
Though definite advantage of targeted excimer therapy is more safety profile with the absence of darkening of non-lesional skin, the cost of therapy can sometimes be prohibitive. It can also be time-consuming if used for large body areas.
Home Phototherapy
This modality is an alternative to in-clinic NB-UVB phototherapy. Though a little inferior in efficacy due to lower intensity of irradiation, it offers an advantage of at-home therapy, and saves patients the enormous physical and financial burden of multiple visits to phototherapy centers. Different-sized panels or hand-held devices are available depending on the BSA involved. Compared to in-clinic treatment, it demonstrates better compliance, similar repigmentation outcomes, similar frequency of adverse effects, and lower time investment.
Upcoming devices are being designed to have higher safety, have easier dose adjustment, and being user-friendly at the same time. Patients should be guided about shielding sensitive sites, recognizing any adverse effects and encouraged for regular follow-up for treatment success.
The shortage of home phototherapy units, high initial investment, reduced energy output of device over time, and unfamiliarity of patients with this modality are important deterrents to the use. Thus, an initial period of in-clinic phototherapy may be helpful to evaluate treatment responsiveness, which can be maintained up by a home unit.
Another upcoming treatment modality for home care is topical band-pass filter cream- (TBFC) assisted home-based NB-UVB therapy. This water-in-oil emulsion filters solar radiation and preferentially absorbs light of wavelength 311–313 nm. It contains two photostable UV-absorbing molecules, namely diethylamino hydroxybenzyl hexyl benzoate (DHHB) and alpha-glucosyl hesperidin (αGH), which attenuate lower wavelengths and allow higher therapeutic wavelengths. The therapeutic effects of TBFC have been found to be comparable to NB-UVB in fairer skin types. Even in skin of color, effective responses have also been documented.
Systemic Management
Oral Corticosteroids
These constitute one of the oldest treatment modalities in unstable vitiligo. The definite advantage of quickly halting the disease, easy availability, and cost effectiveness makes them one of the most preferred classes of drugs for actively spreading vitiligo. The most popular form of treatment with oral corticosteroids is oral mini-pulse (OMP) therapy. This mitigates the adverse effects (AEs) associated with long courses of high-dose corticosteroids to some extent. OMPs can be given using either dexamethasone or betamethasone, dosed at 5–10 mg per week. This modality when given for about 6 months halts disease progression in around 91.8% patients with varying degrees of repigmentation. Low-dose prednisolone (0.3 mg/kg/day) has also been tried for few months with or without phototherapy and found to have encouraging results in most patients. Four good quality studies have been conducted in India with most of them reporting good evidence in halting disease but minimally effective in repigmenting the lesion. Arguably, the side effect profile such as weight gain, hypertrichosis, menstrual abnormalities and acne or concomitant illness like diabetes limit their use.
Low-dose prednisolone (0.3 mg/kg/day) has also been used for vitiligo. In a study by Kim et al., the arrest of progression was noted in 87.7% patients, while repigmentation was noted in 70.4%. Additionally, repigmentation was found to be statistically more in men. Side effects were noted, but were transient, and did not warrant treatment cessation. Imamura and Tagami also conducted a study on generalized (17 patients) and localized (5 patients) vitiligo. They used various oral corticosteroids (prednisolone, methylprednisolone, betamethasone, and paramethasone acetate) at different doses, which were gradually decreased to a maintenance dose. At 6 months, 35% patients with generalized vitiligo had more than 75% pigmentation in at least one patch, and in most cases, repigmentation was evident at 4 weeks.
Cyclosporine
This rapidly acting immunosuppressive drug is another modality for unstable vitiligo. CD8+ T cells are assumed to play an important role in melanocyte destruction. By inhibiting IL-2, cyclosporine inhibits CD8+ and CD4+cells, thus posing as an effective treatment option in vitiligo.
In a case series, cyclosporine given at 3 mg/kg for 12 weeks halted disease progression in 61% patients and significantly improved Vitiligo Area Scoring Index (VASI). Another study employing the use of cyclosporine after autologous non-cultured melanocyte transplantation (NCMKT) in stable vitiligo enabled better repigmentation without the typical peri-lesional halo often seen post-surgically. Additionally, it can serve as a useful adjunctive therapy to surgical treatment if patients are unable to attend NB-UVB.
A recent study compared the use of dexamethasone 5 mg a week OMP therapy versus cyclosporine 3 mg/kg/day for 4 months. Patients receiving cyclosporine were found to have a faster halting in disease activity; however, the patients achieving halt in disease progression were similar. Both groups had similar but poor results in terms of pigmentation, reiterating the fact that the action of immunosuppressives is mainly in halting progression of the disease.
Methotrexate
It is a slow-acting steroid-sparing agent, which could be an alternative treatment to OMP in active or unstable vitiligo. It inhibits T cell activation, downregulates B cells, and inhibits intracellular expression of adhesion molecules. Inhibition of T cells, and thereby TNF-α makes this drug theoretically effective in the management of vitiligo.
In a randomized open-label study, similar results were obtained with low-dose oral methotrexate (10 mg weekly) when compared to OMP (dexamethasone 2.5 mg taken twice weekly) in terms of the number of patients getting newer lesions and reduction in Vitiligo Activity Disease score. In another study, methotrexate-alone group was compared with OMP group and methotrexate with OMP. OMP alone or in combination with methotrexate is a superior modality in arresting the disease progression. However, methotrexate alone as well as in combination with OMP results in better repigmentation. Thus, it was concluded that a combination treatment is highly efficacious and tolerable in treating vitiligo.
Azathioprine
This immunosuppressive has also been used in unstable vitiligo. In a randomized study, azathioprine (50 mg twice daily) was compared with 5 mg betamethasone OMP on two consecutive days. It was noted that the disease stabilized in 19 of 23 patients treated with OMP and only in 4 of 22 treated with azathioprine in the second month of treatment. It was shown that OMP group had a faster arrest of disease at 2 and 4 months, but comparable at 6 months. Better repigmentation rates were seen in OMP group at 6 months in this study. Though patients on azathioprine did not have any adverse effects (barring one who had pancreatitis and leukopenia), it was not found to be very useful as a stand-alone drug due to a delayed initiation of action and can be added as a steroid-sparing agent as maintenance therapy in the absence of or when there is a contraindication of other medications.
Minocycline
This antibiotic drug has been used due to its additional immunomodulatory and anti-inflammatory properties, with a role in free-radical destruction and salvaging melanocytes from oxidative damage. In a study by Parsad and Kanwar, disease progression was halted in 29 of 32 patients treated with minocycline 100 mg/day as monotherapy. Another prospective, randomized study involving 50 patients who were given either minocycline (100 mg/day) or dexamethasone (2.5 mg OMP) showed reduction in mean disease activity and VASI in both sets of patients, without any significant difference in repigmentation. However, in another prospective randomized comparative study, NB-UVB proved to be statistically more efficacious at achieving disease stability compared to minocycline 100 mg once daily monotherapy for the treatment of unstable vitiligo to be statistically more efficacious and better at achieving disease stability than minocycline (100 mg/day) monotherapy for unstable vitiligo (disease stability in 76.1% of NB-UVB group versus 33.3% in minocycline group). Thus, though minocycline is inferior to NB-UVB phototherapy, it is comparable to corticosteroid (OMP) treatment if phototherapy is unavailable.
Simvastatin
This drug is FDA approved for the treatment of hypercholesterolemia. Interestingly, it was shown to inhibit IFNγ-induced STAT1 activation in vitro. When a high-dose simvastatin was administered in patients with vitiligo with hypercholesterolemia, there was a resultant repigmentation of the skin, supporting simvastatin as a potential therapy for vitiligo. In an experimental study on mice, simvastatin was given thrice a week for 5 weeks in therapeutic doses with hypercholesterolemia, which prevented and reversed depigmentation in the Krt14-Kitl* transgenic mice and reduced CD81 T-cells in skin. Thus, the effect of simvastatin in inducing repigmentation, observed at only high doses, suggests its utility as an adjuvant therapeutic alternative. However, this is still an experimental drug with studies with efficacy still uncharted.
Afamelanotide
It is a synthetic analog of α-melanocyte-stimulating hormone and targets melanocortin 1 receptor (MC1R), thereby stimulating the transfer of eumelanin into melanosomes. In one of the largest studies conducted on 28 patients, repigmentation by afamelanotide and NBUVB combination was superior to NBUVB alone. Additionally, repigmentation was seen significantly earlier and in significantly higher number of patients on face and upper extremities and also overall superior at day 84 as compared with day 56 for patients of Fitzpatrick skin types IV–VI.
JAK Inhibitors
Tofacitinib, ruxolitinib, and baricitinib are the three most commonly reported JAK inhibitors used in vitiligo treatment. Tofacitinib 5–10 mg once to twice a day along with phototherapy has demonstrated superior efficacy against vitiligo, with reduced VASI score. Tissue analysis showed a decline in CD8+ T cells and chemokines, like CXCL9 and CXCL10 after tofacitinib treatment, but no changes in melanocyte-specific T cells. Topical tofacitinib cream 2% has also been tried in vitiligo with more significant responses noted for facial lesions and patients with darker skin types; however, it can be associated with systemic side effects such as urinary tract infections, cytopenia, and malignancies.
Ruxolitinib is another JAK inhibitor used for vitiligo. It has recently received US-FDA approval as a first-of-its-kind treatment for non-segmental vitiligo. The facial lesions respond better than non-facial lesions, reinforced by a 20-week, open-label. Similar to tofacitinib, most studies report better repigmentation rates when ruxolitinib treatment is combined with phototherapy. Baricitinib is also being tried in vitiligo, and few case reports have reported significant benefit, mostly with phototherapy even in patients with generalized vitiligo.
IL-23 Inhibitors
Few authors have demonstrated elevation of IL- 23 levels in vitiligo, but such a finding has not been consistently reported. Ustekinumab use has been reported to induce either stabilization or repigmentation of vitiligo in case reports.
Systemic Antioxidants
One of the major contributing pathogenic mechanisms is free-radical oxidative stress. Thus, targeting them adds to the therapeutic management and helps in stabilizing and halting the disease. Few target pathways have been identified, which can reduce melanocytic oxidative damage and even stimulate repigmentation. Though they seldom act in isolation, they can be an excellent adjuvant therapy in managing vitiligo in toto.
Few oral agents have been successfully used in treating vitiligo, including vitamin E alone or in combination to vitamin C, alpha-lipoic acid, carotenoids, Phyllanthus emblica, as well as using Polypodium leucotomos, Silybum marianum, leading to variable improvement. Topically, pseudocatalase, catalase plus superoxide dismutase, and superoxide dismutase plus copper, zinc, vitamin B12, and calcium pantothenate have been used with good to no results individually. Different oxidative pathways are being explored in vitiligo, and many newer agents are being explored based on those pathways, as summarized in Table 5.
Table 5
Targeted pathways and mechanism of action of antioxidants in vitiligo
| Targeted pathway | Mechanism of action | Treatment objective | Clinically used antioxidants | Experimental antioxidants |
|---|---|---|---|---|
| PI3K/AKT | Regulation of melanocyte metabolism, proliferation and differentiation | Reduce melanocytic oxidative damage | Methoxypsoralen, Basic fibroblast growth factor (BFGF), mesenchymal stem cell, chalcones | Geniposide, quercetin |
| p38 MAPK | Melanogenesis, antioxidant action | Reduce melanocytic oxidative damage | Karwiya, glutathione, minocycline | 1,5-dicaffeoylquinic acid, epigallocatechin gallate (EGCG), flumequine, maclurin, cynarine |
| Nrf2/ARE | Upregulating antioxidant gene expression | Reduce melanocytic oxidative damage | Gingko biloba, afamelanotide, simvastatin, aspirin | Cinnamaldehyde, berberine |
| AhR | Regulate mitochondrial biosynthesis and repair mitochondrial oxidative damage | Reduce melanocytic oxidative damage | - | Isopsoralen, Tapinarof, cinnamaldehyde |
| Wnt/β-catenin | Stimulate melanocyte metabolism, proliferation and differentiation | Stimulate repigmentation | Vitamin D | Wnt receptor inducer, adipose tissue extracellular fraction |
Depigmenting Therapy
Depigmentation therapy can be a feasible option in patients with extensive disease, affecting >80% body area. The patients should be told about the permanency of treatment, potential patchy repigmentation, treatment time and cost, as well as color match.
Monobenzyl ether of hydroquinone (MBEH), a hydroquinone derivative, is currently the only FDA-approved topical depigmenting agent for vitiligo. It is structurally homologous to tyrosine and is selectively cytotoxic toward melanocytes, thereby inducing chemical leukoderma. Daily usage of 20% MBEH can start inducing depigmentation in three to 6 months. It should be noted that ocular irritation can ensue, and should therefore be avoided over eyelid area. Furthermore, MBEH destroys only epidermal melanocytes, and follicular melanocytes are retained, which on sun exposure can induce repigmentation. Thus, a strict photoprotection with sunscreen as well as photo-protective clothing is advised.
Other depigmenting agents include monomethyl ether of hydroquinone (MMEH), also known as mequinol and 88% phenol. Similar to MBEH, mequinol can induce depigmentation and can be applied as monotherapy, or in combination to Q-switched ruby laser. However, it can cause pruritus, burning, and contact dermatitis. Phenol 88% is a medium-depth peel, which suppresses melanogenesis and induces epidermal protein coagulation, thereby treating residual pigmentation.
Cryotherapy and lasers like Q-switched ruby and Q-switched alexandrite laser are the physical modalities used for depigmentation. Cryotherapy has an excellent safety profile and is low-cost, but has limited availability. It leads to irreversible tissue damage and is particularly effective for koebnerized lesions. Lasers are selectively melanocyte-specific and effective and can relatively treat large treatment areas. In lighter skin types, Q-switched ruby and alexandrite lasers can be effective. However, for skin of color, these may not be very effective.
Few new agents, which can be tried, include imiquimod, diphencyprone, and imatinib. These agents, when used for their respective indications, were found to induce vitiligo-like depigmented lesions and thus, can be serendipitously used as a treatment option in depigmenting residual pigmentation in vitiligo universalis.
What does the future hold?
Of late, there is a focus on the role of signaling pathways and cytokines involved in vitiligo pathogenesis, and thus targeting them can be future options of tackling vitiligo [Table 6]. There is recognition of IFN-gamma-CXCL9/10-CXCR3 axis along with the JAK-STAT pathway as an essential pathway in vitiligo, leading to inhibition of melanogenesis, triggering melanocyte apoptosis, and recruitment of T cells to the skin. Additionally, cytokines such as TNF-α, IL-15, IL-17/23, Tregs, WNT signaling pathway, and mi-RNAs have also been added to the pathogenesis of vitiligo. Apart from the successful use of JAK inhibitors, TNF-α inhibitors such as infliximab and IL-23 inhibitor have been tried with good response. Most of the other studies have been done on mouse models and are under trials. These new molecules, such as CXCL neutralizing antibodies, CXCR depleting antibodies, IL-17 receptor antibodies, and anti-CD-122 antibodies are in initial stages of trial and can be exciting treatment options in future.
Table 6
New upcoming treatment modalities for vitiligo
| Drug | Mechanism of action | Dose | Remarks |
|---|---|---|---|
| Biologicals | |||
| Etanercept | TNF-α inhibitor | 50 mg subcutaneously once-twice weekly | Stabilization of disease in progressive vitiligo |
| Infliximab | TNF-α inhibitor | 5 mg/kg intravenous (week 0, 2, 6 and then 8 weekly) | Good repigmentation observed |
| Ustekinumab | Inhibits IL-23 | 90 mg subcutaneous injection at week 0, 4 and 8 weekly thereafter | Improvement in facial vitiligo |
| Rituximab | Anti-CD20 antibody | 500 mg intravenous infusions (twice) | Moderate improvement |
| Abatacept | CTLA-4 Immunoglobulin | 125 mg weekly injections for 24 weeks | Not available |
| JAK Inhibitors | |||
| Baricitinib | JAK 1 & 2 inhibitor | 4 mg/day oral | Repigmentation in vitiligo patient with Rheumatoid arthritis |
| Ritlecitinib | JAK 3 inhibitor | 200 mg once daily for 4 weeks; followed by 50 mg once daily for 20 weeks | Improvement in facial vitiligo |
| Ifidancitinib (ATI-50002) | JAK 1 & 3 inhibitor | 0.46% solution applied twice daily | Improvement in facial vitiligo (phase 2 study) |
| Other molecules | |||
| ChMBC7 (Anti-CD122 antibody) | IL-15 inhibitor | 100 mg thrice weekly | Repigmentation seen in treated mice (mouse model) |
| Anti-IL-17A Receptor antibody | IL-17 inhibitor | - | Increased melanin content and cellular proliferation |
| XMG-6 (IFN-gamma Neutralizing antibody) | IFN-gamma inhibitor | 100–500 mg intraperitoneally twice weekly | Inhibition of further depigmentation (mouse model) |
| CXCL10 neutralizing antibodies | Antibodies against CXCL 10 | 100 μg Intraperitonealinjection thrice weekly | Repigmentation seen after 4 weeks of treatment (mouse model) |
| CXCR3 depleting antibodies | Antibodies against CXCR3 | 100 μg intraperitoneal injection thrice weekly | Reversal of vitiligo (mouse model) |
| HSP70i Q435A DNA delivery | HSP70i inducer | 2.5 mg once weekly | Considerable repigmentation |
| SKL2001 (Wnt-specific agonists) | Wnt agonist | - | Melanogenesis associated proteins (MITF, TYR, TRP1, TRP2) were increased |
Acknowledgment
None.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Patients’ consent form
Not applicable.
References
1
van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: Position statement from the International Vitiligo Task Force Part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol 2023;37:2173–84.2
Behl PN, Bhatia RK. 400 cases of vitiligo. A clinico-therapeutic analysis. Indian J Dermatol 1972;17:51–6.3
Mahajan VK, Vashist S, Chauhan PS, Mehta KIS, Sharma V, Sharma A. Clinico-epidemiological profile of patients with vitiligo: a retrospective study from a tertiary care center of North India. Indian Dermatol Online J 2019;10:38–44.4
Eleftheriadou V, Atkar R, Batchelor J, et al., British Association of Dermatologists’ Clinical Standards Unit. British Association of Dermatologists guidelines for the management of people with vitiligo 2021. Br J Dermatol 2022;186:18–29.5
Kumar Jha A, Sonthalia S, Lallas A, Chaudhary RKP. Dermoscopy in vitiligo: diagnosis and beyond. Int J Dermatol 2018;57:50–4.6
Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther 2020;33:e13418.7
Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol 2022;13:986918.8
Shah S, Sakhiya J, Deshpande P, Sakhiya D, Inamadar AC. Safety and efficacy of the combination of 308-nm monochromatic excimer light and topical 0.1% tacrolimus ointment in segmental vitiligo: an open-label study. J Clin Aesthet Dermatol 2020;13:69–75.9
van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin 2017;35:145–50.10
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 2015;386:74–84.11
Seneschal J, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the international Vitiligo Task Force-Part 2: specific treatment recommendations. J Eur Acad Dermatol Venereol 2023;37:2185–95.12
Raju SP, Kaur S, Loganathan E. Management of childhood vitiligo−a brief review. Pigment Int 2022;9:14–24.13
Sisti A, Sisti G, Oranges CM. Effectiveness and safety of topical tacrolimus monotherapy for repigmentation in vitiligo: a comprehensive literature review. An Bras Dermatol 2016;91:187–95.14
Radakovic S, Breier-Maly J, Konschitzky R, et al. Response of vitiligo to once- vs. twice-daily topical tacrolimus: a controlled prospective, randomized, observer-blinded trial. J Eur Acad Dermatol Venereol 2009;23:951–3.15
Dang YP, Li Q, Shi F, Yuan XY, Liu WL. Effect of topical calcineurin inhibitors as monotherapy or combined with phototherapy for vitiligo treatment: a meta-analysis. Dermatol Ther 2016;29:126–33.16
Lepe V, Moncada B, Castanedo-Cazares JP, Torres-Alvarez MB, Ortiz CA, Torres-Rubalcava AB. A double-blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol 2003;139:581–5.17
Köse O, Arca E, Kurumlu Z. Mometasone cream versus pimecrolimus cream for the treatment of childhood localized vitiligo. J Dermatolog Treat 2010;21:133–9.18
Bae JM, Jeong KH, Choi CW, et al. Development of evidence-based consensus on critical issues in the management of patients with vitiligo: a modified Delphi study. Photodermatol Photoimmunol Photomed 2021;37:3–11.19
Cavalié M, Ezzedine K, Fontas E, et al. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol 2015;135:970–4.20
Sheikh A, Rafique W, Owais R, Malik F, Ali E. FDA approves ruxolitinib (opzelura) for vitiligo therapy: a breakthrough in the field of dermatology. Ann Med Surg (Lond) 2022;81:104499.21
Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol 2017;76:1054–60.22
Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 2: applications in psoriasis, atopic dermatitis, and other dermatoses. Actas Dermosifiliogr (Engl Ed) 2021;112:586–600.23
Joge RR, Kathane PU, Joshi SH. Vitiligo: a narrative review. Cureus 2022;14:e29307.24
Sitek JC. Vitiligo-loss of cutaneous pigmentation. Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke 2006;126:2370–2.25
Parsad D, Godse K, Shah B, et al. Basic Fibroblast Growth Factor (bFGF) related decapeptide 0.1% solution, with tacrolimus 0.1% ointment combination therapy compared with tacrolimus 0.1% ointment monotherapy in the treatment of stable vitiligo: a phase IV, randomized 12 months Study. Indian J Clin Exp Dermatol 2020;6:249–53.26
George M, Mallikarjun M, Manjunath P, Gangadhar B. Efficacy of laser dermabrasion followed by topical 5-fluorouracil in the treatment of stable vitiligo. Int J Health Sci Res 2017;7:144–7.27
Jha AK, Sonthalia S. 5-Fluorouracil as an adjuvant therapy along with microneedling in vitiligo. J Am Acad Dermatol 2019;80:e75–6.28
Kumar A, Bharti R, Agarwal S. Microneedling with Dermaroller 192 needles along with 5-fluorouracil solution in the treatment of stable vitiligo. J Am Acad Dermatol 2019;81:e67–9.29
Zahra FT, Adil M, Amin SS, Mohtashim M, Bansal R, Khan HQ. Efficacy of topical 5% 5-fluorouracil with needling versus 5% 5-fluorouracil alone in stable vitiligo: a randomized controlled study. J Cutan Aesthet Surg 2020;13:197–203.30
Yang YS, Cho HR, Ryou JH, Lee MH. Clinical study of repigmentation patterns with either narrow-band ultraviolet B (NBUVB) or 308nm excimer laser treatment in Korean vitiligo patients. Int J Dermatol 2010;49:317–23.31
Wu CS, Yu CL, Wu CS, Lan CC, Yu HS. Narrow-band ultraviolet-B stimulates proliferation and migration of cultured melanocytes. Exp Dermatol 2004;13:755–63.32
Mohammad TF, Al-Jamal M, Hamzavi IH, et al. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol 2017;76:879–88.33
Boniface K, Passeron T, Seneschal J, Tulic MK. Targeting innate immunity to combat cutaneous stress: the vitiligo perspective. Front Immunol 2021;12:613056.34.34
Silpa-archa N, Lim HW. Narrow-band ultraviolet B phototherapy in vitiligo. In: Gupta S, Olsson MJ, Parsad D, Lim HW, van Geel N, Pandya AG, eds. Vitiligo: Medical and Surgical Management. 1st ed. 2018 vol. 13, p. 91–103.35
Bae JM, Ju HJ, Lee RW, et al. Evaluation for skin cancer and precancer in patients with vitiligo treated with long-term narrowband UV-B phototherapy. JAMA Dermatol 2020;156:529–37.36
AlJasser M, Richer V, Ball N, Lui H, Zhou Y. Photolichenoid papules within vitiligo induced by narrowband UVB phototherapy. J Eur Acad Dermatol Venereol 2016;30:1428–9.37
Shenoi SD, Prabhu S. Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol 2014;80:497–504.38
Balasaraswathy P, Kumar U, Srinivas CR, Nair S. UVA and UVB in sunlight, optimal utilization of UV rays in sunlight for phototherapy. Indian J Dermatol Venereol Leprol 2002;68:198–201.39
Sharma CK, Sharma M, Aggarwal B, Sharma V. Different advanced therapeutic approaches to treat vitiligo. J Environ Pathol Toxicol Oncol 2015;34:321–34.40
Taieb A, Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013;168:5–19.41
Mysore V. Targeted phototherapy. Indian J Dermatol Venereol Leprol 2009;75:119–25.42
Meredith F, Abbott R. Vitiligo: an evidence-based update. Report of the 13th Evidence Based Update Meeting, 23 May 2013, Loughborough, U. K. Br J Dermatol 2014;170:565–70.43
Hong SB, Park HH, Lee MH. Short-term effects of 308-nm xenon-chloride excimer laser and narrow-band ultraviolet B in the treatment of vitiligo: a comparative study. J Korean Med Sci 2005;20:273–8.44
Hofer A, Hassan AS, Legat FJ, Kerl H, Wolf P. The efficacy of excimer laser (308nm) for vitiligo at different body sites. J Eur Acad Dermatol Venereol 2006;20:558–64.45
Gupta S, Olsson MJ, Parsad D, Lim HW, van Geel N, Pandya AG, editors. Vitiligo: Medical and Surgical Management. John Wiley & Sons; 2018.46
Sonthalia S. Topical Band-pass Filter Cream (TBFC)-assisted home-based NB-UVB: a must-know Alternative to artificial phototherapy. J CosmetDermatol 2021;20:2141–7.47
Searle T, Al-Niaimi F, Ali FR. Vitiligo: an update on systemic treatments. Clin Exp Dermatol 2021;46:248–58.48
Kanwar AJ, Mahajan R, Parsad D. Low-dose oral minipulse dexamethasone therapy. J Cutan Med Surg 2013;17:259–68.49
Kim SM, Lee HS, Hann SK. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patients. Int J Dermatol 1999;38:546–50.50
Imamura S, Tagami H. Treatment of vitiligo with oral corticosteroids. Dermatologica 1976;153:179–85.51
Taneja A, Kumari A, Vyas K, Khare AK, Gupta LK, Mittal AK. Cyclosporine in treatment of progressive vitiligo: an open-label, single-arm interventional study. Indian J Dermatol Venereol Leprol 2019;85:528–31.52
Mutalik S, Shah S, Sidwadkar V, Khoja M. Efficacy of cyclosporine after autologous noncultured melanocyte transplantation in localized stable vitiligo—a pilot, open label, comparative study. Dermatol Surg 2017;43:1339–47.53
Mehta H, Kumar S, Parsad D, Bishnoi A, Vinay K, Kumaran MS. Oral cyclosporine is effective in stabilizing active vitiligo: results of a randomized controlled trial. Dermatol Ther 2021;34:e15033.54
Singh H, Kumaran MS, Bains A, Parsad D. A randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatol Online J 2015;231:286–90.55
ElGhareeb MI, Metwalli M, AbdelMoneim N. Combination of oral methotrexate and oral mini-pulse dexamethasone vs either agent alone in vitiligo treatment with follow up by dermoscope. Dermatol Ther 2020;33:e13586.56
Dellatorre G, Antelo DAP, Bedrikow RB, et al. Consensus on the treatment of vitiligo − Brazilian Society of Dermatology. An Bras Dermatol 2020;95:70–82.57
Patra S, Khaitan BK, Sharma VK, Khanna N. A randomized comparative study of the effect of betamethasone oral mini-pulse therapy versus oral azathioprine in progressive nonsegmental vitiligo. J Am Acad Dermatol 2021;85:728–9.58
Song X, Xu A, Pan W, et al. Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med 2008;22:9–16.59
Parsad D, Kanwar A. Oral minocycline in the treatment of vitiligo − a preliminary study. Dermatol Ther 2010;23:305–7.60
Singh A, Kanwar AJ, Parsad D, Mahajan R. Randomised controlled study to evaluate the effectiveness of dexamethasone oral minipulse therapy versus oral minocycline in patients with active vitiligo vulgaris. Indian J Dermatol Venereol Leprol 2014;80:29–35.61
Siadat AH, Zeinali N, Iraji F, et al. Narrow-band ultraviolet B versus oral minocycline in treatment of unstable vitiligo: a prospective comparative trial. Dermatol Res Pract 2014;2014:240856.62
Zhao Y, Gartner U, Smith FJ, et al. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol 2011;131:1045–52.63
Noël M, Gagné C, Bergeron J, et al. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis 2004;3:7.64
Agarwal P, Rashighi M, Essien KI, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol 2015;135:1080–8.65
Birlea SA, Goldstein NB, Norris DA. Repigmentation through melanocyte regeneration in vitiligo. Dermatol Clin 2017;35:205–18.66
Minder EI, Barman-Aksoezen J, Schneider-Yin X. Pharmacokinetics and pharmacodynamics of afamelanotide and its clinical use in treating dermatologic disorders. Clin Pharmacokinet 2017;56:815–23.67
Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial JAMA Dermatol 2015;151:42–50.68
Vu M, Heyes C, Robertson SJ, Varigos GA, Ross G. Oral tofacitinib: a promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin Exp Dermatol 2017;42:942–4.69
Mobasher P, Guerra R, Li SJ, Frangos J, Ganesan AK, Huang V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol 2020;182:1047–9.70
McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet 2021;398:803–16.71
Li X, Sun Y, Du J, Wang F, Ding X. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol 2023;16:635–8.72
Vaccaro M, Cannavo SP, Imbesi S, et al. Increased serum levels of interleukin- 23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol 2015;54:672–4.73
Wang L, Yang H, Fan J. Clinical significance of detection of levels of IL-17 and IL-23 in vitiligo patients. Chin J Trop Med 2010;10:591–2.74
Cengiz FP, Emiroğlu N, Çevirgen Cemil B, Erdem ÜGB, Kemeriz F. Serum IL-23 levels in patients with vitiligo. Turkderm 2014;48:204–7.75
Osman AM, Mukhtar MM, Bakheit KH, Hamdan HZ. Plasma levels of Interleukin-17, Interleukin-23, and transforming growth factor- beta in Sudanese patients with Vitiligo: a case- control study. Indian J Dermatol 2015;60:635.76
Zhang J, Hu W, Wang P, Ding Y, Wang H, Kang X. Research progress on targeted antioxidant therapy and vitiligo. Oxid Med Cell Longev 2022;2022:1821780.77
Grimes PE, Nashawati R. Depigmentation therapies for vitiligo. Dermatol Clin 2017;35:219–27.78
Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol 2000;42:760–9.79
Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol 2012;132:2601–9.80
Gupta D, Kumari R, Thappa D. Depigmentation therapies in vitiligo. Indian J Dermatol Venereol Leprol 2012;78:49–58.81
Webb KC, Tung R, Winterfield LS, et al. Tumour necrosis factor-a inhibition can stabilize disease in progressive vitiligo. Br J Dermatol 2015;173:641–50.82
Simon JA, Burgos-Vargas R. Vitiligo improvement in a patient with ankylosing spondylitis treated with infliximab. Dermatology 2008;216:234–5.83
Elkady A, Bonomo L, Amir Y, Vekaria AS, Guttman-Yassky E. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. JAAD Case Rep 2017;3:477–9.84
Ruiz-Arguelles A, Garcia-Carrasco M, Jimenez-Brito G, et al. Treatment of vitiligo with a chimeric monoclonal antibody to CD20: a pilot study. Clin Exp Immunol 2013;174:229–36.85
Open-label pilot study of abatacept for the treatment of vitiligo. Available at: https://clinicaltrials.gov/ct2/show/NCT0228105886
Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs 2014;23:1067–77.87
Robinson MF, Damjanov N, Stamenkovic B, et al. Efficacy and safety of PF-06651600 (Ritlecitinib), a novel JAK3/TEC inhibitor, in patients with moderate-to-Severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2020;72:1621–31.88
A study of ATI-50002 topical solution for the treatment of vitiligo. 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT034688558989
Richmond JM, Strassner JP, Zapata L, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Trans Med 2018;10:eaam7710.90
Bhardwaj S, Bhatia A, Kumaran MS, Parsad D. Role of IL-17A receptor blocking in melanocyte survival: a strategic intervention against vitiligo. Exp Dermatol 2019;28:682–9.91
Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFNgamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol 2012;132:1869–76.92
Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Trans Med 2014;6:223ra23.93
Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 depleting antibodies prevent and reverse vitiligo in mice. J Invest Dermatol 2017;137:982–5.94
Henning SW, Fernandez MF, Mahon JP, et al. HSP70iQ435A-encoding DNA repigments vitiligo lesions in Sinclair swine. J Invest Dermatol 2018;138:2531–9.95
Zou DP, Chen YM, Zhang LZ, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the wnt/beta-catenin signaling. Genes Dis 2021;8:677–88.
