Loneliness has been associated with detrimental effects on mental and physical health and is increasingly recognized as a critical public health issue which may be further exacerbated by societal challenges such as increasing urbanization, an aging society as well as the COVID-19 pandemic. A recent clinical study published in Psychotherapy and Psychosomatics has demonstrated that an internet-based cognitive behavioral therapy (ICBT) can significantly reduce loneliness, and such a preventive intervention may be co-opted to target suicidality in the elderly [, ]. As such, it is now an opportune time to review current conceptualization of chronic loneliness, its detrimental consequences and potential neurocognitive mechanisms as well as initial treatment strategies.
Loneliness is not a clinical diagnosis, but a psychological state with detrimental effects on physiological and mental health if it is experienced chronically. Prevalence estimates vary depending on the assessment criteria, but representative samples surveyed before the onset of the COVID-19 pandemic showed that 22% of inhabitants in the United States and 23% in Britain always or often feel lonely []. Loneliness can occur at any life stage, but elevated levels have been observed during late adolescence and in elderly people []. Various lines of research also indicate that the extended lockdowns and necessary social isolation during the COVID-19 pandemic have increased not only feelings of loneliness but also depression and suicidal ideation [-]. However, of note, loneliness is a subjective feeling which is distinct from objective social isolation [, ]. It is possible to have a large and diverse social network and feel lonely, and vice versa, to live a life with only a few meaningful social connections and experience no loneliness at all. Therefore, loneliness can be best described as a discrepancy between desired and actual social connectedness []. This conceptualization is in line with earlier epidemiological studies differentiating between “availability” and “adequacy” of social support. Increased mortality and risk of cardiovascular diseases have been linked to less perceived adequacy of social support [-]. In humans as a social species, loneliness may have evolved as an adaptive function and evolutionary coping strategy to promote behavioral changes, which increase the chance of survival []. Loneliness can be seen as a social equivalent to hunger, such that the feeling of loneliness triggers the need to form new social relationships, in the same way as hunger triggers the need to eat [-]. If loneliness is an evolutionary signal to form social bonds, the question of why some people stay lonely over extended periods of time arises. Current models of loneliness postulate that lonely individuals exhibit negative social biases which paradoxically lead to even more withdrawal from social connections []. Clearly, the effects of acute loneliness are distinct from the impact of chronic loneliness [, ]. For instance, a recent study found that chronic loneliness was associated with a greater preferred interpersonal distance, whereas acute loneliness was related to smaller preferred distances [], possibly reflecting the evolutionary desire to form social bonds. Although previous studies found that acute social exclusion elicits activations in neural pathways overlapping with those mediating physical pain such as the dorsal anterior cingulate cortex (ACC) and may lead to severe distress [, ], a recent meta-analysis did not detect reliable activation in the dorsal ACC in acute social exclusion but rather found robust engagement of the ventral ACC, posterior insula, posterior cingulate cortex, and lateral prefrontal regions with further co-activation analyses demonstrating a functional co-variance with large-scale networks that resembled the default mode network (DMN) topography []. Nevertheless, acute social isolation should not be confused with chronic loneliness, which exerts more harmful effects such as strongly increased mortality in comparison to acute social isolation []. Chronic loneliness may function as a continuous psychological stressor which increases the allostatic load, characterized as the wear and tear resulting from chronic overreactivity of stress systems [, ]. Several studies linked satisfactory social relationships to reduced allostatic load [-]. Allostatic overload is associated with poor health and should be assessed with an integrated approach including not only clinimetric criteria but also biomarkers []. Several large-scale studies showed that common genetic variants contribute to loneliness in a range from 4 to 27% [-]. Therefore, loneliness seems to interact with a complex system consisting of individual biology, as well as psychosocial status and may lead to a form of biosocial pathogenesis [, ]. Given that the COVID-19 pandemic and the necessary measures of social distancing may facilitate the transition from acute to chronic loneliness [], interventions in vulnerable populations [] may help to reduce the allostatic load and therefore prevent the detrimental health consequences of loneliness.
Detrimental Health Consequences of Loneliness
Accumulating evidence from different lines of research convergently indicates the detrimental impact of chronic loneliness and perceived social isolation on both, somatic and mental health. A number of studies have established associations between chronic loneliness and increased morbidity and mortality mirroring the negative impact of well-established risk factors such as obesity or smoking. Thus, loneliness and social disconnection are increasingly recognized as major public health concerns [-]. Increasing evidence suggests associations between chronic loneliness and an impaired integrity of the immune system, including reduced numbers of natural killer cells [, ] and diminished immune responses to acute stressors [] in lonely individuals. Chronic loneliness has also been linked to heightened blood pressure [, ] and an increased risk for coronary heart diseases and stroke [, ]. In addition, feelings of social isolation are a risk factor for obesity [-] and impaired sleep quality [, ]. Sleep deprivation in turn can trigger feelings of loneliness, starting a self-reinforcing cycle of social withdrawal []. Importantly, the detrimental effects of loneliness are not restricted to somatic disorders but extend to mental health. Perceived social isolation has been identified as a significant predictor for cognitive decline in dementia and Alzheimer disease [, ] and is associated with higher levels of depressive symptoms [, ], anxiety [, ], and psychosocial stress []. Furthermore, patients with substance abuse [-], borderline personality disorder [, ], and schizoid personality disorder [] report more loneliness and social disconnection than healthy controls. In addition, loneliness is a potential risk factor for post-traumatic stress disorder [, ] and enhances intrusive thoughts after trauma exposure [, ]. Overall, loneliness and social isolation are critical risk factors for several somatic and mental disorders and thus should be considered in therapeutic protocols. The development of neurobiologically informed interventions for loneliness critically requires a better understanding of the brain structural and functional neural changes related to chronic feeling of social isolation.
Brain Structural Adaptations Associated with Loneliness
Prolonged periods of social isolation have been linked to broad changes in brain morphology. For instance, participants of a 14-month long Antarctica expedition exhibited significant reductions in brain-derived neurotrophic factor concentrations and gray matter volume in the dorsolateral and orbitofrontal cortex and hippocampus compared to controls []. While these findings are consistent with animal studies showing an association between social isolation and hippocampal neurogenesis [], it is also conceivable that the expedition-related changes are a byproduct of sensory deprivation. Previous studies also observed that larger and more diverse social networks positively correlate with amygdala volume [], but a recent study failed to replicate this association []. Along these lines, a rare patient with bilateral amygdala damage showed a normal size and complexity of her social network [], indicating that an intact amygdala is not necessary to maintain social relationships or at least can be compensated for []. Several years after the first assessment of the social network, the woman with amygdala lesion developed severe treatment-resistant depression along with a reduction in the size of her social network, and she reported strong feelings of loneliness [], demonstrating that the experience of loneliness may not require an intact amygdala either. However, recent large-scale brain morphology studies suggest that there are sex-dependent brain volume effects of loneliness, especially in the amygdala and the ventromedial prefrontal cortex (vmPFC) []. Smaller amygdala volumes were detected for lonely men, but not lonely women, and this pattern was reversed for the vmPFC volume. Thus, prospective longitudinal studies are required to monitor sex-specific morphological changes that accompany chronic loneliness. Sex and loneliness interactions are not restricted to brain structural effects. A recent large databank study found that lonely individuals display volume deviations and functional communication changes in the DMN, identifying the DMN as a key component of perceived social isolation []. Interestingly, this loneliness-related effect was more prominent in men than women.
Furthermore, individual differences in loneliness correlated with gray matter density in the left posterior superior temporal sulcus, and this association was mediated by social perception skills []. Interestingly, the correlation remained significant after controlling for trait anxiety and social network size, thus providing further support for the notion that loneliness and social anxiety are characterized by distinct neural phenotypes [] and for the dissociation of loneliness and social isolation. Importantly, loneliness has also been linked to altered neural processing in various neurocognitive domains (Fig. 1), including negative cognitive biases, sensory processing, executive functioning, reward-related processes, and memory.
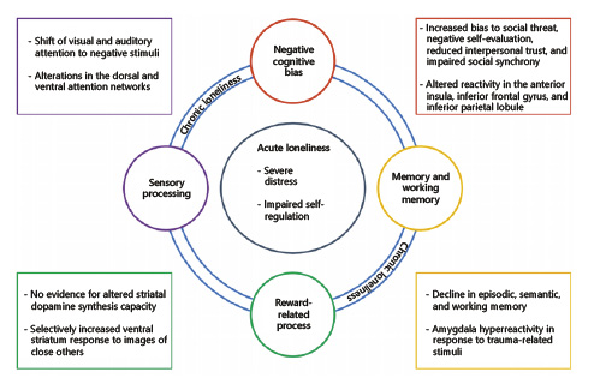
Fig. 1
Theoretical model illustrating the impact of acute and chronic loneliness. Acute effects of loneliness are shown in the inner circle. Chronic loneliness may affect functional domains which are illustrated in the outer circles. Exemplary findings for the domains are listed in the circles: negative cognitive biases (red), memory and working memory (yellow), reward-related processes (green), and sensory processing (purple).
Negative Cognitive Biases
It has been hypothesized that the maintenance of loneliness is fueled by negative cognitive biases which make positive social interactions less rewarding and may foster even more social withdrawal [, ]. Mechanistically, lonely individuals may be more likely to perceive social stimuli as threatening and to evaluate themselves and others more negatively []. Feelings of alienation may result from larger self-other dissimilarity of activation patterns in the medial prefrontal cortex []. Furthermore, loneliness is associated with reduced interpersonal trust and a preference for larger social distances from unfamiliar others, and this behavioral phenotype is paralleled by reduced trust-associated activity in the anterior insula. Importantly, blunted functional connectivity between the anterior insula and occipito-parietal regions predicts diminished affective and oxytocinergic responses to positive social interaction []. Given that the anterior insula plays a key role in self-awareness and interoceptive processing [], we hypothesize that the negative cognitive biases in loneliness are mediated by an external attention focus due to reduced generation of, or sensitivity to, internal physiological signals in social situations []. Further supporting evidence for this notion comes from a study showing that insula responses to emotional faces mediate the association between alexithymia and subjective isolation stress []. Additionally, the DMN has been recently identified as a key system involved in loneliness through large-scale UK biobank studies. Increased functional connectivity of the DMN [] and overall increased network integration between the DMN and the attentional and visual networks in lonely subjects [] may reflect exaggerated rumination during rest []. Furthermore, it has been suggested that negative cognitive biases such as the expectation of more negative social interactions may be based on the association between loneliness and distinct divergences in the structural covariation of DMN and hippocampus subregions [].
In addition, loneliness may affect synchronization during social interactions, such that lonely people may require stronger activation of their observation execution system including the inferior frontal gyrus (IFG) and the inferior parietal lobule for alignment to compensate for some deficiency in their synchronization ability []. Further studies are warranted to probe possible causal pathways of how disrupted interoceptive processes and impaired synchronization may lead to social withdrawal and the chronicity of loneliness.
Sensory Processing and Executive Functioning
Loneliness-induced hypervigilance can be observed in a shift of visual and auditory attention to negative or threatening stimuli. These changes in sensory processing could be induced by alterations in the dorsal and ventral attention networks [, ]. Furthermore, there appears to be a bidirectional relationship between tactile processing and loneliness. Touch deprivation during COVID-19-related restrictions has been linked to higher anxiety and greater loneliness [], but loneliness also positively correlated with touch avoidance []. The excitatory transcranial direct current stimulation to the right IFG slowed responses to observed touch in lonely individuals [], indicating that the IFG may contribute to the perpetuation of loneliness by enhancing the avoidance of positive social cues. Likewise, olfactory impairment can severely disrupt close relationships []. Loneliness is higher in participants who experienced childhood maltreatment, which correlates with amygdala hyperreactivity and hippocampal deactivation in response to social stress odors []. Whether and how loneliness may affect the sensory integration of multiple modalities is still elusive. In addition, it has been hypothesized that reduced functional connectivity of the right middle/superior frontal gyrus to the cingulo-opercular network during rest may reflect diminished executive functioning in loneliness [], but evidence for an association between loneliness and impaired executive functioning across the life span is scarce.
Reward-Related Processes
The activation patterns evoked by acute social isolation in the ventral tegmental area are similar to the craving-related activation pattern observed after fasting []. By contrast, dissociable responses were evident in the striatum, with fasting enhancing responses to food cues in the nucleus accumbens and social isolation increasing responses to social cues in the caudate nucleus. Cacioppo et al. [] showed reduced ventral striatum (VS) activity in lonely individuals while viewing pleasant pictures with social connotation, but other studies found no significant correlation between loneliness and VS responses to pleasant social stimuli [], nor between loneliness and striatal dopamine synthesis capacity in healthy controls or patients with autism spectrum disorder []. These contradictory findings may be reconciled by taking the familiarity of the social context into account. For instance, another functional magnetic resonance imaging study reported selectively increased VS responses to images of close others compared to strangers in lonely individuals, possibly reflecting fear of alienation or rejection [].
Memory and Working Memory
In line with the above-mentioned association between loneliness and cognitive decline, several studies have reported loneliness-related declines in episodic, semantic, and working memory in older adults [-]. In patients with major depressive disorder, loneliness had no significant effect on working memory performance, but it was linked to increased functional connectivity between the dorsolateral prefrontal cortex and inferior parietal cortex, indicating that loneliness may be associated with altered neural regulatory functioning in self-referential processing []. Of note, a recent study found that loneliness may influence trauma memory in a sex-dependent manner. Specifically, lonely men, but not lonely women, exhibited more intrusive thoughts after experimental trauma and this phenotype was related to amygdala hyperreactivity during both fear conditioning and habituation processes, suggesting that the limbic system is a potential target for interventions that increase social connectedness []. Furthermore, the above-mentioned alterations in hippocampus-DMN covariation may reflect the neurobiological basis for an increased negative memory retrieval []. Interestingly, these alterations seem to have distinct links to genetic components of loneliness [, ].
Neurocognitive Mechanisms Underlying Loneliness-Related Vulnerability
The current lack of longitudinal studies probing the trajectories of loneliness-associated neural changes hamper conclusions about causal mechanisms. However, given the strong involvement of the DMN in loneliness, it is conceivable that DMN dysregulation also contributes to the detrimental health effects of loneliness. For instance, loneliness has consistently been associated with cognitive decline in patients with Alzheimer’s disease [, ], and DMN dysregulation has not only been linked to Alzheimer pathology and cognitive decline [, ], but also to psychiatric disorders such as substance abuse [], depression [, ], and post-traumatic stress disorder [, ]. Perceived social isolation could therefore influence different pathologies by changing the structural and functional integrity of the DMN.
A second possible mechanism mediating detrimental health effects of loneliness could be based on disrupted interoceptive processes. Loneliness has been linked with an “attentional switch” leading to a shift in the direction of heightened exteroceptive attention rather than interoceptive processes which may foster the negative cognitive bias in loneliness []. A perceptual insensitivity to the modulation of interoceptive signals has been observed across several common psychiatric disorders such as depression and anxiety disorder [, ]. This way, loneliness-dependent activity and connectivity changes in the anterior insula may reflect heightened subjective isolation stress and could convey increased vulnerability in lonely individuals to psychological disorders [, ].
Furthermore, amygdala hyperreactivity might be another mechanism underlying the elevated prevalence of psychiatric disorders in high-lonely individuals. Recently, we found amygdala hyperreactivity and increased intrusive thought formation after trauma exposure in high-lonely men []. Heightened amygdala reactivity predicts depressive [] and post-traumatic stress disorder symptoms []. Furthermore, amygdala connectivity with the DMN is decreased in patients with major depressive disorder []. All of these hypothesized neurocognitive mechanisms might be possible targets for specific therapeutic interventions to reduce loneliness-related vulnerability, but rigorous randomized clinical trials are required to probe causal effects.
Therapeutic Interventions for Loneliness and Integration with Neurocognitive Mechanisms
Social interventions should be considered in new therapeutic concepts to effectively reduce feelings of loneliness. Several studies support the effectiveness of social interventions in a non-clinical environment [-]. Intervention types range from group-based physical activities [-], internet and app-based group interventions [-] to the use of robotic agents [, ]. In addition, a positive social climate and community programs can further prevent loneliness [-]. A recent meta-analysis showed that psychological interventions were more effective than measures to increase access to other people to improve the perceived quality of social connections []. For example, cognitive-behavioral therapies targeting maladaptive cognition can reduce loneliness levels and the elevated blood pressure associated with loneliness in older individuals [, ]. Furthermore, mindfulness training has been demonstrated to be effective in reducing loneliness and related pro-inflammatory gene expression [-]. Further studies have focused on designing and evaluating internet- or tele-delivered approaches that may facilitate more scalable and accessible interventions for chronic loneliness. A recent randomized controlled trial compared ICBT and internet-based interpersonal psychotherapy (IIPT) and demonstrated a significantly greater efficacy of ICBT than IIPT in reducing loneliness []. Similarly, a short-term tele-delivered intervention that aimed at facilitating social connectedness showed promising results in older adults by reducing feelings of loneliness and depression []. CBT and group therapy sessions also significantly increased social support and decreased depression scores after coronary heart disease []. Nevertheless, one-to-one peer support did not significantly reduce readmission rates in the year after discharge from inpatient psychiatric care [], indicating that more specific interventions may be required. Overall, there is growing evidence that behavioral and psychological interventions targeting loneliness are an effective way to increase the feeling of social connectedness and additionally reduce harmful health effects. Despite increasing evidence demonstrating the efficacy of behavioral interventions for loneliness, the brain-based mechanisms mediating interventional effects have not been examined. Future prospective studies are needed to differentiate predisposing brain alterations that render individuals vulnerable to chronic loneliness from alterations as a consequence of prolonged loneliness and those that normalize during the course of successful treatment. Based on the notion of loneliness as biosocial pathogenesis, longitudinal studies are required to distinguish whether loneliness-related neural changes reflect damage as a direct consequence of excessive exposure to this stressor or adaptive processes which shape the brain in an experience-dependent plastic manner to cope with the negatively perceived social environment []. Similar approaches lead to a better understanding of the neural mechanisms in childhood maltreatment and should be adapted in future loneliness research [].
Moreover, a better understanding of the neurocognitive mechanisms mediating chronic loneliness opens up novel opportunities to enhance the efficacy of loneliness interventions by targeting the underlying brain circuits. Loneliness-related functional and structural brain changes are evident in various neural circuits of social and affective brain systems, including limbic regions such as the amygdala, hippocampus, and the anterior insula, as well as striatal, prefrontal, and temporal regions (Fig. 2). Alterations in the underlying brain circuits have been associated with detrimental effects of loneliness in various functional domains, which appear to be distinct from the consequences of depression [] and social anxiety []. Therapy outcomes may be improved when interventions focus on multiple functional domains and the related neural targets. For instance, accumulating evidence from basic research and proof-of-concept studies suggests that targeting hormonal systems such as the oxytocin or vasopressin system may have the potential to facilitate social functioning in relevant domains in both healthy individuals and patients with mental disorders []. A single intranasal dose of oxytocin reduced aversive anticipation in high anxious individuals [] and prevented sensitization towards angry faces [] via reducing amygdala reactivity. Furthermore, oxytocin was found to enhance approach behavior towards positive social stimuli by modulating responsivity of the anterior insula [, ]. Both single-dose administrations of oxytocin and vasopressin may enhance the salience of social stimuli and decrease reactivity towards negative social feedback [, ]. Although neuropeptide treatment effects in these domains may vary as a function of dosage [, ], treatment expectation [-], and sex [-], the adjunct administration in combination with behavioral interventions may represent a promising venue to enhance the efficacy of loneliness interventions. Likewise, the endogenous oxytocin response to positive social interactions seems to be attenuated in high-lonely individuals [], but repeated exposure to situations that have been found to induce the release of endogenous oxytocin such as massage, choir singing, or interpersonal synchronized behavior may reinstate normal neurohormonal responses [-].
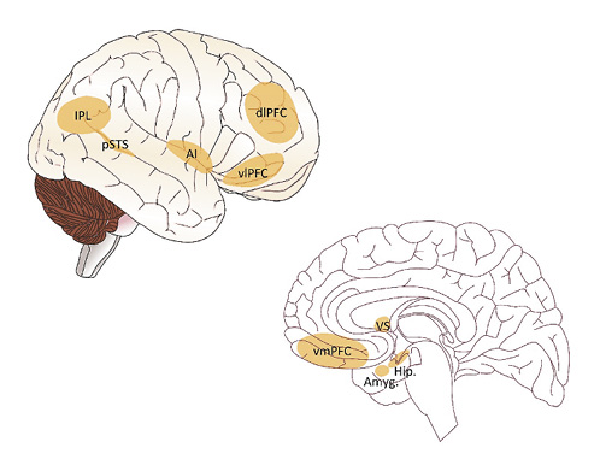
Fig. 2
Illustration of brain areas involved in loneliness. Chronic loneliness has been associated with functional and structural changes in various neural circuits of social and affective brain systems, including limbic regions such as the amygdala, hippocampus, and the anterior insula, as well as striatal, prefrontal, and temporal regions. Amyg., amygdala; dlPFC, dorsolateral prefrontal cortex; Hip., hippocampus; IPL, inferior parietal lobule; AI, anterior insula; VS, ventral striatum; vlPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; pSTS, posterior superior temporal sulcus. Source of the brain template picture used to display the brain regions from https://scidraw.io/ (shared under the creative commons license CC-BY license).
Conclusion
Taken together, loneliness is a crucial and modifiable risk factor for physical and mental health. A better understanding of the neural underpinnings of social (dis)connectedness can help boost the efficiency of loneliness interventions not only in healthy participants but also in patients with mental disorders.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Dr. Scheele is supported by an Else-Kröner-Fresenius-Stiftung grant (2017_A35).
Author Contributions
M.M., X.L., R.H., B.B and D.S. all made substantial contributions to the conception of this paper. M.M. and D.S. drafted this paper, and X.L. and B.B. contributed critical revisions for intellectual content. All of the authors had final approval of all of the submitted versions, and all are in agreement to be accountable for all aspects of this work. A preprint of the manuscript has been posted on the Psyarxiv repository (DOI: 10.31234/osf.io/r4f9e).
References
- 1. Siu HC, Lee SH, Au JS, Lo APK, Huang CM, Tsai YF, et al. Loneliness and major depressive disorder in the elderly with a history of suicidal ideation or attempt: a comment on “therapist-guided internet-based treatments for loneliness” by Käll et al. Psychother Psychosom. 2022;91(2):142–4.
- 2. Käll A, Bäck M, Welin C, Åman H, Bjerkander R, Wänman M, et al. Therapist-guided internet-based treatments for loneliness: a randomized controlled three-arm trial comparing cognitive behavioral therapy and interpersonal psychotherapy. Psychother Psychosom. 2021;90(5):351–8.
- 3. DiJulio B, Hamel L, Munana C, Brodie M. Loneliness and social isolation in the United States, the United Kingdom, and Japan: an international survey. San Francisco, CA: Kaiser Family Foundation; 2018.
- 4. Luhmann M, Hawkley LC. Age differences in loneliness from late adolescence to oldest old age. Dev Psychol. 2016 Jun;52(6):943–59.
- 5. Killgore WDS, Cloonan SA, Taylor EC, Lucas DA, Dailey NS. Loneliness during the first half-year of COVID-19 lockdowns. Psychiatry Res. 2020 Dec;294:113551.
- 6. Killgore WDS, Cloonan SA, Taylor EC, Dailey NS. Loneliness: a signature mental health concern in the era of COVID-19. Psychiatry Res. 2020 Aug;290:113117.
- 7. Killgore WDS, Cloonan SA, Taylor EC, Miller MA, Dailey NS. Three months of loneliness during the COVID-19 lockdown. Psychiatry Res. 2020 Nov;293:113392.
- 8. Cacioppo JT, Cacioppo S. The growing problem of loneliness. Lancet. 2018 Feb;391:426.
- 9. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010 Oct;40:218–27.
- 10. Peplau LA, Perlman D. Loneliness: a sourcebook of current theory, research and therapy. Hoboken, NJ: John Wiley & Sons Inc.; 1982.
- 11. House JS, Kahn RL, McLeod JD, Williams D. Measures and concepts of social support; social support and health. New York: Academic Press; 1985. p. 83–108.
- 12. Woloshin S, Schwartz LM, Tosteson ANA, Chang CH, Wright B, Plohman J, et al. Perceived adequacy of tangible social support and health outcomes in patients with coronary artery disease. J Gen Inter Med. 1997 Oct;12:613–8.
- 13. Hanson BS, Isacsson SO, Janzon L, Lindell SE. Social network and social support influence mortality in elderly men: prospective population study of “men born in 1914” Malmö, Sweden. Am J Epidemiol. 1989 Jul;130:100–11.
- 14. Hedblad B, Östergren PO, Hanson BS, Janzon L. Influence of social support on cardiac event rate in men with ischaemic type ST segment depression during ambulatory 24-h long-term ECG recording: the prospective population study “Men born in 1914,” Mahnö, Sweden. Eur Heart J. 1992 Apr;13:433–9.
- 15. Cacioppo JT, Cacioppo S, Boomsma DI. Evolutionary mechanisms for loneliness. Cogn Emot. 2014;28(1):3–21.
- 16. Inagaki TK, Muscatell KA, Moieni M, Dutcher JM, Jevtic I, Irwin MR, et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc Cogn Affect Neurosci. 2016 Jul;11(7):1096–101.
- 17. Qualter P, Vanhalst J, Harris R, Van Roekel E, Lodder G, Bangee M, et al. Loneliness across the life span. Perspect Psychol Sci. 2015 Mar;10(2):250–64.
- 18. Tomova L, Wang KL, Thompson T, Matthews GA, Takahashi A, Tye KM, et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat Neurosci. 2020 Dec;23(12):1597–605.
- 19. Spithoven AWM, Bijttebier P, Goossens L. It is all in their mind: a review on information processing bias in lonely individuals. Clin Psychol Rev. 2017 Dec;58:97–114.
- 20. Roddick CM, Chen FS. Effects of chronic and state loneliness on heart rate variability in women. Ann Behav Med. 2021 May;55(5):460–75.
- 21. Vanhalst J, Soenens B, Luyckx K, Van Petegem S, Weeks MS, Asher SR. Why do the lonely stay lonely? Chronically lonely adolescents’ attributions and emotions in situations of social inclusion and exclusion. J Pers Soc Psychol. 2015 Nov;109(5):932–48.
- 22. Saporta N, Scheele D, Lieberz J, Stuhr-Wulff F, Hurlemann R, Shamay-Tsoory SG. Opposing association of situational and chronic loneliness with interpersonal distance. Brain Sci. 2021 Aug;11(9):1135.
- 23. Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003 Oct;302(5643):290–2.
- 24. MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005 Mar;131(2):202–23.
- 25. Mwilambwe-Tshilobo L, Spreng RN. Social exclusion reliably engages the default network: a meta-analysis of cyberball. NeuroImage. 2021 Feb;227:117666.
- 26. Shiovitz-Ezra S, Ayalon L. Situational versus chronic loneliness as risk factors for all-cause mortality. Int Psychogeriatr. 2010 May;22(3):455–62.
- 27. McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993 Sep;153(18):2093–101.
- 28. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998 Jan;338(3):171–9.
- 29. Seeman T, Glei D, Goldman N, Weinstein M, Singer B, Lin YH. Social relationships and allostatic load in Taiwanese elderly and near elderly. Soc Sci Med. 2004 Dec;59(11):2245–57.
- 30. Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002 May–Jun;64(3):395–406.
- 31. Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, Seeman TE. Social relationships and allostatic load in the MIDUS study. Health Psychol. 2014 Nov;33(11):1373–81.
- 32. Guidi J, Lucente M, Sonino N, Fava GA. Allostatic load and its impact on health: a systematic review. Psychother Psychosom. 2021;90(1):11–27.
- 33. Day FR, Ong KK, Perry JRB. Elucidating the genetic basis of social interaction and isolation. Nat Commun. 2018 Jul;9(1):2457.
- 34. Gao J, Davis LK, Hart AB, Sanchez-Roige S, Han L, Cacioppo JT, et al. Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology. 2017 Mar;42(4):811–21.
- 35. Abdellaoui A, Chen HY, Willemsen G, Ehli EA, Davies GE, Verweij KJ, et al. Associations between loneliness and personality are mostly driven by a genetic association with neuroticism. J Pers. 2019 Apr;87(2):386–97.
- 36. Horwitz RI, Singer BH, Hayes-Conroy A, Cullen MR, Mawn M, Colella K, et al. Biosocial pathogenesis. Psychother Psychosom. 2022;91(2):73–7.
- 37. Quadt L, Esposito G, Critchley HD, Garfinkel SN. Brain-body interactions underlying the association of loneliness with mental and physical health. Neurosci Biobehav Rev. 2020 Sep;116:283–300.
- 38. Luchetti M, Lee JH, Aschwanden D, Sesker A, Strickhouser JE, Terracciano A, et al. The trajectory of loneliness in response to COVID-19. Am Psychol. 2020 Oct;75(7):897–908.
- 39. Hopf D, Schneider E, Aguilar-Raab C, Scheele D, Ditzen B, Eckstein M. Loneliness and diurnal cortisol levels during COVID-19 lockdown: the roles of living situation, relationship status and relationship quality. medRxiv. 2022 Feb.
- 40. Rico-Uribe LA, Caballero FF, Martín-María N, Cabello M, Ayuso-Mateos JL, Miret M. Association of loneliness with all-cause mortality: a meta-analysis. PLoS One. 2018 Jan;13(1):e0190033.
- 41. Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006 Aug;160(8):805–11.
- 42. Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000 Jul–Aug;14(6):362–70.
- 43. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010 Jul;7(7):e1000316.
- 44. Kiecolt-Glaser JK, Garner W, Speicher C, Penn GM, Holliday J, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosom Med. 1984 Jan–Feb;46(1):7–14.
- 45. Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005 May;24(3):297–306.
- 46. Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004 Jun;29(5):593–611.
- 47. Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006 Mar;21(1):152–64.
- 48. Hawkley LC, Thisted RA, Masi CM, Cacioppo JT. Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol Aging. 2010 Mar;25(1):132–41.
- 49. Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016 Jul;102(13):1009–16.
- 50. Golaszewski NM, LaCroix AZ, Godino JG, Allison MA, Manson JE, King JJ, et al. Evaluation of social isolation, loneliness, and cardiovascular disease among older women in the US. JAMA Netw Open. 2022 Feb;5(2):e2146461.
- 51. Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychol. 2009 May;28(3):354–63.
- 52. Mason TB. Loneliness, eating, and body mass index in parent-adolescent dyads from the Family Life, Activity, Sun, Health, and Eating Study. Pers Relationship. 2020;27(2):420–32.
- 53. Lauder W, Mummery K, Jones M, Caperchione C. A comparison of health behaviours in lonely and non-lonely populations. Psychol Health Med. 2006 May;11(2):233–45.
- 54. Matthews T, Danese A, Gregory AM, Caspi A, Moffitt TE, Arseneault L. Sleeping with one eye open: loneliness and sleep quality in young adults. Psychol Med. 2017 Sep;47(12):2177–86.
- 55. Segrin C, Burke TJ. Loneliness and sleep quality: dyadic effects and stress effects. Behav Sleep Med. 2015;13(3):241–54.
- 56. Ben Simon E, Walker MP. Sleep loss causes social withdrawal and loneliness. Nat Commun. 2018 Aug;9(1):3146.
- 57. Holwerda TJ, Deeg D, Beekman ATF, van Tilburg TG, Stek ML, Jonker C, et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL). J Neurol Neurosurg Psychiatry. 2014 Feb;85(2):135–42.
- 58. Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007 Feb;64(2):234–40.
- 59. Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, et al. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 2017 Mar;17(1):97.
- 60. Erzen E, Çikrikci Ö. The effect of loneliness on depression: a meta-analysis. Int J Soc Psychiatry. 2018 Aug;64(5):427–35.
- 61. Eres R, Lim MH, Lanham S, Jillard C, Bates G. Loneliness and emotion regulation: implications of having social anxiety disorder. Aust J Psychol. 2021;73(1):46–56.
- 62. Richardson T, Elliott P, Roberts R. Relationship between loneliness and mental health in students. J Public Ment Health. 2017;16(2):48–54.
- 63. Morr M, Lieberz J, Dobbelstein M, Philipsen A, Hurlemann R, Scheele D. Insula reactivity mediates subjective isolation stress in alexithymia. Sci Rep. 2021 Jul;11(1):15326.
- 64. Åkerlind I, Hörnquist JO. Loneliness and alcohol abuse: a review of evidences of an interplay. Soc Sci Med. 1992 Feb;34(4):405–14.
- 65. García-Montes JM, Zaldívar-Basurto F, López-Ríos F, Molina-Moreno A. The role of personality variables in drug abuse in a Spanish University population. Int J Ment Health Addict. 2009;7:475–87.
- 66. Bragard E, Giorgi S, Juneau P, Curtis BL. Loneliness and daily alcohol consumption during the COVID-19 pandemic. Alcohol Alcohol. 2021 Mar;57(2):198–202.
- 67. Liebke L, Bungert M, Thome J, Hauschild S, Gescher DM, Schmahl C, et al. Loneliness, social networks, and social functioning in borderline personality disorder. Pers Disord. 2017 Oct;8(4):349–56.
- 68. Hauschild S, Winter D, Thome J, Liebke L, Schmahl C, Bohus M, et al. Behavioural mimicry and loneliness in borderline personality disorder. Compr Psychiatry. 2018 Apr;82:30–6.
- 69. Martens WHJ. Schizoid personality disorder linked to unbearable and inescapable loneliness. Eur J Psychiat. 2010;24(1):38–45.
- 70. O’Connor M. A longitudinal study of PTSD in the elderly bereaved: prevalence and predictors. Aging Ment Health. 2010 Apr;14(3):310–8.
- 71. Lee B, Youm Y. Social network effects on post-traumatic stress disorder (PTSD) in female North Korean immigrants. J Prev Med Public Health. 2011 Sep;44(5):191–200.
- 72. Scheele D, Lieberz J, Goertzen-Patin A, Engels C, Schneider L, Stoffel-Wagner B, et al. Trauma disclosure moderates the effects of oxytocin on intrusions and neural responses to fear. Psychother Psychosom. 2019;88(1):61–4.
- 73. Morr M, Noell J, Sassin D, Daniels J, Philipsen A, Becker B, et al. Lonely in the dark: trauma memory and sex-specific dysregulation of amygdala reactivity to fear signals. Adv Sci. 2022;e2105336. DOI:10.1002/advs.202105336
- 74. Stahn AC, Gunga HC, Kohlberg E, Gallinat J, Dinges DF, Kühn S. Brain changes in response to long Antarctic expeditions. N Engl J Med. 2019 Dec;381(23):2273–5.
- 75. Biggio F, Mostallino MC, Talani G, Locci V, Mostallino R, Calandra G, et al. Social enrichment reverses the isolation-induced deficits of neuronal plasticity in the hippocampus of male rats. Neuropharmacology. 2019 Jun;151:45–54.
- 76. Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011 Feb;14(2):163–4.
- 77. Lin C, Keles U, Tyszka JM, Gallo M, Paul L, Adolphs R. No strong evidence that social network index is associated with gray matter volume from a data-driven investigation. Cortex. 2020 Apr;125:307–17.
- 78. Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012 Jul;72(1):70–7.
- 79. Mihov Y, Kendrick KM, Becker B, Zschernack J, Reich H, Maier W, et al. Mirroring fear in the absence of a functional amygdala. Biol Psychiatry. 2013 Apr;73(7):e9–11.
- 80. Scheele D, Zimbal S, Feinstein JS, Delis A, Neumann C, Mielacher C, et al. Treatment-resistant depression and ketamine response in a patient with bilateral amygdala damage. Am J Psychiatry. 2019 Dec;176(12):982–6.
- 81. Kiesow H, Dunbar RI, Kable JW, Kalenscher T, Vogeley K, Schilbach L, et al. 10,000 Social brains: sex differentiation in human brain anatomy. Sci Adv. 2020 Mar;6(12):eaaz1170.
- 82. Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, et al. The default network of the human brain is associated with perceived social isolation. Nature Commun. 2020 Dec;11(1):1–11.
- 83. Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. Brain structure links loneliness to social perception. Curr Biol. 2012 Oct;22(20):1975–9.
- 84. Lieberz J, Shamay-Tsoory SG, Saporta N, Kanterman A, Gorni J, Esser T, et al. Behavioral and neural dissociation of social anxiety and loneliness. J Neurosci. 2022;42:2570–83.
- 85. Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009 Oct;13(10):447–54.
- 86. Courtney AL, Meyer ML. Self-other representation in the social brain reflects social connection. J Neurosci. 2020 Jul;40(29):5616–27.
- 87. Lieberz J, Shamay-Tsoory SG, Saporta N, Esser T, Kuskova E, Stoffel-Wagner B, et al. Loneliness and the social brain: how perceived social isolation impairs human interactions. Adv Sci. 2021 Nov;8(21):e2102076.
- 88. Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012 May;13(6):421–34.
- 89. Arnold AJ, Winkielman P, Dobkins K. Interoception and social connection. Front Psychol. 2019 Nov;10:2589.
- 90. Mwilambwe-Tshilobo L, Ge T, Chong M, Ferguson MA, Misic B, Burrow AL, et al. Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain. Soc Cogni Affect Neurosci. 2019 May;14(4):423–33.
- 91. Zhou H-X, Chen X, Shen Y-Q, Li L, Chen N-X, Zhu Z-C, et al. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. NeuroImage. 2020 Feb;206:116287.
- 92. Zajner C, Spreng RN, Bzdok D. Loneliness is linked to specific subregional alterations in hippocampus-default network covariation. J Neurophysiol. 2021 Dec;126(6):2138–57.
- 93. Saporta N, Scheele D, Lieberz J, Nevat M, Kanterman A, Hurlemann R, et al. Altered activation in the action observation system during synchronization in high loneliness individuals. Cerebral Cortex. 2022 Feb:bhac073. Epub ahead of print.
- 94. Tian Y, Yang L, Chen S, Guo D, Ding Z, Tam KY, et al. Causal interactions in resting-state networks predict perceived loneliness. PLoS One. 2017 May;12(5):e0177443.
- 95. von Mohr M, Kirsch LP, Fotopoulou A. Social touch deprivation during COVID-19: effects on psychological wellbeing and craving interpersonal touch. R Soc Open Sci. 2021 Sep;8(9):210287.
- 96. Saporta N, Peled-Avron L, Scheele D, Lieberz J, Hurlemann R, Shamay-Tsoory SG. Touched by loneliness – how loneliness impacts the response to observed human touch: a tDCS Study. Soc Cogn Affect Neurosci. 2021 Feb;17(1):142–50.
- 97. Blomkvist A, Hofer M. Olfactory impairment and close social relationships. a narrative review. Chem Senses. 2021 Jan;46:bjab037.
- 98. Maier A, Heinen-Ludwig L, Güntürkün O, Hurlemann R, Scheele D. Childhood maltreatment alters the neural processing of chemosensory stress signals. Front Psychiatry. 2020 Aug;11:783.
- 99. Layden EA, Cacioppo JT, Cacioppo S, Cappa SF, Dodich A, Falini A, et al. Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. NeuroImage. 2017 Jan;145(Pt A):58–73.
- 100. Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J Cogn Neurosci. 2009 Jan;21(1):83–92.
- 101. D’Agostino AE, Kattan D, Canli T. An fMRI study of loneliness in younger and older adults. Soc Neurosci. 2019 Apr;14(2):136–48.
- 102. Schalbroeck R, van Velden FHP, de Geus-Oei LF, Yaqub M, van Amelsvoort T, Booij J, et al. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: an [(18)F]-FDOPA PET/CT study. Transl Psychiatry. 2021 Jan;11(1):47.
- 103. Yin J, Lassale C, Steptoe A, Cadar D. Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: the English longitudinal study of ageing. Int J Epidemiol. 2019 Dec;48(6):1937–48.
- 104. McHugh Power JE, Steptoe A, Kee F, Lawlor BA. Loneliness and social engagement in older adults: a bivariate dual change score analysis. Psychol Aging. 2019 Feb;34(1):152–62.
- 105. Rosenstreich E, Margalit M. Loneliness, mindfulness, and academic achievements: a moderation effect among first-year college students. Open Psychol J. 2015 May;8(1):138–45.
- 106. Gao M, Shao R, Huang C-M, Liu H-L, Chen Y-L, Lee S-H, et al. The relationship between loneliness and working-memory-related frontoparietal network connectivity in people with major depressive disorder. Behav Brain Res. 2020 Sep;393:112776.
- 107. Spithoven AWM, Cacioppo S, Goossens L, Cacioppo JT. Genetic contributions to loneliness and their relevance to the evolutionary theory of loneliness. Perspect Psychol Sci. 2019 May;14(3):376–96.
- 108. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009 Apr;62(1):42–52.
- 109. Spreng RN, Bzdok D. Loneliness and neurocognitive aging. Adv Geriatr Med Res. 2021;3(2):e210009.
- 110. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. NeuroImage. 2019 Oct;200:313–31.
- 111. Yan C-G, Chen X, Li L, Castellanos FX, Bai T-J, Bo Q-J, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci USA. 2019 Apr;116(18):9078–83.
- 112. Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. NeuroImage. 2018 Aug;176:489–98.
- 113. Viard A, Mutlu J, Chanraud S, Guenolé F, Egler P-J, Gérardin P, et al. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage Clin. 2019;22:101731.
- 114. Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010 Jun;214(5–6):451–63.
- 115. Smith R, Feinstein JS, Kuplicki R, Forthman KL, Stewart JL, Paulus MP, et al. Perceptual insensitivity to the modulation of interoceptive signals in depression, anxiety, and substance use disorders. Sci Rep. 2021 Jan;11(1):2108.
- 116. Mattson WI, Hyde LW, Shaw DS, Forbes EE, Monk CS. Clinical neuroprediction. Amygdala reactivity predicts depressive symptoms 2 years later. Soc Cogn Affect Neurosci. 2016;11:892–8.
- 117. Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017 Jun;81(12):1023–9.
- 118. Wackerhagen C, Veer IM, Erk S, Mohnke S, Lett TA, Wüstenberg T, et al. Amygdala functional connectivity in major depression: disentangling markers of pathology, risk and resilience. Psychol Med. 2020 Dec;50(16):2740–50.
- 119. Williams T, Lakhani A, Spelten E. Interventions to reduce loneliness and social isolation in rural settings: a mixed-methods review. J Rural Stud. 2022 Feb;90:76–92.
- 120. Osborn T, Weatherburn P, French RS. Interventions to address loneliness and social isolation in young people: a systematic review of the evidence on acceptability and effectiveness. J Adolesc. 2021 Dec;93:53–79.
- 121. Williams CYK, Townson AT, Kapur M, Ferreira AF, Nunn R, Galante J, et al. Interventions to reduce social isolation and loneliness during COVID-19 physical distancing measures: a rapid systematic review. PLoS One. 2021 Feb;16(2):e0247139.
- 122. Fakoya OA, McCorry NK, Donnelly M. Loneliness and social isolation interventions for older adults: a scoping review of reviews. BMC Public Health. 2020 Feb;20(1):129.
- 123. Gyasi RM, Phillips DR, Asante F, Boateng S. Physical activity and predictors of loneliness in community-dwelling older adults: the role of social connectedness. Geriatr Nurs. 2021 Mar–Apr;42(2):592–8.
- 124. Sebastião E, Mirda D. Group-based physical activity as a means to reduce social isolation and loneliness among older adults. Aging Clin Exp Res. 2021 Jul;33(7):2003–6.
- 125. Franke T, Sims-Gould J, Nettlefold L, Ottoni C, McKay HA. It makes me feel not so alone”: features of the choose to move physical activity intervention that reduce loneliness in older adults. BMC Public Health. 2021 Feb;21(1):1–15.
- 126. Boucher EM, McNaughton EC, Harake N, Stafford JL, Parks AC. The impact of a digital intervention (happify) on loneliness during COVID-19: Qualitative Focus Group. JMIR Ment Health. 2021 Feb;8(2):e26617.
- 127. Shapira S, Yeshua-Katz D, Cohn-Schwartz E, Aharonson-Daniel L, Sarid O, Clarfield AM. A pilot randomized controlled trial of a group intervention via Zoom to relieve loneliness and depressive symptoms among older persons during the COVID-19 outbreak. Internet Interv. 2021 Apr;24:100368.
- 128. Kramer LL, Mulder BC, van Velsen L, de Vet E. Use and effect of web-based embodied conversational agents for improving eating behavior and decreasing loneliness among community-dwelling older adults: protocol for a randomized controlled trial. JMIR Res Protoc. 2021 Jan;10(1):e22186.
- 129. Gasteiger N, Loveys K, Law M, Broadbent E. Friends from the future: a scoping review of research into robots and computer agents to combat loneliness in older people. Clin Interv Aging. 2021 May;16:941–71.
- 130. Follmann A, Schollemann F, Arnolds A, Weismann P, Laurentius T, Rossaint R, et al. Reducing loneliness in stationary geriatric care with robots and virtual encounters: a contribution to the COVID-19 pandemic. Int J Environ Res Public Health. 2021 May;18(9):4846.
- 131. Nieminen T, Prättälä R, Martelin T, Härkänen T, Hyyppä MT, Alanen E, et al. Social capital, health behaviours and health: a population-based associational study. BMC Public Health. 2013 Jun;13:613.
- 132. Mays AM, Kim S, Rosales K, Au T, Rosen S. The leveraging exercise to age in place (LEAP) study: engaging older adults in community-based exercise classes to impact loneliness and social isolation. Am J Geriatr Psychiatry. 2021 Aug;29(8):777–88.
- 133. Kotwal AA, Fuller SM, Myers JJ, Hill D, Tha SH, Smith AK, et al. A peer intervention reduces loneliness and improves social well-being in low-income older adults: a mixed-methods study. J Am Geriatr Soc. 2021 Dec;69(12):3365–76.
- 134. Zagic D, Wuthrich VM, Rapee RM, Wolters N. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2021 Nov. Epub ahead of print. http://dx.doi.org/10.1007/s00127-021-02191-w.
- 135. Jarvis MA, Padmanabhanunni A, Chipps J. An evaluation of a low-intensity cognitive behavioral therapy mhealth-supported intervention to reduce loneliness in older people. Int J Environ Res Public Health. 2019 Apr;16(7):1305.
- 136. Theeke LA, Mallow JA, Moore J, McBurney A, Rellick S, VanGilder R. Effectiveness of LISTEN on loneliness, neuroimmunological stress response, psychosocial functioning, quality of life, and physical health measures of chronic illness. Int J Nurs Sci. 2016 Sep;3(3):242–51.
- 137. Lindsay EK, Young S, Brown KW, Smyth JM, Creswell JD. Mindfulness training reduces loneliness and increases social contact in a randomized controlled trial. Proc Natl Acad Sci U S A. 2019 Feb;116(9):3488–93.
- 138. Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JMG, Ma J, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012 Oct;26(7):1095–101.
- 139. Zhang N, Fan FM, Huang SY, Rodriguez MA. Mindfulness training for loneliness among Chinese college students: a pilot randomized controlled trial. Int J Psychol. 2018 Oct;53(5):373–8.
- 140. Choi NG, Pepin R, Marti CN, Stevens CJ, Bruce ML. Improving social connectedness for homebound older adults: randomized controlled trial of tele-delivered behavioral activation versus tele-delivered friendly visits. Am J Geriatr Psychiatry. 2020 Jul;28(7):698–708.
- 141. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA. 2003 Jun;289(23):3106–16.
- 142. Gillard S, Bremner S, Patel A, Goldsmith L, Marks J, Foster R, et al. Peer support for discharge from inpatient mental health care versus care as usual in England (ENRICH): a parallel, two-group, individually randomised controlled trial. Lancet Psychiatry. 2022 Feb;9(2):125–36.
- 143. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016 Sep;17(10):652–66.
- 144. Shao R, Liu H-L, Huang C-M, Chen Y-L, Gao M, Lee S-H, et al. Loneliness and depression dissociated on parietal-centered networks in cognitive and resting states. Psychol Med. 2020 Dec;50(16):2691–701.
- 145. Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol Psychiatry. 2021 Jan;26(1):80–91.
- 146. Xin F, Zhou X, Dong D, Zhao Z, Yang X, Wang Q, et al. Oxytocin differentially modulates amygdala responses during top‐down and bottom‐up aversive anticipation. Adv Sci. 2020 Jul;7(16):2001077.
- 147. Liu C, Lan C, Li K, Zhou F, Yao S, Xu L, et al. Oxytocinergic modulation of threat-specific amygdala sensitization in humans is critically mediated by serotonergic mechanisms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 Nov;6(11):1081–9.
- 148. Yao S, Zhao W, Geng Y, Chen Y, Zhao Z, Ma X, et al. Oxytocin facilitates approach behavior to positive social stimuli via decreasing anterior insula activity. Int J Neuropsychopharmacol. 2018 Oct;21(10):918–25.
- 149. Scheele D, Kendrick KM, Khouri C, Kretzer E, Schläpfer TE, Stoffel-Wagner B, et al. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology. 2014 Aug;39(9):2078–85.
- 150. Gozzi M, Dashow EM, Thurm A, Swedo SE, Zink CF. Effects of oxytocin and vasopressin on preferential brain responses to negative social feedback. Neuropsychopharmacology. 2017 Jun;42(7):1409–19.
- 151. Zhuang Q, Zheng X, Becker B, Lei W, Xu X, Kendrick KM. Intranasal vasopressin like oxytocin increases social attention by influencing top-down control, but additionally enhances bottom-up control. Psychoneuroendocrinology. 2021 Nov;133:105412.
- 152. Kou J, Zhang Y, Zhou F, Gao Z, Yao S, Zhao W, et al Anxiolytic effects of chronic intranasal oxytocin on neural responses to threat are dose-frequency dependent. Psychother Psychosom. 2022:1–12. Epub ahead of print. (doi: doi: DOI: 10.1159/000521348 )
- 153. Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, et al. Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biol Psychiatry. 2017 Dec;82(12):885–94.
- 154. Liu C, Huang Y, Chen L, Yu R. Lack of evidence for the effect of oxytocin on placebo analgesia and nocebo hyperalgesia. Psychother Psychosom. 2020;89(3):185–7.
- 155. Zhao W, Becker B, Yao S, Ma X, Kou J, Kendrick KM. Oxytocin enhancement of the placebo effect may be a novel therapy for working memory impairments. Psychother Psychosom. 2019;88(2):125–6.
- 156. Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry. 2016 May;79(10):794–802.
- 157. Ma X, Zhao W, Luo R, Zhou F, Geng Y, Xu L, et al. Sex-and context-dependent effects of oxytocin on social sharing. NeuroImage. 2018 Dec;183:62–72.
- 158. Lieberz J, Scheele D, Spengler FB, Matheisen T, Schneider L, Stoffel-Wagner B, et al. Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology. 2020 Jun;45(7):1134–40.
- 159. Coenjaerts M, Trimborn I, Adrovic B, Stoffel-Wagner B, Cahill L, Philipsen A, et al. Estradiol and oxytocin modulate sex differences in hippocampal reactivity and episodic memory. bioRxiv. 2021 Nov.
- 160. Kreutz G. Does singing facilitate social bonding?Music Med. 2014;6(2):51–60.
- 161. Spengler FB, Scheele D, Marsh N, Kofferath C, Flach A, Schwarz S, et al. Oxytocin facilitates reciprocity in social communication. Soc Cogn Affect Neurosci. 2017 Aug;12(8):1325–33.
- 162. Li Q, Becker B, Wernicke J, Chen Y, Zhang Y, Li R, et al. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology. 2019 Mar;101:193–203.
B.B. and D.S. contributed equally.
