Introduction
The increase in life expectancy and the progressive aging of the general population are phenomena occurring worldwide (). Although cohort comparisons suggest that the debilitating effects of senescence are delayed by a decade, advanced adulthood continues to be associated with functional decline and health status problems, with important implications for individuals, society, and healthcare systems ().
In this scenario, emerging preventive approaches highlighted the need to address the promotion of brain and body health, in contrast with the classical risk reduction model aimed at reducing disease incidence (; ). This paradigm shift is further supported by the fact that some of the most prevalent age-related diseases share the same risk factors, such as stroke, heart disease, dementia, and disability (; ). Thus, it is of great scientific interest and public health utility to investigate the determinants of positive health outcomes in very old individuals (over 80 years of age). Aging is a universal and deleterious process characterized by the progressive accumulation of biological changes that occur over time and which increase the probability of disease and death (). Thus, maintaining health in very old age requires a continuous process of compensation and adaptation to low-intensity biological and psychosocial stressors.
Resilience is the ability to cope with and adapt to adverse events, diseases, and stressful situations. To assess resilience, at least two key factors must be considered: the presence of a stressor and a positive response/adaptation (). The definition of stressor should be chosen depending on the context and goal of resilience research (). A recent systematic review of the conceptual literature on resilience in aging research highlighted the presence of two chief perspectives: the most widely applied, defines resilience as a positive response to a highly intense stressor; whereas the most recent one describes it as the dynamic process of adapting and regaining equilibrium following low-intensity stressors over time (). In line with this latter perspective, we conceptualized resilience during the aging process as the dynamic and multidimensional ability to counteract accumulating physical damage and psychosocial stressors, including COVID-19 infection, maintaining cognitive and mental health, and functional independence in later life. This definition of positive adaptation reflects a multidimensional conceptualization of health.
Conversely, previous studies investigating resilience in older adults have usually considered only one of these aspects (cognitive, psychological, or physical health). Broadly, we can identify three corresponding primary definitions of resilience in aging research (). The psychological resilience construct is derived from developmental psychology and is mostly studied as a protective factor against mental illness, such as depression (). The American Psychological Association defines resilience as “the process and outcome of successfully adapting to difficult or challenging life experiences, especially through mental, emotional, and behavioral flexibility and adjustment to external and internal demands” (). Cognitive resilience is a property of the brain that allows for sustained cognitive performance in the face of age-related changes and neurodegenerative diseases (; ) and accounts for the discrepancy between observed and expected cognitive impairment associated with age or with a given degree of neuropathology (; ). Finally, the construct of physical resilience, which has recently emerged in the biomedical literature, is the ability to recover or optimize function in the face of age-related losses or illnesses (; ).
However, regardless of the outcome considered (cognitive, psychological, and physical), similar methodological limitations have been highlighted in each of the research areas. First, there is no consensus on the terminology and instruments used to assess resilience (; ; ). Most evidence comes from cross-sectional analyses of convenience samples. Finally, overlap and interrelations exist between the different predictors and proxies of resilience; thus, the study designs in the field must address the issues of low specificity, multidirectionality, and collinearity between measurements ().
The present project addresses these limitations by jointly considering cognition, mental health, and functional autonomy as a single comprehensive multidimensional outcome, that we refer to here as the “resilience phenotype.” We were also able to concurrently assess the unique contributions of the main resilience-enhancing factors identified in previous studies. This notable aim was pursued by adopting a multidimensional approach on a population-based sample of the oldest-old (80+), an understudied but growing portion of the global population with increased vulnerabilities and specific care needs ().
Specifically, we formulated two main research questions to achieve our aim:
RQ1. What is the underlying structure of the cognitive, psychological, and physical resilience-enhancing factors in the oldest-old without dementia?
RQ2. To what extent do the factors considered predict the resilience phenotype, controlling for proxies of cumulative exposure to physical and psychosocial stressors?
Methods
Study Design
This was a cross-sectional study on a population-based sample of older adults without dementia and aged between 83 and 87 years who participated in the fifth wave of the InveCe.Ab study, a population-based cohort enrolled in 2009 (Invecchiamento Cerebrale in Abbiategrasso, i.e., Brain Aging in Abbiategrasso; ClinicalTrials.gov, NCT01345110). Here, we mainly used data from the last assessment performed in 2022, which included all relevant variables for the resilience construct. However, in light of our aim, we reconstructed indices of cumulative exposure to stressors using data from the entire study period. Figure 1 shows the study design and measurements used for the present study, within the framework of the InveCe.Ab project.
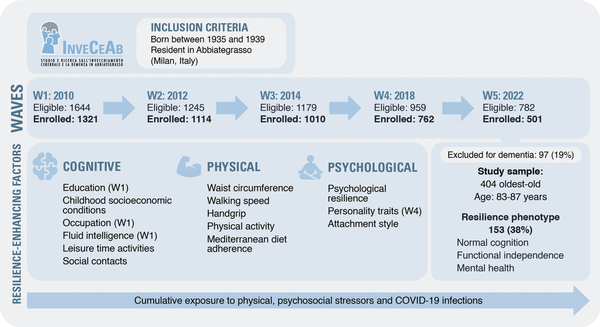
Figure 1
Flow diagram showing the InveCe.Ab study waves, participants selection, and measures of interest of the present study. In the upper part, we show the inclusion criteria for the population-based cohort study “InveCe.Ab.” In the middle, the flow chart of the participants in the five waves is shown. Below, there are the criteria used to define the resilience phenotype and the specific features of the study sample, selected among the participants to the fifth wave. In the resilience-enhancing factors box, we report the measures used as predictors of the resilience phenotype, grouped according to the corresponding domain (cognitive, physical, and psychological). Unless otherwise specified (in brackets), the measures were collected during the fifth wave (W5). In the bottom arrow, “Cumulative exposure to physical and psychosocial stressors” refers to the indices that combine the measures collected during the entire observation period (see “Stressors” subparagraph for details). InveCe.Ab = Invecchiamento Cerebrale in Abbiategrasso (i.e., Brain Aging in Abbiategrasso); COVID-19 = coronavirus disease 2019; W = wave.
Participants and Procedures
Participants were recruited from the InveCe.Ab study, a population-based cohort of people born between 1935 and 1939 who lived in the city of Abbiategrasso when the study began (November 1, 2009). The aim was to estimate the incidence of dementia and to explore sociodemographic, clinical, and lifestyle factors associated with aging and dementia (). Participants enrolled underwent multidimensional assessments at baseline (n = 1,321), and after 2, 4, and 8 years; they engaged in blood sampling, geriatric visits, neuropsychological assessments, and social and lifestyle interviews.
For our purposes, all InveCe.Ab participants (age range: 83–87 years) who were alive and underwent at least one follow-up assessment after baseline were contacted again in 2022 to participate in the fifth wave of the study. When the study began (mid-February 2022), there were 782 eligible participants.
All the eligible individuals received a personalized letter explaining the study’s aims and procedures for the fifth wave. Then, trained personnel called each participant to provide further explanations and, in the case of acceptance, to schedule the appointments for a multidimensional assessment comprising blood sampling at home by a nurse, a social and lifestyle survey by phone lasting approximately 30 min, and in-person visits to the Golgi Cenci Foundation with the neuropsychologist and the physician (geriatrician or neurologist) on the same day, lasting a total of 3 hr. Visits could also be provided at home or in a nursing home to meet older adults’ needs.
The study procedures were carried out in accordance with the principles outlined in the Declaration of Helsinki of 1964 and the relevant amendments. The study was approved by the Ethics Committee of Milano Area 3 on January 19, 2022 (number: 26-19012022). Informed consent was signed before the study visits.
Multidimensional Assessment
The geriatrician collected a guided and detailed medical history supported by clinical documentation, recorded current medications, reviewed the results of the blood tests, and performed a physical examination with special attention to neurologic signs and symptoms. Comorbidity was assessed with the Cumulative Illness Rating Scale (CIRS; ). Functional status was assessed with the Basic Activity of Daily Living (BADL) and Instrumental Activity of Daily Living (IADL) scales (; ).
Neuropsychologists administered the 15-item form of the Geriatric Depression Scale (GDS) and a comprehensive neuropsychological test battery to evaluate global cognition (Mini-Mental State Examination, MMSE) and the main cognitive domains: language (category fluency), memory (Rey-Auditory Verbal Learning Test), attention (attentional matrices), executive functions (Trail Making Test, Raven Coloured Progressive Matrices), visuospatial abilities, and constructional praxis (Clock Drawing Test; Rey-Osterrieth Complex Figure Test). Age- and education-adjusted scores were used according to Italian norms to determine the presence of impairment for each test. See the study protocol for more details on the instruments used ().
After the visits, the neuropsychologist and the physician made independent working diagnoses within the following categories: dementia, based on DSM-V criteria (); mild cognitive impairment, based on the Petersen criteria (); cognitive impairment—not dementia (); clinically relevant depression or subthreshold depression (); and psychosis (ICD10: F20–F29). Participants without cognitive impairment and without major mental disorders (clinically relevant depression or psychosis) were classified as cognitively normal.
After multidimensional assessment completion, an expert geriatrician (A.G.) together with a clinical neuropsychologist (L.P.) reviewed all individual records, and a final diagnosis within the aforementioned categories was reached.
Resilience Phenotype
For the present study, we included all participants without dementia based on the multidimensional assessment performed. Then, we classified resilient individuals as those who maintained normal cognition, mental health, and functional independence in very old age (83–87 years). This represents a positive adaptation to the aging process, considering that all of the participants are oldest-old in a specific age quintile according to the study design. Normal cognition was ascertained by the agreement of the geriatrician and the neuropsychologist who reviewed the individual charts, as specified in the subparagraph on multidimensional assessment. Functional independence was determined if all the BADLs were preserved. Mental health was ascertained by the absence of clinically relevant depression or psychosis at the final diagnosis.
Resilience-Enhancing Factors
During the fifth wave, to enrich the characterization of resilience in aging, we added specific scales to the standard InveCe.Ab instruments are detailed in the study protocol ().
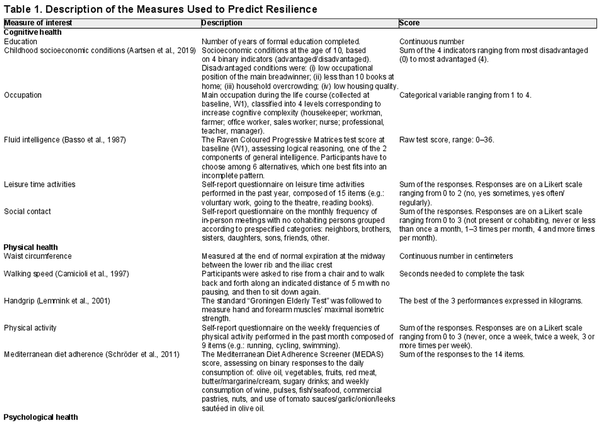
Table 1 displays the measures used as predictors of resilience in the present study. Unless otherwise specified, we used the data collected during the fifth wave (2022). We grouped the measures into the three chief dimensions of cognitive, physical, and psychological health, based on the a priori hypothesis about their potential actions in relation to the different dimensions. In the cognitive domain, we included variables classically used as indirect proxies of cognitive reserve, representing the amount and complexity of cognitive-stimulating activity performed throughout one’s life, such as years of education, occupational level, leisure time cognitive activities, and social contact later in life (). As a measurement of early-life cognitive enrichment, we used the childhood socioeconomic condition indicator (). Moreover, because the intelligence quotient is frequently employed as a cognitive reserve proxy, we used the raw baseline score of the Raven Coloured Progressive Matrices (), which assesses logical reasoning and is considered a measure of fluid intelligence (one of the two components of general intelligence). In the physical health domain, we included self-reported measures of physical activity and Mediterranean diet adherence () due to their established protective effects against several aging-related diseases. We also included waist circumference, handgrip test (), and walking speed test (), in line with recent contributions that suggest measuring physical functionality directly through standardized performance and strength tests, to better capture the real biology of the function ().
Finally, as predictors of psychological health, we chose measurements known to increase individuals’ ability to adapt to adverse and stressful situations and protect against mental illness. The 14-item Resilience Scale measures self-reported adaptive coping styles to counteract adversity, with specific questions on five dimensions (): purpose, perseverance, self-reliance, equanimity, and existential aloneness (authenticity). These are all psychosocial characteristics associated with high resilience among older adults (; ). Personality traits and attachment styles are stable individual traits shaped by gene[FIGURE DASH]environment interactions in early life and are associated with the risk of mental illness during one’s life (). We measured three personality traits using the Eysenck Personality Questionnaire–Revised (EPQ-SF-R; ): extraversion (a tendency to be sociable, carefree, convivial, easy-going, and impulsive), neuroticism (low emotional stability and a predisposition toward experiencing negative affect), and psychoticism (a tendency to be hostile, untrusting, unemotional, lacking in empathy, and unfriendly). Adult attachment styles refer to an individual’s emotional regulation abilities, which arise from the intimate bonds built with caregivers very early during infancy, modulating the internal state and subsequent behaviors in close relationships (). An early supportive and responsive social environment, leading to a secure attachment profile, encourages both mental and cognitive health (). Conversely, insecure attachment has been linked to dysregulated physiological responses to stress, risky health-related behaviors, and susceptibility to serious physical illness ().
Stressors
We used indices based on instruments administered in each wave to gauge the cumulative exposure to physical and psychosocial stressors during the aging process. We also include COVID-19 infection as an important source of stress, especially for the oldest individuals, who are at increased risk of hospitalization and death due to COVID-19 ().
Physical stressors were based on two comorbidity measures: the CIRS severity index and the number of drugs used. The CIRS () is a standardized tool compiled by a physician to rate the level of pathology and impairment of 13 major organ groups and in the psychiatric/behavioral domain on a scale ranging from 1 (no impairment) to 5 (extremely severe impairment). The severity of overall physical illness was computed as the mean of the 13 items (excluding the psychiatric/behavioral domain). For our purposes, we used the average CIRS severity score of all available assessments for each participant.
Moreover, during the geriatric visit, the number of pharmacologically active ingredients consumed in the past 2 weeks was recorded, and a list was compiled of 53 prespecified principles chosen among those drug categories most likely to interfere with cerebral activity. We used the average number of pharmacologically active ingredients recorded in all available assessments.
Psychosocial stressors were represented by the score of the Geriatric Adverse Life Events Scale (GALES) which investigates the stressful life events experienced over the previous 12 months with a 26-item checklist (). For the present study, we measured cumulative exposure to psychosocial stressors as the sum of the adverse events reported in all available assessments.
Finally, as a potential stressor, we included contracting COVID-19, which was assessed as a yes/no question during the geriatric visit in 2022.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics 20.0 (IBM SPSS Statistics for Windows, version 20.0, BM Corp. Released 2011, Armonk, NY, US). Preliminarily, descriptive univariate analysis was performed to compare the resilient and non-resilient subgroups using Student’s t-test for continuous variables and the chi-squared test for categorical ones.
To address our research questions, we performed several steps of analysis. First, we were interested in exploring the underlying structure of the measures considered to be predictors of resilience (see Table 1 for details). To make the statistical analyses more robust and to include the entire sample evaluated, we handled missing data by performing a data imputation through Predictive Mean Matching with Full Conditional Specification (MCMC), which specifies a predictive model for each missing variable considering all other relevant variables to be predictors (i.e., the six factors, sociodemographics, stressors, and the main clinical scales of MMSE, GDS, BADL). This ensures a plausible posterior predictive distribution. Then, we performed an exploratory factor analysis (EFA) because, to the best of our knowledge, no previous study has concurrently assessed all these physical, cognitive, and psychological factors in the same sample of oldest-old individuals. We examined the correlation matrix for randomness using Bartlett’s test of sphericity, and the KMO statistic had to be greater than or equal to 0.50. The correlation matrix was factored using the principal axis factoring method after we determined that it could be factored. We assumed that the factors considered would be correlated, so an oblimin rotation was used. We applied Kaiser’s criterion (an eigenvalue greater than 1) to establish how many factors should be retained. We defined a threshold of 0.3 for variable loadings to justify the inclusion of the factors obtained and eventually rerun the analysis accordingly (). To analyze the factors as new variables, we extracted the scores using Bartlett’s method.
This step of analysis provided answers to RQ1 and reduced the dimensions of the dataset by obtaining a limited number of factors with higher internal consistency and low intercorrelation, thus addressing the issue of multicollinearity among the predictors.
Second, to investigate the relative importance of each factor in explaining resilience (RQ2), we constructed a logistic regression model with the resilience phenotype (1 = yes; 0 = no) as the dependent variable and factor scores as the independent variables, with sociodemographics (age, sex) and stressor indices as covariates. To test the performance of the models, we studied sensitivity and specificity using ROC curves.
Results
Study Sample
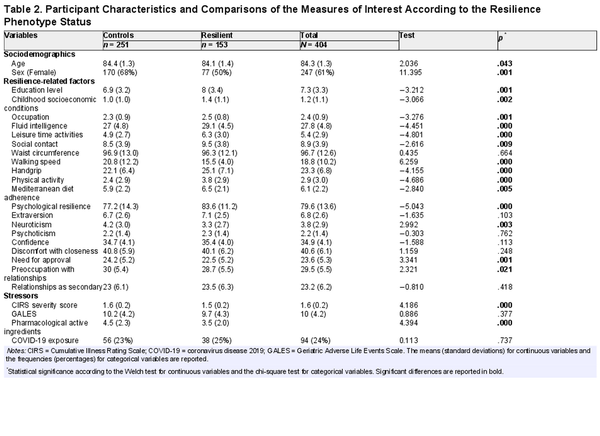
Recruitment took place between mid-February 2022 and January 2023. Among the 782 eligible individuals, 27 died (3.5%), 52 were unreachable (6.6%), and 202 refused to take part (25.8%). Thus, 501 participants (64.1% of the eligible population) participated in the fifth wave, and 97 received a final diagnosis of dementia (19.4%), resulting in a sample of 404 oldest-old individuals without dementia who were eligible for the present investigation. Among them, 153 were categorized as resilient (38%) based on prespecified criteria. Before imputation, 11% of the values in the dataset were missing. Table 2 shows the comparisons of the measures of interest according to resilience status. Resilient individuals were more likely to be male and slightly younger. Overall, they showed a more favorable profile for most of the measures used as predictors of resilience. They were relatively healthier based on the CIRS index and on the number of drugs assumed, whereas no differences were found in terms of exposure to stressful life events or COVID-19 infection.
The Structure of the Resilience-Related Factors
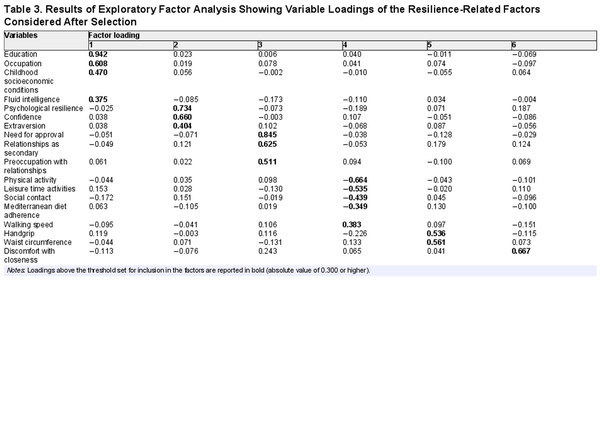
The first EFA on the 20 selected variables resulted in six factors, with two variables showing loadings of less than 0.3 (neuroticism and psychoticism). We reran the EFA excluding these variables, confirming the number and composition of factors resulting from the first analysis.
The correlation matrix was not random according to the results of Bartlett’s test of sphericity: χ2(190) = 1,322.818, p < .001; in addition, the KMO statistic was 0.672, above the minimal threshold for factor analysis. As a result, we decided that factor analysis would be appropriate for the correlation matrix.
Kaiser’s criterion suggests retaining six factors that, after rotation, accounted for 59% of the total variance. Table 3 shows the factor loadings from the pattern matrix. Factor 1 (17% of the total variance) included all the variables related to cognitive enrichment and stimulation in early and middle life, namely, education, occupation, childhood socioeconomic conditions, and fluid intelligence; thus, it was named “cognitive reserve.” Factor 2 (12% of the total variance) grouped psychological resilience, confidence, and extraversion; thus, we named it “affective reserve.” Factor 3 (9% of the total variance) included the need for approval, relationship as secondary, and preoccupation with relationships subscales of the Attachment Style Questionnaire (ASQ); this factor was named “insecure attachment.” Factor 4 (8% of the total variance) was named “current lifestyle” because it included physical activity, leisure time activities, social contact, adherence to the Mediterranean diet, and walking speed. Considering the direction of the loadings, we changed the sign of this factor to improve the interpretative reading of its relationship with the outcome (i.e., higher scores represent a more active lifestyle). Factor 5 (7% of the total variance), including handgrip strength and waist circumference, can be defined as “physical reserve.” Finally, Factor 6 (6% of the total variance) included only the ASQ subscale of discomfort with closeness; thus, it can be considered a measure of “Avoidant Attachment.” The correlation among the factors after rotation ranged between −0.216 and 0.183 (see Supplementary Table S1).
Predictors of Resilience Phenotype
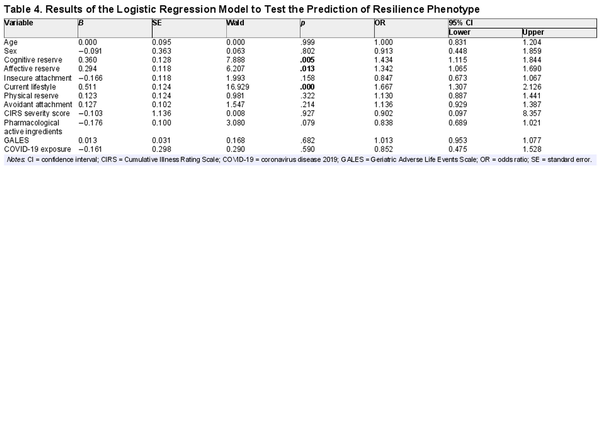
Table 4 displays the results of the logistic regression model. Cognitive reserve, affective reserve, and current lifestyle are the factors that significantly and independently predict the resilience phenotype (p < .05), controlling for sociodemographics and cumulative exposure to physical stressors, stressful life events during the aging process, and COVID-19 infection, with an AUC of 0.70 (95% CI: 0.64–0.76).
Discussion
The present study aimed to explore the structure and impact of the predictors of a resilience phenotype in aging, conceptualized as the multidimensional and dynamic ability to counteract the accumulating damage and stressors that characterize the aging process, maintaining normal cognition, psychological health, and functional independence in very old age.
Among the participants, 38% were resilient. Compared with non-resilient individuals, resilient ones are not spared from stressful life events, including COVID-19 infection. The independent predictors of the resilience phenotype were cognitive reserve, affective reserve, and current lifestyle, controlling for cumulative exposure to physical and psychosocial stressors across a 12-year period.
Features of Resilient Participants
The “resilience phenotype” was directly ascertained through a comprehensive multidimensional assessment in 38% of the sample under study, which was composed of individuals aged between 82 and 87 years who were without dementia. Resilient individuals were more likely to be male and slightly younger, despite the narrow age range considered. As expected, and in line with our hypothesis, they showed a more favorable profile in most of the measures considered to be predictors of resilience. Moreover, they were slightly healthier based on comorbidity measures. At the same time, they did not differ from the non-resilient subgroup for exposure to stressful life events during the observation period (12 years) or for COVID-19 infection.
The Structure of the Resilience-Related Factors
The first finding of the study pertains to the structure of the main cognitive, psychological, and physical resilience-enhancing factors in old age (RQ1). For the exploratory factor analysis, we grouped the selected variables into six separate factors in a predictable and plausible way, favoring a straightforward interpretation of the underlying constructs. The cognitive reserve factor is composed of variables related to early-life cognitive enrichment, with education showing the highest loading, in line with previous research (). The affective reserve factor contains both trait-like factors shaped by gene—environment interactions in early life (attachment and personality traits) and state-like factors (i.e., belief and coping measured by the RS-14) known to protect against depression later in life (). All self-report questionnaires on current habits and activities load primarily on the same factor, together with the walking speed test, a key indicator of physical health in older adults strictly related to neurosensory, muscle-skeletal, and cardiometabolic functions (). Hence, this could be viewed as a comprehensive measure of adherence to a healthy lifestyle later in life, an established protective factor against dementia and other age-related diseases (). Finally, the factor “physical reserve” contains two measures exclusively related to physical health and functionality, namely, waist circumference and handgrip strength.
The loading values indicate direct relationships of both waist circumference and strength with the underlying factor, implying that a common proxy for overweight could be protective in our sample. This finding is in line with prior studies reporting an age-dependent relationship between body mass index and dementia risk over the course of life (; ; ).
Taken together, these findings contribute to advancing theoretical knowledge on the structure of physical, lifestyle, cognitive, and psychosocial protective factors in old age. Indeed, to the best of our knowledge, no previous study has concurrently measured all these independent but interrelated resilience-enhancing factors in the same sample of older adults. From a methodological standpoint, this first step of the analysis was crucial to reduce the dimensionality of our dataset to obtain a limited number of relevant predictors for the resilience phenotype, with high internal consistency and low intercorrelation, thus addressing the issue of multicollinearity among the measures of interest ().
Predicting the Resilience Phenotype
Cognitive reserve, affective reserve, and current lifestyle significantly and independently predicted the resilience phenotype in our sample of oldest-old people, with similar impacts on the probability of being resilient and controlling for cumulative exposure to physical and psychosocial stressors during the study period, from 70–75 years to 83–87 years of age (see Figure 1). Notably, we derived these findings from multivariable models of primary data, allowing us to directly control for the main confounding factors and to estimate the unique contribution of each predictor.
Cognitive reserve and modifiable lifestyles were established as protective factors against dementia; several theoretical and empirical models have highlighted their interrelationship in determining better cognitive outcomes during the aging process (; ).
The original finding of the present study is that the affective reserve factor—composed of variables typically related to mental health and psychological wellbeing—exerts a comparable and independent protective effect in determining resilient aging. A recent review focused on the protective effect of secure attachment on dementia introduced the concept of affective reserve to explain how favorable affective resources might protect against cognitive decline (). Our results could provide the first experimental insight into the existence of such protective factor in the context of aging.
Our results also have crucial translational implications. First, they further support the importance of being ambitious about disease prevention and promotion of successful aging, in line with the life-course approach for reducing dementia risk (). Indeed, both the cognitive and affective reserve factors identified here are mostly composed of measures related to early-life exposure, which may be targeted by specific public health interventions to encourage an enriched, safe environment for younger generations. In this view, a new preventive model termed “young adult brain capital” focused on the importance of optimizing young adults’ brains and mental health to empower the next generation to become active agents in reducing their own risk of developing age-related diseases ().
At the same time, we further confirm that it is never too late to start prevention by showing that the maintenance of an active, healthy lifestyle in very old age (80+) is an independent contributor to the resilience phenotype. This age group is a fast-growing portion of the population, posing an enormous challenge for health and social care systems but receiving limited attention in research studies. In line with our findings, a recent systematic review—which included 27 observational cohort studies—showed that eating a healthy diet with plenty of fruits and vegetables, engaging in physical and leisure/social activities, or having a combination of these lifestyle habits is associated with improved cognitive health in the oldest-old individuals (). Targeting lifestyle habits to improve health and wellbeing (rather than to reduce disease incidence) throughout the life course may be the most effective and parsimonious approach, with positive outcomes for a broad range of age-related diseases ().
Strengths and Limitations
The present study has several methodological strengths. To the best of our knowledge, this is the first attempt to concurrently assess the structure and impact of the chief resilience-enhancing factors in the same cohort, thus allowing us to measure their direct and independent effects on the outcome of interest. Another original aspect is the operationalization of the resilient outcome as a multidimensional construct encompassing physical, cognitive, and psychological health. This is due to the opportunity to rely on a population-based cohort prospectively followed with comprehensive geriatric and neuropsychological assessments. At the same time, we bridge theoretical knowledge from different but interrelated research fields, offering practical suggestions to prevent age-related diseases and disabilities. Finally, we were able to recruit and evaluate hundreds of individuals aged between 83 and 87 years through a personalized approach and reciprocal trust consolidated over 12 years (). As such, we have provided important information on a selected portion of the aged population, which has displayed the greatest growth in recent years due to increased life expectancy but is rarely or marginally included in studies on aging ().
Some limitations should also be discussed. First, although relying on a longitudinal cohort, for our aim, we were able to perform only cross-sectional analysis because we included some measures of interest only in the most recent waves of assessment (2018: personality traits, adherence to the Mediterranean diet; 2022: childhood socioeconomic conditions, handgrip strength, psychological resilience, and attachment style). Moreover, we focused on a narrow age range in a specific geographic area, thus reducing the generalizability of our findings. Finally, the current study adopted a new definition of resilience, thus it is important to replicate the results in other national contexts/samples whereas using a similar operationalization.
Conclusions and Future Directions
The present project enriched the understanding of how lifestyle, cognitive, and psychological factors independently affect individuals’ ability to adapt to the aging process by maintaining physical, cognitive, and mental health. We confirmed that cognitive reserve and current lifestyle are vital drivers of resilient outcomes in a population-based sample of oldest-old individuals. We further found that a new emerging construct, termed affective reserve, exerts a comparable protective effect. These findings, as previously discussed, have direct practical implications for the prevention of dementia and age-related diseases. Future studies on longitudinal data and in different contexts are needed to further confirm these findings because facing the challenge of an aged population is a global issue. Moreover, the present study, by revealing the shared and specific variance of a wide array of resilience-enhancing factors, may inform future research on the biological mechanisms underlying the protective effects of these factors in aging. Finally, this approach to investigating different factors pertinent to graceful or pathological aging has inspired the development of a cognitive and biological model from the perspective of a historical lifespan.
Acknowledgments
The authors would like to thank all the collaborators and professionals who participated in the fifth wave of the InveCe.Ab study for their valuable work. Recruitment and telephone survey: Valeria Marzagalli, Valeria Terzoli, Valentina Biagioli, and Francesca Conti. Geriatric visits: Arcangelo Ceretti, Mauro Ceroni, Maria Cottino, Riccardo Perelli, Emanuele Poloni, and Silvia Vitali. Blood sampling: Annalisa Davin, Valentina Fantini, Riccardo Rocco Ferrari, and Xhulja Profka; Administrative staff: Federica Zagari and Luciana Mordocco. We further thank Riccardo Rocco Ferrari for Figure 1 graphical editing.
References
- Aartsen M. J., Cheval B., Sieber S., Van der Linden B. W., Gabriel R., Courvoisier D. S., Guessous I., Burton-Jeangros C., Blane D., Ihle A., Kliegel M., Cullati S. (2019). Advantaged socioeconomic conditions in childhood are associated with higher cognitive functioning but stronger cognitive decline in older age. Proceedings of the National Academy of Sciences of the United States of America, 116(12), 5478–5486. https://doi.org/10.1073/pnas.1807679116
- Abadir P. M., Bandeen-Roche K., Bergeman C., Bennett D., Davis D., Kind A., LeBrasseur N., Stern Y., Varadhan R., Whitson H. E. (2023). An overview of the resilience world: Proceedings of the American geriatrics society and national institute on aging state of resilience science conference. Journal of the American Geriatrics Society, 71(8), 2381–2392. https://doi.org/10.1111/jgs.18388
- Angevaare M. J., Roberts J., van Hout H. P. J., Joling K. J., Smalbrugge M., Schoonmade L. J., Windle G., Hertogh C. M. P. M. (2020). Resilience in older persons: A systematic review of the conceptual literature. Ageing Research Reviews,63, 101144. https://doi.org/10.1016/j.arr.2020.101144
- APA Dictionary of Psychology. (n.d.). Retrieved January 18, 2024, from https://dictionary.apa.org/resilience
- Arenaza-Urquijo E. M., Vemuri P. (2018). Resistance vs resilience to Alzheimer disease. Neurology, 90(15), 695–703. https://doi.org/10.1212/WNL.0000000000005303
- Arenaza-Urquijo E. M., Wirth M., Chételat G. (2015). Cognitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Frontiers in Aging Neuroscience, 7(August), 134. https://doi.org/10.3389/fnagi.2015.00134
- Bartholomew K., Horowitz L. M. (1991). Attachment styles among young adults: A test of a four-category model. Journal of Personality and Social Psychology, 61(2), 226–244. https://doi.org/10.1037//0022-3514.61.2.226
- Basso A., Capitani E., Laiacona M. (1987). Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Functional Neurology, 2(2), 189–194.
- Callegari C., Bertù L., Lucano M., Ielmini M., Braggio E., Vender S. (2016). Reliability and validity of the Italian version of the 14-item resilience scale. Psychology Research and Behavior Management, 9, 277–284. https://doi.org/10.2147/PRBM.S115657
- Camicioli R., Howieson D., Lehman S., Kaye J. (1997). Talking while walking. Neurology, 48(4), 955–958. https://doi.org/10.1212/wnl.48.4.955
- Chertkow H., Massoud F., Nasreddine Z., Belleville S., Joanette Y., Bocti C., Drolet V., Kirk J., Freedman M., Bergman H. (2008). Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ, 178(10), 1273–1285. https://doi.org/10.1503/cmaj.070797
- Christensen K., Doblhammer G., Rau R., Vaupel J. W. (2009). Ageing populations: The challenges ahead. Lancet (London, England), 374(9696), 1196–1208. https://doi.org/10.1016/S0140-6736(09)61460-4
- Cosco T. D., Kaushal A., Richards M., Kuh D., Stafford M. (2016). Resilience measurement in later life: A systematic review and psychometric analysis. Health and Quality of Life Outcomes, 14(1), 1–6. https://doi.org/10.1186/s12955-016-0418-6
- Dazzi C., Pedrabissi L., Santinello M. (2004). Adattamento Italiano delle Scale di Personalità Eysenck per Adulti [Italian Adaptation of the Eysenck Personality Scales for Adults]. Organizzazioni Speciali.
- Devanand D. P., Kim M. K., Paykina N., Sackeim H. A. (2002). Adverse life events in elderly patients with major depression or dysthymic disorder and in healthy-control subjects. The American Journal of Geriatric Psychiatry, 10(3), 265–274. https://doi.org/10.1097/00019442-200205000-00005
- Escourrou E., Durrieu F., Chicoulaa B., Dupouy J., Oustric S., Andrieu S., Gardette V. (2020). Cognitive, functional, physical, and nutritional status of the oldest old encountered in primary care: A systematic review. BMC Family Practice, 21(1), 58. https://doi.org/10.1186/S12875-020-01128-7
- Farina F. R., Booi L., Occhipinti J. A., Quoidbach V., Destrebecq F., Muniz-Terrera G., Eyre H. A. (2023). Young adult brain capital: A new opportunity for dementia prevention. Journal of Alzheimer's Disease, 94(2), 415–423. https://doi.org/10.3233/JAD-230260
- Fossati A., Feeney J. A., Donati D., Donini M., Novella L., Bagnato M., Acquarini E., Maffei C. (2003). On the dimensionality of the attachment style questionnaire in Italian clinical and nonclinical participants. Journal of Social and Personal Relationships, 20(1), 55–79. https://doi.org/10.1177/02654075030201003
- Fratiglioni L., Marseglia A., Dekhtyar S. (2020). Ageing without dementia: Can stimulating psychosocial and lifestyle experiences make a difference? The Lancet Neurology, 19(6), 533–543. https://doi.org/10.1016/S1474-4422(20)30039-9
- Guaita A., Colombo M., Vaccaro R., Fossi S., Vitali S. F., Forloni G., Polito L., Davin A., Ferretti V. V., Villani S. (2013). Brain aging and dementia during the transition from late adulthood to old age: Design and methodology of the “Invece.Ab” population-based study. BMC Geriatrics, 13(1), 98. https://doi.org/10.1186/1471-2318-13-98
- Hachinski V., Avan A. (2022). From dementia to eumentia: A new approach to dementia prevention. Neuroepidemiology, 56(3), 151–156. https://doi.org/10.1159/000525219
- Katz S., Downs T. D., Cash H. R., Grotz R. C. (1970). Progress in development of the index of ADL. The Gerontologist, 10(1), 20–30. https://doi.org/10.1093/geront/10.1_part_1.20
- Kivipelto M., Mangialasche F., Ngandu T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews Neurology, 14(11), 653–666. https://doi.org/10.1038/s41582-018-0070-3
- Laird K. T., Krause B., Funes C., Lavretsky H. (2019). Psychobiological factors of resilience and depression in late life. Translational Psychiatry, 9(1), 88. https://doi.org/10.1038/s41398-019-0424-7
- Lawton M. P., Brody E. M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. https://doi.org/10.1093/GERONT/9.3_PART_1.179
- Lemmink K. A. P. M., Han K., De Greef M. H. G., Rispens P., Stevens M. (2001). Reliability of the Groningen fitness test for the elderly. Journal of Aging and Physical Activity, 9(2), 194–212. https://doi.org/10.1123/JAPA.9.2.194
- Lisko I., Kulmala J., Annetorp M., Ngandu T., Mangialasche F., Kivipelto M. (2021). How can dementia and disability be prevented in older adults: Where are we today and where are we going? Journal of Internal Medicine, 289(6), 807–830. https://doi.org/10.1111/joim.13227
- Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S. G., Dias A., Fox N., Gitlin L. N., Howard R., Kales H. C., Kivimäki M., Larson E. B., Ogunniyi A., Mukadam N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- MacLeod S., Musich S., Hawkins K., Alsgaard K., Wicker E. R. (2016). The impact of resilience among older adults. Geriatric Nursing (New York, N.Y.), 37(4), 266–272. https://doi.org/10.1016/j.gerinurse.2016.02.014
- Minnai F., De Bellis G., Dragani T. A., Colombo F. (2022). COVID-19 mortality in Italy varies by patient age, sex and pandemic wave. Scientific Reports, 12(1), 1–9. https://doi.org/10.1038/s41598-022-08573-7
- Mount S., Ferrucci L., Wesselius A., Zeegers M. P., Schols A. M. W. J. (2019). Measuring successful aging: an exploratory factor analysis of the InCHIANTI Study into different health domains. Aging, 11(10), 3023–3040. https://doi.org/10.18632/AGING.101957
- Osborne J. W. (2014). Best practices in exploratory factor analysis. CreateSpace Independent Publishing.
- Parmelee P. A., Thuras P. D., Katz I. R., Lawton M. P. (1995). Validation of the cumulative illness rating scale in a geriatric residential population. Journal of the American Geriatrics Society, 43(2), 130–137. https://doi.org/10.1111/j.1532-5415.1995.tb06377.x
- Patrizio E., Calvani R., Marzetti E., Cesari M. (2021). Physical functional assessment in older adults. The Journal of Frailty & Aging, 10(2), 141–149. https://doi.org/10.14283/jfa.2020.61
- Petersen R. C., Morris J. C. (2004). Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology, 62(7), 1160–1163; discussion 1167. https://doi.org/10.1001/archneur.62.7.1160
- Pietromonaco P. R., Beck L. A. (2019). Adult attachment and physical health why attachment matters for health. Current Opinion in Psychology, 25, 115–120. https://doi.org/10.1016/j.copsyc.2018.04.004
- Pinto J. O., Peixoto B., Dores A. R., Barbosa F. (2024). Measures of cognitive reserve: An umbrella review. The Clinical Neuropsychologist, 38, 42–115. https://doi.org/10.1080/13854046.2023.2200978
- Resnick B., Galik E., Dorsey S., Scheve A., Gutkin S. (2011). Reliability and validity testing of the physical resilience measure. The Gerontologist, 51(5), 643–652. https://doi.org/10.1093/geront/gnr016
- Rolandi E., Zaccaria D., Vaccaro R., Abbondanza S., Pettinato L., Davin A., Guaita A. (2020). Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: A population-based study. Alzheimer’s Research and Therapy, 12(1), 94. https://doi.org/10.1186/s13195-020-00661-y
- Sachdev P. S., Blacker D., Blazer D. G., Ganguli M., Jeste D. V., Paulsen J. S., Petersen R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology, 10(11), 634–642. https://doi.org/10.1038/nrneurol.2014.181
- Schröder H., Fitó M., Estruch R., Martínez‐González M. A., Corella D., Salas‐Salvadó J., Lamuela‐Raventós R., Ros E., Salaverría I., Fiol M., Lapetra J., Vinyoles E., Gómez‐Gracia E., Lahoz C., Serra‐Majem L., Pintó X., Ruiz‐Gutierrez V., Covas M. (2011). A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. The Journal of Nutrition, 141(6), 1140–1145. https://doi.org/10.3945/jn.110.135566
- Song S., Stern Y., Gu Y. (2022). Modifiable lifestyle factors and cognitive reserve: A systematic review of current evidence. Ageing Research Reviews, 74, 101551. https://doi.org/10.1016/j.arr.2021.101551
- Stern Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology, 11(11), 1006–1012. https://doi.org/10.1016/S1474-4422(12)70191-6
- Vaccaro R., Borrelli P., Abbondanza S., Davin A., Polito L., Colombo M., Francesca Vitali S., Villani S., Guaita A. (2017). Subthreshold depression and clinically significant depression in an Italian population of 70–74-year-olds: Prevalence and association with perceptions of self. Biomed Research International, 2017, 1–8. https://doi.org/10.1155/2017/3592359
- Vaupel J. W. (2010). Biodemography of human ageing. Nature, 464(7288), 536–542. https://doi.org/10.1038/nature08984
- Viña J., Borrás C., Miquel J. (2007). Theories of ageing. IUBMB Life, 59(4–5), 249–254. https://doi.org/10.1080/15216540601178067
- Walsh E., Blake Y., Donati A., Stoop R., Von Gunten A. (2019). Early secure attachment as a protective factor against later cognitive decline and dementia. Frontiers in Aging Neuroscience, 11(JUL), 1–20. https://doi.org/10.3389/fnagi.2019.00161
- Whitson H. E., Duan-Porter W., Schmader K. E., Morey M. C., Cohen H. J., Colón-Emeric C. S. (2016). Physical resilience in older adults: Systematic review and development of an emerging construct. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(4), 489–495. https://doi.org/10.1093/gerona/glv202
- Ye K. X., Sun L., Wang L., Khoo A. L. Y., Lim K. X., Lu G., Yu L., Li C., Maier A. B., Feng L. (2023). The role of lifestyle factors in cognitive health and dementia in oldest-old: A systematic review. Neuroscience and Biobehavioral Reviews, 152, 105286. https://doi.org/10.1016/j.neubiorev.2023.105286