Introduction
The diagnosis of emphysema, a form of chronic obstructive pulmonary disease (COPD), is based on the pathological findings. It is characterized by permanent destruction of airway walls, lung parenchyma, and enlargement of airspaces distal to the terminal bronchioles []. Since the 2 well-known forms of COPD, emphysema and chronic bronchitis, can coexist in many patients, COPD is used more frequently in the literature when discussing the epidemiology of the disease []. COPD is a leading cause of morbidity and mortality worldwide. Its burden on economies and societies keeps growing [, ]. Large-scale epidemiological projects like the BOLD study estimated there were 384 million COPD patients in 2010, with a global prevalence of 11.7%. Currently, it causes are >3 million deaths per year worldwide [].
Body mass index (BMI) has been widely used for a long time to describe the nutritional status of patients. There is also an association between BMI and nutritional status in patients with emphysema []. When comparing low-attenuation areas on computed tomography (CT), BMI is significantly lower in the emphysema phenotype than in the airway phenotype in male patients with COPD []. However, no difference has been found between these 2 groups in the forced expiratory volume <1 s (FEV1) predicted value. Men with a low BMI have a higher risk of developing COPD than those with a high BMI []. The association between low BMI and advanced COPD is well established and is termed “pulmonary cachexia syndrome.” Accepted causative mechanisms include poor nutritional status related to early satiety, increased energy expenditure related to the work of breathing as well as the known associated chronic systemic inflammation [, ].
BMI has a strong association with all-cause mortality. In a 2009 study by the Prospective Studies Collaboration, a connection was found between BMI and mortality in900,000 adults []. The lowest mortality rate was found in those with a BMI in the range of 22.5–25 kg/m2 and it increased with a BMI of >25 and <22.5 kg/m2. The latter association is mainly due to the strong associations with respiratory diseases. Low BMI is also an independent factor in predicting the mortality of patients with severe emphysema caused by α1-antitrypsin deficiency []. In this disease group, a higher risk of death has been found in COPD patients with a BMI of <21.75 kg/m2 []. The study also found that an increase in BMI was associated with a lower risk of death. There is a plausible link between low BMI and worse outcomes, and between poor nutritional status and decreased survival [].
Endoscopic lung volume reduction (ELVR) with endobronchial valves (EBV) is an innovative, effective, and safe treatment for patients with severe emphysema undergoing optimal medical therapy. Several clinical trials have evaluated the safety and effectiveness of different EBV. The REACH trial found a significant improvement in clinical and quality of life (QoL) measurements in the group treated with EBV compared to the control group that received medical therapy only []. The LIBERATE trial showed ELVR with Zephyr® valves to be a safe procedure resulting in a clinical improvement in lung function, exercise tolerance, dyspnea, and QoL []. The EMPROVE trial highlights that careful selection by high-resolution CT of patients undergoing ELVR with Spiration® valves resulted in the effectiveness of the procedure lasting 12 months []. The Spiration valve system showed an improvement in pulmonary function, QoL, and dyspnea score while the risk profile remained acceptable in patients with severe heterogeneous emphysema and hyperinflation without collateral ventilation []. There is an associated survival benefit for patients with severe emphysema who undergo endoscopic valve therapy and achieve lobar atelectasis [].
There is a paucity of data on the associations between low BMI in patients living with COPD and clinical outcomes after ELVR. In this study, we explored this relationship and its effects on outcomes in patients with severe heterogeneous emphysema. Our hypothesis was that there is a plausible link in severe heterogeneous emphysema between changes in BMI and the ELVR procedure.
Materials and Methods
We performed a retrospective cohort review of patients with Zephyr EBV installation for lung volume reduction from December 2016 to July 2018. Data were collected at the Thorax Clinic, University of Heidelberg, Germany. We included patients with severe emphysema and without collateral ventilation measured by the Chartis® system. All patients had heterogeneous emphysema with a difference in emphysema severity of >50% between lobes. Exclusion criteria were incomplete data or refusal to sign the consent form.
Procedure Protocol
Valves were placed according to the LIBERATE study protocol requirements []. Collateral ventilation status between the targeted lobe and the adjacent lobes was determined to assess patient eligibility according to the Chartis system. Eligible subjects who had no collateral ventilation underwent ELVR with EBV to achieve complete lobar occlusion. The procedure was performed under general anesthesia.
Data Extraction
We gathered data on demographics (age, gender, pack-year index, and BMI) and the procedure (the number of EBV and identification of the target lobe). Pulmonary function and 6-min-walk test (6MWT) results, modified Medical Research Council (mMRC) dyspnea score, and complications were recorded. Patients were divided into 2 groups based on their baseline BMI: a higher BMI group (BMI ≥21 kg/m2) and lower BMI group (BMI <21 kg/m2). We chose the threshold of 21 kg/m2 because, according to the BODE (BMI, airflow obstruction, dyspnea, exercise capacity) index, this value is associated with the inflection point in the inverse relationship between BMI and survival []. Other data in the literature also found association between lower BMI and higher mortality [-]. However, some reference used the threshold of 21.75 kg/m2 [].
Patients were followed up at 6 months. The primary outcome was a change in BMI after ELVR (dBMI). Secondary outcomes were changes in weight (dWeight), FEV1 (dFEV1), residual volume (dRV), distance during 6MWT (d6MWT), dyspnea score (dmMRC), and BODE index (dBODE index). Procedure-related complications were also recorded.
Statistical Analysis
Results are expressed as mean (±SD) (range). Quantitative data were evaluated with a nonparametric test (the Mann-Whitney U test) and qualitative with the Fisher exact test. We also explored a potential correlation between changes in BMI and major outcomes. For this, we used a linear regression model and R2 was calculated for each major outcome in order to explore any association between initial BMI and the following outcomes: dWeight, dBMI, dFEV1, dRV, d6MWT, dmMRC, and dBODE index after ELVR. As a confounder assessment, we performed a multivariate regression analysis between significant outcomes and the following prespecified variables at baseline: age, male gender, pulmonary function testing (VC, FEV1, RV, and TLC), 6MWT, and BODE index. Analyses were performed using SPSS v17.0 (IBM, Chicago, IL, USA) and p < 0.05 was considered statistically significant.
Results
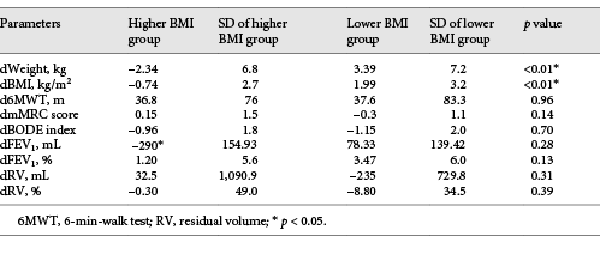
Eighty-two patients were included in total (higher BMI group: n = 62; lower BMI group: n = 20 patients). Median age was 61.2 (range 28–81) years. Mean baseline BMI was 25.79 (range 21.54–36.29) and 19.57 (range 16.99–20.83) kg/m2 in the higher BMI and lower BMI group, respectively. After ELVR with EBV, the mean dWeight in the higher BMI group was –2.34 kg (±6.8) (–7.8 to 2.6) and the lower BMI group reported an improvement of 3.39 kg (±7.2) (–2.3 to 8.4) (p < 0.01). After ELVR with EBV, BMI decreased in the higher BMI group by –0.74 kg/m2 (±2.7) (–3.02 to 3.8); in the lower BMI group, it improved by +1.99 kg/m2(±3.2) (–0.7 to 4.2) (p < 0.01) (Fig. 1).
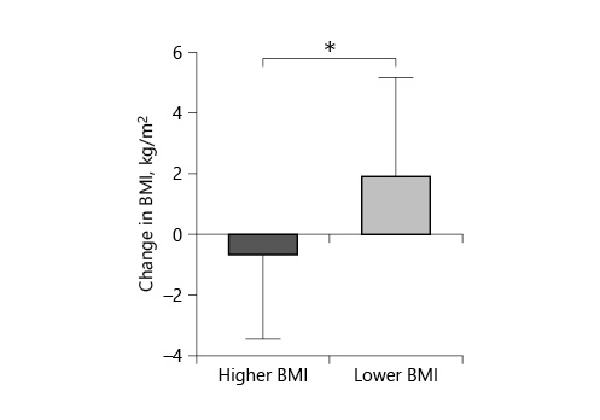
Fig. 1
Change in BMI after endobronchial valve placement. In the higher BMI group, BMI decreased, while in the lower BMI group, it increased (p < 0.01).
Other objective parameters are shown in Table 1. We found no statistical significance between dFEV1in the lower BMI group (3.47% ± 6.0) and higher BMI group (1.2% ± 5.6) (p = 0.13) (Fig. 2).
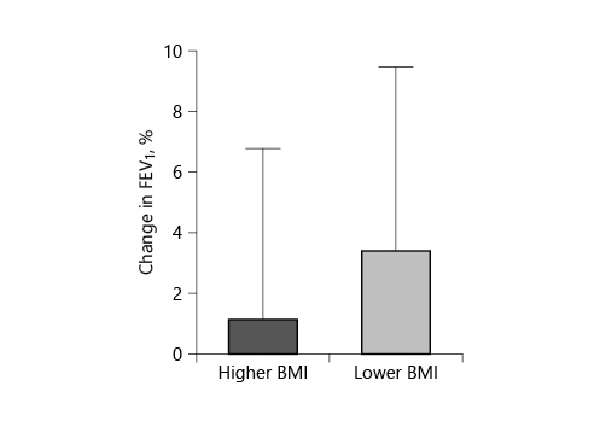
Fig. 2
The change in FEV1 after endobronchial valve placement. FEV1 increased more in the lower BMI group than in the higher BMI group (p = 0.13).
In the higher BMI group, d6MWT was 36.8 m (±76); in the lower BMI group, it was 37.6 m (±83.3) (p = 0.96). The dmMRC was –0.15 (±1.5) in the higher BMI group and –0.3 (±1.1) in the lower BMI group (p = 0.14). dBODE index was –0.96 points (±1.8) in the higher BMI group and –1.15 (±2.0) in the lower BMI group (p =0.7) (Fig. 3).
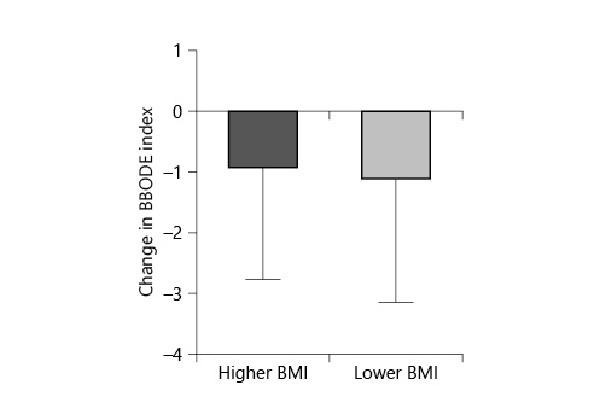
Fig. 3
The change in BODE index after endobronchial valve placement. The BODE index in the lower BMI group decreased more than in the higher BMI group (p = 0.7).
Linear regression analysis found an R2 value of 0.99 (p < 0.01) between dBMI and dWeight in the lower BMI group and an R2 value of 0.96 (p < 0.01) between dBMI and dWeight in the higher BMI group. In the lower BMI group, 40% of patients had complications; in the higher BMI group, 24% had complications. The specific complications and their occurrence rates are listed in Figures 4 and 5.
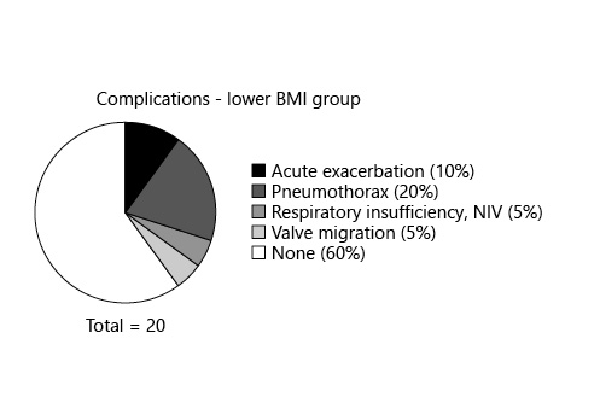
Fig. 4
The rate of complications in the higher BMI group after endobronchial valve placement.
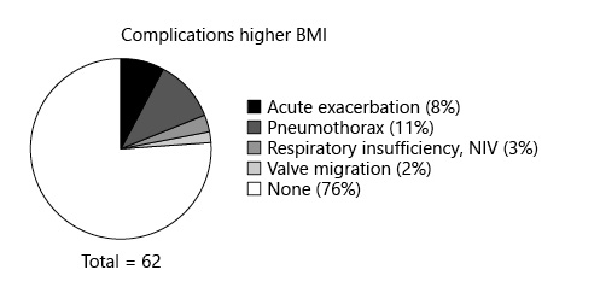
Fig. 5
The rate of complications in the lower BMI group after endobronchial valve placement.
Multivariate regression analysis used the following confounders at baseline: age, male gender, VC, FEV1, RV, TLC, 6MWT, BODE index. The candidate variable was dBMI. As a result, female gender at baseline was independently associated with dBMI with a B coefficient of –0.14 (confidence interval: 3.3 to –0.4) (p = 0.01). Finally, we found no statistically significant difference between dBMI and other prespecified variables.
Table 1 summarizes changes in parameters after endoscopic valve placement.
Discussion
Emphysema is associated with a lower BMI (<18.5 kg/m2) []. We found a correlation between initial BMI, change in BMI, and weight after ELVR with EBV. Our results show that COPD patients with a lower BMI gained a significant amount of weight and increased their BMI after the procedure. On the other hand, patients with a higher BMI lost weight, so their BMI decreased. However, after our confounder analysis, the principal change in BMI was independently associated with age. Younger participants reported better results than older ones. Our results show that only BMI changed significantly 6 months after the procedure in patients with severe heterogeneous emphysema. We did not find any other significant changes in lung function variables. This suggests that ELVR might affect other parameters, e.g., metabolic functions, thus causing the BMI change. All patients underwent a lifestyle consultation 3 months after the procedure which included information about nutrition, vaccination, exercise, etc. This can contribute to the degree of weight change after the procedure. Spelta et al. []raised awareness about the obesity paradox possibly present with COPD. According to their review, several studies have reported obesity to be a protective factor against all-cause mortality, including COPD. However, they highlighted that several confounding factors might be present in the association of COPD, obesity, and mortality. The lower FEV1 in obese people may be due to restrictive defects, not due to obstructive ones. COPD patients with increased fat mass/obesity have modified mechanical properties of their chest wall, and therefore a hyperinflation in the lower lungs which is associated with a higher mortality rate. Another possible bias mentioned in this review is the extent of emphysema and its link to mortality, which can bias the connection between survival and weight. Using body composition would be a better predictor of mortality than BMI []. Others suggest using a dynamic index of nutrition status, i.e., the temporal body weight change, which could be more accurate than BMI for detecting malnutrition []. However, most studies have used BMI to evaluate connections between weight, mortality, and COPD. Finally, there is a possibility of a reverse bias, i.e., that unintentional weight loss could be the major factor increasing the mortality rate, rather than reflecting on obesity as the protective factor [].
As stated above, 1 study identified lower BMI as an important risk factor in the development of COPD in men []. According to the hypothesis in that study, early nutritional supplementation could prevent or delay the occurrence of COPD. The Hertfordshire Study identified malnutrition as a probable major cause of poor gestational growth []. Animal studies have shown that a deprived nutritional intake during the neonatal period in rats causes emphysematous changes like a deterioration in lung growth and elastin production, enlargement of the alveoli, and a reduction in elastic recoil [-]. The resting energy requirement of COPD patients with malnutrition is relatively greater; this includes more energy needed for ventilation. The reason for this might be the decreased respiratory muscle efficiency caused by severe COPD [].
Lung volume reduction surgery will result in weight gain by nonobese patients with emphysema, possibly related to enhanced lung function, exercise capacity, and the ability to strengthen muscles including respiratory muscles, and thus increased ventilation capacity []. By supplementing for nutritional deficiencies, patients undergoing lung volume reduction surgery might not only benefit from decreased morbidity and length of stay in hospital, but also decreased overall costs [].
Baseline BMI is relevant in order to select candidates for EBV. Our results show that patients undergoing ELVR with EBV with a higher BMI reported fewer complications. There is not much research on whether weight affects the postoperative complication rate. Nezu et al. []evaluated COPD patients with different nutritional status and the complication rate after bilateral lung volume reduction. They calculated ideal body weight and BMI, and measured fat-free mass and fat mass using a bioelectrical impedance analyzer. Those patients with low fat-free mass index experienced a higher rate of complications. Obese patients when compared to “normal weight” patients were found to have no increased risk for intra- or postoperative complications (except for pneumonia) [].
For patients living with chronic diseases, symptom severity is a major factor contributing to their QoL. In COPD, dyspnea is this crucial factor. COPD patients with a lower BMI are more dyspneic based on their mMRC score, pulmonary function test and arterial blood gas results, and respiratory muscle strength [].
In other studies, the BODE index significantly improved in those with COPD undergoing EBV therapy compared to conservative therapy []. In one of our reviews on ELVR with different techniques, including valves compared to surgical therapy, we found that ELVR can result in improvements in lung function, exercise tolerance, and QoL, with fewer associated complications []. Despite an increased risk for procedure-related adverse events found with EBV, observational studies report an improved long-term outcome []. In a systematic review of Zephyr® valves, we found an improvement in QoL and spirometry results for up to 12 months in patients with severe homogeneous or heterogeneous emphysema with no collateral ventilation []. Though other studies have found that lung functions test and QoL results change significantly after ELVR in this limited number of patients, we did not find any significant difference in these variables (d6MWT, dBODE index, dFEV1, and dmMRC). A possible solution could be increasing the number of patients.
The main limitations of this study are the following. First, the data were retrospectively collected from a single center. Second, the study may have been underpowered due to the small sample size. A pooled analysis including data from ELVR using Zephyr® valves, or a post hoc patient level meta-analysis could confirm our findings. Third, the included data were restricted to patients with heterogeneous emphysema and no collateral ventilation was measured by the Chartis® system. Fourth, no exams (e.g., bioimpedance, quantitative magnetic resonance, etc.) were done to determine body composition to see what type of tissue (e.g., muscle or fat) was lost or gained during the study. Finally, not all the patients had radiologic outcomes recorded after the procedure. Whether the conclusion is generalizable to other emphysema types or valve devices remains unknown.
To date, there is a paucity of research regarding the relationship between BMI and ELVR. Our results showed that, in patients with lower BMI, there is a higher rate of complications directly after the procedure, and that EBV are associated with increased BMI 6 months after treatment. We suggest supplemental nutrition for emphysematous patients with lower BMI undergoing ELVR. Even though this practice comes at extra cost, the advantages of improved BMI and its outcomes can contribute to lower overall costs for the individual as well as decrease the enormous economic and social burden of COPD. Most importantly, it can help patients with COPD to have a better QoL. We would like to highlight that the association between BMI and long-term outcomes of ELVR should be studied further. Our findings suggest a potential nutritional benefit in emphysematous patients with low BMI undergoing ELVR with EBV.
Statement of Ethics
The subjects gave their written informed consent. No institutional review board approval was required for this research as this was a retrospective review of medical records and no patient identification information was kept after the research.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
The research had no funding.
Author Contributions
S.F.-B. made substantial contributions to study conception and design and the interpretation of data. A.K. made substantial contributions to the analysis and interpretation of data and drafted the submitted article. G.L., D.A.-T., and N.M.P. made substantial contributions to the analysis and interpretation of data. F.J.F.H. made substantial contributions to study conception and design and the acquisition and interpretation of data. All authors revised the article critically and substantially for important intellectual content, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
References
- 1. Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest. 1998;113(4Suppl):235S–41S. 0012-3692
- 2. Weinberger S, Cockrill B, Mandel J. Chronic Obstructive Pulmonary Disease. Principles of Pulmonary Medicine. 7th ed.Elsevier; 2019. pp. 93–112.
- 3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. 0140-6736
- 4. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. 0140-6736
- 5. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. 0140-6736
- 6. Mazolewski P, Turner JF, Baker M, Kurtz T, Little AG. The impact of nutritional status on the outcome of lung volume reduction surgery: a prospective study. Chest. 1999;116(3):693–6. 0012-3692
- 7. Ogawa E, Nakano Y, Ohara T, Muro S, Hirai T, Sato S, et al Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64(1):20–5. 0040-6376
- 8. Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest. 2002;121(2):370–6. 0012-3692
- 9. Jung JW, Yoon SW, Lee GE, Shin HG, Kim H, Shin JW, et al Poor nutritional intake is a dominant factor for weight loss in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2019;23(5):631–7. 1027-3719
- 10. Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, et al Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1414–8. 1073-449X
- 11. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et alProspective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. 0140-6736
- 12. Seersholm N. Body mass index and mortality in patients with severe alpha 1-antitrypsin deficiency. Respir Med. 1997;91(2):77–82. 0954-6111
- 13. Guo Y, Zhang T, Wang Z, Yu F, Xu Q, Guo W, et al Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore). 2016;95(28):e4225. 0025-7974
- 14. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–61. 1073-449X
- 15. Li S, Wang G, Wang C, Gao X, Jin F, Yang H, et al The REACH Trial: A Randomized Controlled Trial Assessing the Safety and Effectiveness of the Spiration(R) Valve System in the Treatment of Severe Emphysema. Respiration.2018;•••:1–12.
- 16. Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et alLIBERATE Study Group. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med. 2018;198(9):1151–64. 1073-449X
- 17. European Respiratory Journal 2018 52: Suppl. 62, OA4928.
- 18. Majid A, Labarca G, Uribe JP, Kheir F, Pacheco C, Folch E, et al Efficacy of the Spiration Valve System in Patients with Severe Heterogeneous Emphysema: A Systematic Review and Meta-Analysis. Respiration. 2020;99(1):62–72. 1423-0356
- 19. Gompelmann D, Benjamin N, Bischoff E, Kontogianni K, Schuhmann M, Hoffmann H, et al Survival after Endoscopic Valve Therapy in Patients with Severe Emphysema. Respiration. 2019;97(2):145–52. 1423-0356
- 20. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. 0028-4793
- 21. Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122(4):1256–63. 0012-3692
- 22. Spelta F, Fratta Pasini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. 2018;23(1):15–22. 1124-4909
- 23. Nakatsuka Y, Handa T, Kokosi M, Tanizawa K, Puglisi S, Jacob J, et al The Clinical Significance of Body Weight Loss in Idiopathic Pulmonary Fibrosis Patients. Respiration. 2018;96(4):338–47. 1423-0356
- 24. Syddall HE, Aihie Sayer A, Dennison EM, Martin HJ, Barker DJ, Cooper C. Cohort profile: the Hertfordshire cohort study. Int J Epidemiol. 2005;34(6):1234–42. 0300-5771
- 25. Das RM. The effects of intermittent starvation on lung development in suckling rats. Am J Pathol. 1984;117(2):326–32.0002-9440
- 26. Kalenga M, Eeckhout Y. Effects of protein deprivation from the neonatal period on lung collagen and elastin in the rat. Pediatr Res. 1989;26(2):125–7. 0031-3998
- 27. Matsui R, Thurlbeck WM, Fujita Y, Yu SY, Kida K. Connective tissue, mechanical, and morphometric changes in the lungs of weanling rats fed a low protein diet. Pediatr Pulmonol. 1989;7(3):159–66. 8755-6863
- 28. Sahebjami H, MacGee J. Effects of starvation on lung mechanics and biochemistry in young and old rats. J Appl Physiol (1985). 1985;58(3):778-84.
- 29. Donahoe M, Rogers RM, Wilson DO, Pennock BE. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(2):385–91. 0003-0805
- 30. Kim V, Kretschman DM, Sternberg AL, DeCamp MM Jr, Criner GJNational Emphysema Treatment Trial Research Group. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. Am J Respir Crit Care Med. 2012;186(11):1109–16. 1073-449X
- 31. Nezu K, Yoshikawa M, Yoneda T, Kushibe K, Kawaguchi T, Kimura M, et al The effect of nutritional status on morbidity in COPD patients undergoing bilateral lung reduction surgery. Thorac Cardiovasc Surg. 2001;49(4):216–20. 0171-6425
- 32. De Oliveira GS Jr, McCarthy RJ, Davignon K, Chen H, Panaro H, Cioffi WG. Predictors of 30-Day Pulmonary Complications after Outpatient Surgery: Relative Importance of Body Mass Index Weight Classifications in Risk Assessment. J Am Coll Surg. 2017;225(2):312-23 e7.
- 33. Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(3 Pt 1):886–90. 1073-449X
- 34. Valipour A, Herth FJ, Burghuber OC, Criner G, Vergnon JM, Goldin J, et alVENT Study Group. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J. 2014;43(2):387–96. 0903-1936
- 35. Fernandez-Bussy S, Labarca G, Herth FJ. Bronchoscopic Lung Volume Reduction in Patients with Severe Emphysema. Semin Respir Crit Care Med. 2018;39(6):685–92. 1069-3424
- 36. Gompelmann D, Herth FJ, Slebos DJ, Valipour A, Ernst A, Criner GJ, et al Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration. 2014;87(6):485–91. 1423-0356
- 37. Labarca G, Uribe JP, Pacheco C, Folch E, Kheir F, Majid A, et al Bronchoscopic Lung Volume Reduction with Endobronchial Zephyr Valves for Severe Emphysema: A Systematic Review and Meta-Analysis. Respiration. 2019;98(3):268–78. 1423-0356