Introduction
Emphysema is a severe and debilitating form of chronic obstructive pulmonary disease characterized by dyspnea, poor exercise capacity, and impaired quality of life [-]. Endobronchial valve treatment is currently a well-established treatment option for patients with advanced emphysema. Valves are positioned into the target lobe after exclusion of collateral ventilation (CV) to inhibit airflow into the lobe but allowing trapped air to exit. The resulting atelectasis of the occluded lobe leads to a lung volume reduction improving breathing mechanics [-].
However, in many patients, lung volume reduction does not occur due to the existence of CV, where air bypasses the normal anatomical airways through channels between the target and adjacent lobe [-]. According to current standards, CV is excluded prior to endobronchial valve treatment by a bronchoscopy with Chartis assessment (PulmonX Inc., ) and a high-resolution computed tomography scan of the thorax (HRCT) with interlobar fissure analysis [, -]. Previously, 4 Chartis phenotypes have been described in spontaneous breathing: CV− (CV negative) (absence of CV), CV+ (CV positive) (presence of CV), and the inconclusive phenotypes low (LF)/no flow or collapse phenotype (characterized as immediate collapse of the lobe with minimal airflow) and less often low plateau (LP) (defined as gradual decrease in airflow to a LP). Quantitative CT (computed tomography) analysis is a noninvasive tool defining fissure integrity with a fissure completeness score (FCS), but exact cut-offs remain subject to research. Recent studies suggest that the combination of Chartis and CT assessments is more precise excluding CV, resulting in a higher rate of endobronchial valve treatment responses []. Interestingly, FCS might be less accurate in the evaluation of both fissures of the right lung in comparison to the left lung fissure [].
Regarding the exact diagnostic approach for Chartis assessment, many aspects are still in debate. Recently, studies demonstrated that general anesthesia with volume-controlled ventilation and procedural sedation with spontaneous breathing are both accurate for Chartis assessment [, ]. However, the impact of high-frequency (HF) jet ventilation, which is widely used during bronchoscopies, is still unclear. Therefore, we conducted the current study to assess whether the ventilation mode has an impact on Chartis assessment outcome.
Material and Methods
Patients
Patients with Chartis assessments and software-based quantifications of FCS were analyzed retrospectively in this single-center study. All data were derived from prospective open-label clinical studies in our institution which were approved by the Ethics Committee of the Charité-Universitätsmedizin Berlin, Germany (EA2/149/17 and EA1/136/13). All patients consented to participation. Patients were admitted at the Charité-Universitätsmedizin Berlin, Germany, for evaluation of endobronchial valve treatment from February 2012 to December 2019. Inclusion criteria were the diagnosis of advanced chronic obstructive pulmonary disease with lung emphysema characterized by forced expiratory volume in the first second <45% predicted and a residual volume >150% predicted, despite optimal medical therapy. All patients were nonsmokers proven by carboxyhemoglobin levels <2%. Exclusion criteria were a significant pulmonary hypertension (systolic pulmonary arterial pressure >50 mm Hg), relevant hypercapnia (pCO2 >55 mm Hg), and the inability to sign the consent form. At baseline, all patients underwent a detailed medical history and clinical examination. Additional tests prior to treatment included lung function test, blood gas analysis, 6-min walk test, HRCT (1 mm slides thickness), and a bronchoscopy with Chartis assessment. A steering committee (emphysema board) consisting of pneumologists, thoracic surgeons, and radiologists determined the final treatment strategies.
Evaluation of FCS on HRCT
The FCS was calculated on thin-section CT scans using an automated software quantification system (StratX; PulmonX Inc.). A complete fissure was defined as an FCS >95%, an incomplete fissure was characterized with an FCS <80%, and an intermediate/inconclusive fissure when FCS was between 80% and 95%, as previously described [].
Evaluation of CV by Chartis Assessment
All patients underwent a flexible bronchoscopy with Chartis assessment for evaluation of CV per lobe. Beforehand, all patients received iv atropine if heart frequency was below 100 bpm to avoid intraprocedural secretion and iv Pethidine to reduce coughing. Patients were sedated intravenously with 2.5-mg midazolam at the beginning and propofol boluses until a sufficient level of sedation was achieved. To maintain a secure airway, patients were intubated with a 7.5-mm endotracheal tube (Bronchoflex; Rüsch GmbH, Rems-Murr, Germany) and were breathing spontaneously during bronchoscopy.
The left major fissure (LMF) was assessed via the left upper lobe. Assessments of the left lower lobe were rarely taken because of the high likelihood of inconclusive measurements due to collapse phenomena (LF phenotype). For this reason, Chartis assessments obtained in the left lower lobe were not included in the statistical analysis. The right upper lobe is separated from the right middle lobe and right lower lobe by the right minor fissure and parts of the right major fissure (RMF). The right upper lobe fissure (RUF) was assessed via the right upper lobe bronchus, while the RMF was measured by occluding the right lower lobe bronchus.
The Chartis pulmonary assessment was performed first under spontaneous breathing and then again under HF jet ventilation. In spontaneously breathing patients, oxygen was administered to maintain a peripheral oxygenation >92%. Subsequently, the same patients were ventilated with a HF jet ventilator (Acutronic Medical Systems, Monsoon III Jet Ventilator; frequency 150/min, pause pressure 1.5 bar, and airway pressure 35 mbar).
During Chartis assessment, the primary and secondary target lobes were measured first. In most patients, the right upper lobe, left upper lobe, and right lower lobe were mostly assessed together in order to have a comprehensive analysis of all fissures. Chartis measurements were classified according to visual assessments into four phenotypes as recently described CV−, CV+, LF, and LP []. In CV+, the expiratory flow remained at least over 50% from initial measurement and no significant elevation of the resistance index was seen. In case of a CV+ visual pattern, Chartis assessment was stopped after 5 min without decrease in airflow or if the total volume of expired air exceeded >750 mL. CV− was characterized by a gradual decrease in expiratory flow below 20% (which means >80% from baseline) together with an increase in the resistance index of >3-cm H2O × s/mL within 5 min of measurement and <750 mL of air was ventilated. The LP phenotype showed a decrease of expiratory flow to a plateau at 20–50% (which means a 50–80% decrease from baseline) and no increase of the resistance above 3 cm H2O × s/mL. In case of LF phenotype, a collapse of the occluded target lobe shortly after starting the assessment occurred and only a small amount (<50 mL) of air was ventilated, shown by a sudden drop in the airflow curve. The exclusion of CV is characterized by CV−, the presence of CV by CV+. These Chartis phenotypes were considered conclusive because they clearly define CV status. LF and LP were considered inconclusive results since the CV status could not be determined and their clinical relevance has not been established yet.
Statistical Analysis
Cross tabulation was used for the comparison of Chartis phenotypes in spontaneous breathing and HF jet ventilation. Then, results were correlated to the FCS with cross tabulation. Concordance rates between Chartis phenotypes of spontaneous breathing and HF jet ventilation were assessed using Cohen’s kappa coefficient (κ). The area under the receiver operating characteristic (ROC) curve was used to examine the ability of FCS to predict the conclusive Chartis phenotypes (CV+/CV−). SPSS Statistics version 26 (IBM, Corporation, Armonk, NY, USA) was used for statistical analysis. Data were presented as mean +/− standard deviation. A p value <0.05 was considered statistically significant.
Results
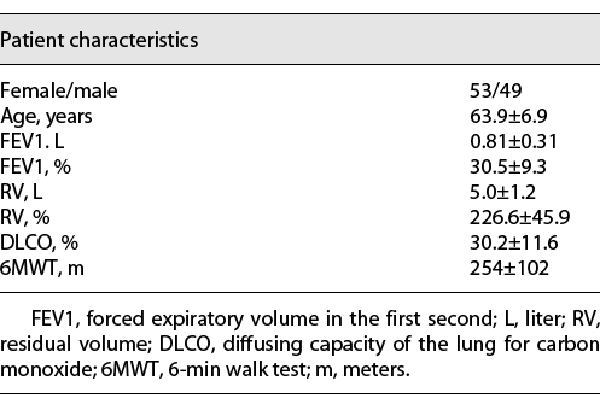
In total, 102 patients with 497 Chartis assessments were analyzed. In detail, 190 Chartis assessments of the LMF, 184 of the RUF, and 123 of the RMF were performed. All patients underwent CT-based fissure integrity analysis. Only in 6 patients, software-based FCS quantification was not available due to technical issues. The baseline patients´ characteristics are summarized in Table 1.
With both ventilation modes, Chartis phenotypes (CV−, CV+, LF, and LP) showed similar appearance and the overall characteristics of the Chartis phenotypes remained, as summarized before (Fig. 1a–d). Nevertheless, a notable difference between both ventilation modes was sometimes that in HF jet ventilation no inspiratory pressure curve was seen and a continuous broad expiratory flow was detected during measurement (see * in Fig. 1 b, d).
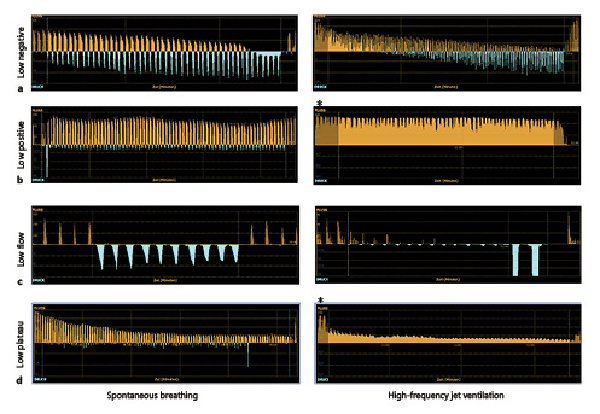
Fig. 1
Examples of Chartis phenotypes in spontaneous breathing (left column) and HF jet ventilation (right column). a CV− phenotype: continuous decrease in expiratory flow below 20% of baseline. b CV+ phenotype: no decrease in expiratory flow below 50% from baseline. c LF (collapse) phenotype: immediate drop in expiratory flow down to zero within 30 s. d LP phenotype: decrease of expiratory flow to a plateau at 20–50% (which means a 50–80% decrease from baseline). Orange curves: expiratory flow. Blue curves: inspiratory pressure. * represents continuous expiratory flow without inspiration mode only detected in HF jet ventilation. CV, collateral ventilation; CV+, CV positive; CV−, CV negative; LF, low flow; LP, low plateau; HF, high frequency.
Figure 2 depicts the frequency of Chartis phenotypes (CV+, CV−, LF, and LP) according to the lung fissures in spontaneous breathing and HF jet ventilation. In most cases, the LMF was assessed as CV− in both ventilation modes (Fig. 2a), while the RUF was seen more often as CV+ (Fig. 2b). The LF phenotype was most prevalent when assessing the RMF (Fig. 2c) in both ventilation modes. The LP was a rare phenotype with both ventilation modes and present for all analyzed fissures (Fig. 2d).
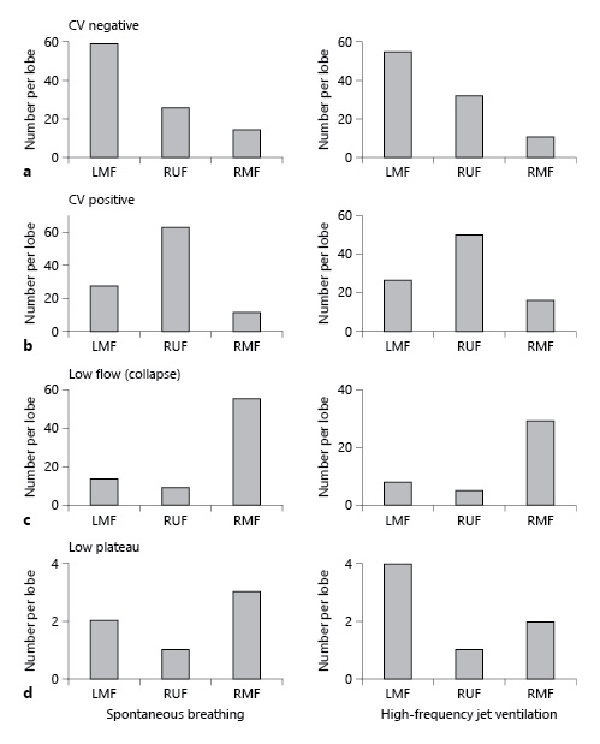
Fig. 2
Distribution of Chartis phenotypes in spontaneous breathing (left column) and in HF jet ventilation (right column) in the lung. a CV− phenotype: most frequently associated with the LMF in both modes. b CV+ phenotype: most frequently associated with the RMF in both modes. c LF (collapse) phenotype: most frequently associated with RMF in both modes. d LP phenotype: rarely seen evenly distributed in the lung in both modes. CV, collateral ventilation; RUF, right upper lobe fissure; RMF, right major fissure; LMF, left major fissure; CV+, CV positive; CV−, CV negative; LF, low flow; LP, low plateau; HF, high frequency.
Matching Chartis phenotypes in spontaneous breathing and HF jet ventilation were found with high concordance rate in assessments of the LMF (86.4%), RUF (88.6%), and RMF (87.3%) (Fig. 3). Cohen´s kappa coefficient was calculated to describe the agreement of Chartis assessments on both ventilation modes. High kappa values were reached, in detail: RUF κ = 0.783, RMF κ = 0.781, and LMF κ = 0.744.
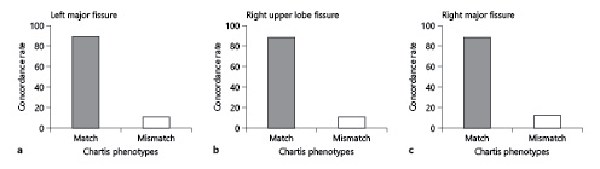
Fig. 3
High concordance rates of Chartis phenotypes in spontaneous breathing and HF jet ventilation in a LMF, b RUF, and c RMF. RUF, right upper lobe fissure; RMF, right major fissure; LMF, left major fissure; HF, high frequency.
In agreement, ROC analysis of both major fissures revealed comparable sensitivity and specificity of the FCS to predict conclusive Chartis phenotypes (CV− and CV+) under both ventilation modes (Fig. 4a, c). However, the area under the curve (AUC) was smaller for the RUF than that of both major fissures, where the AUC of HF jet ventilation was slightly higher than that of spontaneous breathing to discriminate FCS according to conclusive Chartis phenotypes (0.681 vs. 0.781, Fig. 4b).
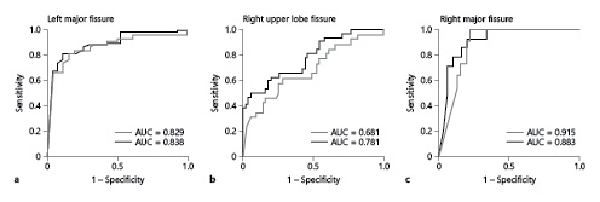
Fig. 4
Similar ROC curves of fissure integrity predicting CV− or CV+ status in spontaneous breathing (gray) and HF jet ventilation (black). a LMF. b RUF. c RMF. AUC, area under the curve; ROC, receiver operating characteristic; RUF, right upper lobe fissure; RMF, right major fissure; LMF, left major fissure; HF, high frequency; CV+, CV positive; CV−, CV negative.
No correlation between mismatching Chartis outcomes and the FCS was found. Furthermore, Chartis mismatches did not have a predilection for any of the analyzed fissure. All Chartis mismatches and their respective FCS are shown in Table 2. Three patient examples of these Chartis mismatches can be seen in Figure 5.
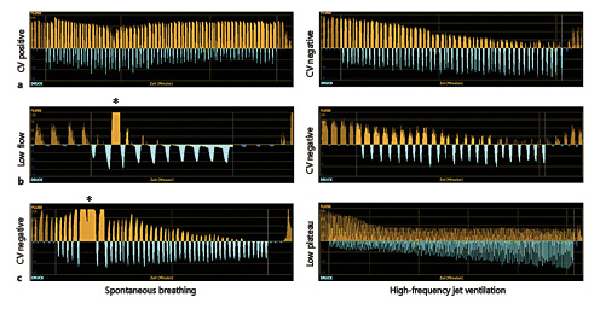
Fig. 5
Patient examples of mismatching Chartis phenotypes in spontaneous breathing (left column) and HF jet ventilation (right column) all measured in the left upper lobe. a Change from CV+ (left column) to CV− (right column). The FCS for the LMF was 86.1%. b Change from LF (left column) to CV− (right column). The FCS for the LMF was 100%. c Change from CV− (left column) to LP (right column). The FCS for the LMF was 86.1%. Orange curves: expiratory flow. Blue curves: inspiratory pressure. * indicates deeper expiration maneuvers most likely caused by pressing. CV, collateral ventilation; FCS, fissure completeness score; CV+, CV positive; CV−, CV negative; LF, low flow; LP, low plateau; HF, high frequency; LMF, left major fissure.
Discussion
The most important predictor for endobronchial valve treatment success is the exclusion of CV in the target lobe []. For CV assessment, Chartis and CT-based quantitative fissure analysis are used in clinical routine [-, ]. To our knowledge, studies analyzing the impact of ventilation mode on Chartis assessment outcome are lacking.
This study showed as its key finding that the ventilation mode during Chartis assessment, whether being spontaneous breathing or HF jet ventilation, has no effect on the resulting measurements. The frequency of Chartis phenotypes did not change, and there were high concordance rates among all phenotypes using either ventilation mode. In addition, ROC analyses revealed almost same predictive values for conclusive Chartis phenotypes among both major fissures to FCS independent of the ventilation modes. Nevertheless, the AUC of RUF was minimally higher for HF jet ventilation than in spontaneous breathing suggesting that HF jet ventilation might be more precise in predicting FCS in RUF.
Endobronchial valve treatment has become a well-established treatment for lung volume reduction in severe emphysema. In numerous studies, Chartis assessment has proven to be safe and effective in evaluating CV in vivo, using rigid or flexible bronchoscopy [, -]. The CV status can be classified according to recently described Chartis phenotypes (CV−, CV+, LF, and LP), with low inter- and interobserver variability []. Key factors affecting the outcome are adequate sedation, analgesia, and secretion management []. In flexible bronchoscopies, sedation must be sufficient to keep periprocedural coughing and movements to an absolute minimum, while apneic episodes should be avoided. HF jet ventilation is a safe and frequently used ventilation mode during flexible and rigid bronchoscopies, as it delivers a gas mix as a high-pressure stream to the bronchial system using rates of 60–300 breaths/min, thereby keeping airways and alveoli almost motionless [-]. Even though data about the use of HF jet ventilation are lacking, in our experience it is widely used in most German centers specialized in emphysema treatment.
Procedural sedation in spontaneous breathing usually allows lighter sedation depths, while an advantage of high jet ventilation is the possibility to better sedate the patient thereby improving conditions for Chartis assessments. However, sedation technique and ventilation mode should be chosen according to the patients cardio-/respiratory needs.
CV− was more commonly found at the LMF, while CV + appeared to be more prevalent with the RUF (formed by the right minor fissure and parts of the RMF). In line with previous studies, the FCS was less accurate predicting the CV status for the RUF compared to both major fissures []. The exact reason why FCS is inferior at discriminating Chartis phenotypes for the RUF is not completely understood and still under investigation []. In our study, HF jet ventilation was able to predict FCS more accurately for the RUF. Data showing clinical outcomes of valve treatment in lobes with discordant FCS and Chartis measurements are lacking. Until then, our study results should be used with caution as they do not necessarily indicate Chartis measurements of the RUF with jet ventilation to be superior to spontaneous breathing.
In an earlier study, Gieserich et al. [] found a significantly higher number of the inconclusive LF phenotype when performing Chartis measurements using rigid bronchoscopy combined with HF jet ventilation as compared to flexible bronchoscopy and spontaneous breathing. This might be due to deeper sedation used for this setting, potentially causing more collapse phenomena in the bronchi. As a consequence, the investigators suggested that Chartis assessment should be performed under flexible bronchoscopy and spontaneous breathing as opposed to rigid bronchoscopy and HF jet ventilation []. However, data were drawn from two different patient cohorts at two different time periods and not by intraindividual comparisons during the same bronchoscopy, unlike our study. Another study showed that general anesthesia did not affect Chartis results compared to periodic sedation [].
Independent of the ventilation mode, inconclusive Chartis phenotypes remain a challenge in clinical practice. In our study, the LF phenotype appeared more commonly in the lower lobes, both with jet ventilation and spontaneous breathing, making assessment of CV status more difficult there [, ]. The underlying mechanisms of the LF/collapse phenotype are poorly understood. In emphysema, chronic inflammatory changes cause narrowing of the airways, thus increasing small airway resistance []. It has been suggested that Chartis assessment might increase airflow in obstructive airways during expiration where valves in the Chartis console are open, resulting in a sudden collapse of distal airways []. A possible explanation why LF appears more commonly in the lower lobes might be a combination of airway instability in emphysema and the close contact to the diaphragm causing an early collapse of the adjacent lobe. The second inconclusive Chartis phenotype LP is visually characterized by a continuous decrease in airflow, finally stabilizing at a plateau and is a rare phenotype equally distributed among all analyzed fissure. A possible explanation could be that microcollaterals open toward the end of Chartis assessment placing this phenotype as an intermediate between CV+ and CV− []. Due to the more frequent appearance of inconclusive phenotypes in the lower lobes, we recommend to assess the upper lobes first. If an inconclusive phenotype is obtained, the adjacent lobes should be investigated.
In our study, high concordance rates between Chartis measurements in spontaneous breathing and HF jet ventilation were reached. Nevertheless, approximately 10–15% of Chartis assessments were discordant irrespective of the assessed fissure and no correlation to the FCS was found. The exact pathomechanisms of discordant results remain unclear. Probably they have a multifactorial etiology. For example, periprocedural coughing, muscle pressing, endobronchial secretions, or changes in the sedation depths or slightly different balloon placements have an effect on Chartis assessment. In addition, peripheral mucous movement or changes of the muscular airway tonus might cause discordant results. To increase diagnostic accuracy, we recommend to assess a target lobe at least twice, whereby the value of multiple Chartis assessments of the same lobe should be addressed in further studies. However, the Chartis results should always be interpreted in the context of the FCS according to Koster et al. [] suggested.
Due to the retrospective nature of this study, selection bias might have been present. However, the large number of included Chartis assessments performed by various experienced examiners reduces this theoretical bias. Another limitation was the lack of functional data linking endobronchial valve treatment response and Chartis outcome in spontaneous breathing and HF jet ventilation, even though additional insight might be limited, since no relevant difference between the two ventilation modes was found. Unfortunately, not every lobe was assessed in all patients, which would have allowed a more complete picture of fissure integrity and the distribution of Chartis phenotypes. Chartis assessments were performed in the same order (first assessment in spontaneous breathing and second assessment in HF jet ventilation); however, at least theoretically, a random order might have impact on the results.
In conclusion, Chartis assessment is a useful and well-reproducible tool to determine CV status. The ventilation mode does not impact Chartis outcome, and ventilation should be mainly be selected according to the patients’ cardio-/respiratory needs. In our experience, the target lobe should be assessed at least twice for better accuracy.
Acknowledgments
The authors sincerely thank Leonore Erdmann and Enrico Schneemann for data management and technical support.
Statement of Ethics
The research presented in this article was conducted according to the standards of the World Medical Association Declaration of Helsinki and the appropriate guidelines for human studies. All data were derived from prospective open-label clinical studies in our institution which were approved by the Ethics Committee of the Charité Universitätsmedizin Berlin, Germany (EA2/149/17 and EA1/136/13). All patients consented to participation. Inability to sign the consent form was an exclusion criterion.
Conflict of Interest Statement
Jacopo Saccomanno, Christoph Ruwwe-Glösenkamp, Konrad Neumann, Felix Doellinger, Pavlina Lenga, Eva Pappe, and Norbert Suttorp have nothing to disclose.
Martin Witzenrath received funding for research from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Actelion, Bayer Health Care, Biotest, Boehringer Ingelheim, Noxxon, Pantherna, Quark Pharma, Silence Therapeutics, Takeda Pharma, and Vaxxilon and for lectures and advisory from Actelion, Aptarion, Astra Zeneca, Bayer Health Care, Berlin Chemie, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Novartis, Noxxon, Pantherna, Silence Therapeutics, Sinoxa, Teva, and Vaxxilon. Ralf-Harto Hübner reports personal fees and nonfinancial support from Olympus.
Funding Sources
No funding was received for this study.
Author Contributions
Jacopo Saccomanno, Christoph Ruwwe-Glösenkamp, and Ralf-Harto Hübner drafted, wrote, and critically revised the manuscript and contributed to data acquisition, analysis, and interpretation. Konrad Neumann contributed to data analysis and interpretation and critically revised the manuscript. Felix Doellinger contributed to data acquisition, analysis, and interpretation and critically revised the manuscript. Pavlina Lenga, Eva Pappe, Norbert Suttorp, and Martin Witzenrath contributed to data analysis and interpretation and wrote and critically revised the manuscript.
Data Availability Statement
The data that support the findings of this study are openly available in figshare.com at https://doi.org/10.6084/m9.figshare.14573082.
References
- 1. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–73. http://dx.doi.org/10.1056/NEJMoa030287.
- 2. Kenn K, Gloeckl R, Soennichsen A, Sczepanski B, Winterkamp S, Boensch M, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–7. https://journals.lww.com/transplantjournal/Fulltext/2015/05000/Predictors_of_Success_for_Pulmonary_Rehabilitation.30.aspxhttp://dx.doi.org/10.1097/TP.0000000000000472.
- 3. Janssen DJ, Wouters EF, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9(3):232–7. http://dx.doi.org/10.1097/SPC.0000000000000146.
- 4. Hartman JE, Vanfleteren LEGW, van Rikxoort EM, Klooster K, Slebos DJ. Endobronchial valves for severe emphysema. Eur Respir Rev. 2019;28(152). http://dx.doi.org/10.1183/16000617.0121-2018.
- 5. Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J. 2013;41(2):302–8. http://dx.doi.org/10.1183/09031936.00015312.
- 6. Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. Efficacy predictors of lung volume reduction with zephyr valves in a European cohort. Eur Respir J. 2012;39(6):1334–42. http://dx.doi.org/10.1183/09031936.00161611.
- 7. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A Randomized Study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–44. http://dx.doi.org/10.1056/NEJMoa0900928.
- 8. Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. Endobronchial valve therapy in patients with homogeneous emphysema. results from the IMPACT Study. Am J Respir Crit Care Med. 2016;194(9):1073–82. http://dx.doi.org/10.1164/rccm.201607-1383OC.
- 9. Gompelmann D, Eberhardt R, Herth FJ. Collateral ventilation. Respiration. 2013;85(6):515–20. http://dx.doi.org/10.1159/000348269.
- 10. Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax. 2006 May;61(5):371–3.
- 11. Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med. 1978;298(1):10–5. http://dx.doi.org/10.1056/NEJM197801052980103.
- 12. Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJ. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a Feasibility Study. Respiration. 2010;80(5):419–25. http://dx.doi.org/10.1159/000319441.
- 13. Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis. 2016;11:765–73. http://dx.doi.org/10.2147/COPD.S103807.
- 14. Koster TD, van Rikxoort EM, Huebner RH, Doellinger F, Klooster K, Charbonnier JP, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration. 2016;92(3):150–7. http://dx.doi.org/10.1159/000448849.
- 15. Klooster K, Koster TD, Ruwwe-Glösenkamp C, Theilig D, Doellinger F, Saccomanno J, et al. An Integrative approach of the fissure completeness score and Chartis assessment in endobronchial valve treatment for emphysema. Int J Chron Obstruct Pulmon Dis. 2020;15:1325–34. http://dx.doi.org/10.2147/COPD.S242210.
- 16. Welling JBA, Hartman JE, Ten Hacken NHT, Franz I, Charbonnier J-P, van Rikxoort EM, et al. Chartis measurement of collateral ventilation: conscious sedation versus general anesthesia – a retrospective comparison. Respiration. 2018;96(5):480–7.
- 17. Welling JBA, Klooster K, Hartman JE, Kerstjens HAM, Franz I, Struys MMRF, et al. Collateral ventilation measurement using Chartis: procedural sedation vs general anesthesia. Chest. 2019;156(5):984–90. http://dx.doi.org/10.1016/j.chest.2019.07.025.
- 18. Herzog D, Thomsen C, Poellinger A, Doellinger F, Schreiter N, Froeling V, et al. Outcomes of endobronchial valve treatment based on the precise criteria of an endobronchial catheter for detection of collateral ventilation under spontaneous breathing. Respiration. 2016;91(1):69–78. http://dx.doi.org/10.1159/000442886.
- 19. Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (transform). Am J Respir Crit Care Med. 2017;196(12):1535–43. http://dx.doi.org/10.1164/rccm.201707-1327OC.
- 20. Klooster K, Hartman JE, Ten Hacken NH, Slebos DJ. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration. 2016 Dec;93(2):112–21. http://dx.doi.org/10.1159/000453529.
- 21. Criner GJ, Delage A, Voelker KGfor the EMPROVE Trial Investigator Group. The EMPROVE trial – a Randomized, Controlled Multicenter Clinical Study to evaluate the safety and effectiveness of the spiration? valve system for single lobe treatment of severe emphysema. C24 New technologies for managing COPD [Internet]New York: American Thoracic Society; 2018. [zitiert 7 Jan 2021]. (American Thoracic Society International Conference Abstracts). S. A7753. https://doi.org/10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A7753.
- 22. Klooster K, Ten Hacken NHT, Hartman JE, Kerstjens HAM, Van Rikxoort EM, Slebos D-J. Endobronchial valve treatment versus standard medical care in patients with emphysema without interlobar collateral ventilation. Eur Respir J. 2015;46(Suppl 59):PA792. http://dx.doi.org/10.1183/13993003.congress-2015.pa792.
- 23. Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138–50. http://dx.doi.org/10.1159/000453588.
- 24. Pathak V, Welsby I, Mahmood K, Wahidi M, MacIntyre N, Shofer S. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc. 2014;11(4):628–34. http://dx.doi.org/10.1513/AnnalsATS.201309-302FR.
- 25. Rezaie-Majd A, Bigenzahn W, Denk DM, Burian M, Kornfehl J, Grasl MC, et al. Superimposed high-frequency jet ventilation (SHFJV) for endoscopic laryngotracheal surgery in more than 1500 patients. Br J Anaesth. 2006;96(5):650–9. http://dx.doi.org/10.1093/bja/ael074.
- 26. Brice JW, Davis WB. High frequency ventilation in the adult. Clin Pulm Med [Internet]. 2004;11(2).
- 27. Gesierich W, Samitas K, Reichenberger F, Behr J. Collapse phenomenon during Chartis collateral ventilation assessment. Eur Respir J. 2016;47(6):1657–67. http://dx.doi.org/10.1183/13993003.01973-2015.
- 28. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53. http://dx.doi.org/10.1056/NEJMoa032158.
- 29. Hubner RH, Herzog D. COPD treatment: about collateral channels and collapsing airways. Eur Respir J. 2016;47(6):1606. http://dx.doi.org/10.1183/13993003.00343-2016.