Introduction
Pulmonary hypertension (PH) affects 15 people per million inhabitants []. PH is defined by an increase in mean pulmonary arterial pressure (pulmonary pressure equal or superior to 25 mm Hg) with normal or decreased cardiac output (CO). PH is divided into 5 groups (Nice classification 2013) []: group 1 with idiopathic, family-related, and other causes such as connective diseases, HIV, and anorectic treatment; group 2 with left heart disease; group 3 with chronic respiratory diseases; group 4 with chronic thromboembolic disease; and group 5 with various causes. Echocardiography is the reference screening technique. Arterial pulmonary pressure is calculated according to the tricuspid insufficiency flow rate. However, it can prove difficult to measure CO outside of optimal conditions. Right heart catheterisation and measurements of pulmonary pressure are carried out to confirm the disease []. Catheterisation also measures CO, which is a prognostic factor in PH. A cardiac index below 2 L/min/m2 is linked to a poor prognosis []. Right heart catheterisation using the direct Fick method is the reference technique for CO measurement, although thermodilution (TD) is often preferred for disease follow-up and must be carried out every year to measure CO and adapt treatment []. However, right heart catheterisation is an invasive method with possible side effects []. A new and simple non-invasive method is required to record CO measurements at rest for initial management and monitoring pharmacological procedures.
Impedance cardiography (IPc) is a known non-invasive method, comprising an electric representation of cardiac flow. Six electrodes are placed on the chest. Impedance variations following injection of a weak electrical current (3.8 mA) with high frequency (75 Hz) are recorded, and represent variations in blood flow in the chest. IPc is already used to measure CO in patients with heart failure. Measuring cardiac haemodynamics with IPc during cardiopulmonary exercise testing has a prognostic value in patients with heart failure [, ]. In PH, numerous studies show that there is a good correlation between IPc and TD. Comparison with the gold standard, the Fick method, is rarely made. A modern IPc system with new software set to improve the accuracy and precision of the measurements has been developed in recent years, known as the PHYSIOFLOW® system. This technique is independent of baseline thoracic impedance with a newly developed filtering technique, so its precision is better than the previous IPc systems. PHYSIOFLOW® has been compared to TD in patients with PH or with a suspicion of PH [], with a good correlation. The purpose of this study is to compare the measurement of CO by IPc using PHYSIOFLOW® to the gold standard, the Fick method, and to TD in a select population with PH: patients with arterial PH or chronic thromboembolic PH.
Material and Methods
Study Population
A prospective single-centre study was carried out at the University Hospital of Toulouse. A total of 91 consecutive patients followed up by the Arterial Pulmonary Hypertension Competency Centre and who underwent right heart catheterisation were invited to participate. The inclusion criteria were as follows: patients over 18 years of age, with arterial PH (group 1) or chronic thromboembolic PH (group 4), undergoing right heart catheterisation as part of the disease follow-up procedure. This study was approved by a local ethic committee (Comité d’éthique, CHU Toulouse, France).
Objective
The primary objective of the study was to compare CO measurements by IPc, PHYSIOFLOW®, and the direct Fick method. The secondary objectives were the comparison of CO measurements between IPc and TD, and the determination of factors which affected the accuracy and precision of the CO measurement by IPc.
The patients were hospitalised for right heart catheterisation following the administration of a new treatment in order to estimate its efficiency or as part of the standard disease follow-up procedure. CO levels were measured simultaneously by impedance and by using the direct Fick method and TD.
Right Heart Catheterisation
Haemodynamic evaluation was performed in the supine position and in ambient air. A 7-Fr quadruple-lumen, balloon-tipped, flow-directed Swan-Ganz catheter (Ref. 131F7, Edwards Lifesciences, Irvine, CA, USA) was advanced through a 7-Fr introducer sheath inserted into the right or left femoral vein and advanced under fluoroscopic guidance into the pulmonary circulation. The catheter was advanced into the pulmonary artery until the pressure recording through the proximal lumen revealed a pulmonary artery pressure curve. In all patients, simultaneous measurements of the CO by IPc and the direct Fick method were performed. Haemodynamic evaluation included right atrial pressure (RAP), mean pulmonary artery pressure (mPAP), pulmonary artery wedge pressure (PAWP), and CO (Fick and TD). PVR was calculated as (mPAP – PAWP)/CO. Cardiac index was calculated as CO/body surface area.
The TD Technique
CO levels were measured using the TD technique, whereby 10 mL of sterile isotonic (0.9%) saline was injected at room temperature through the proximal lumen of the pulmonary artery catheter. The positioning of the Swan-Ganz probe was confirmed by radioscopic control. We averaged at least 5 stable (< 10%) CO determinations.
The Direct Fick Method
CO was measured using the direct Fick method. A face mask was placed over each patient’s face. Average steady-state oxygen consumption was recorded using a portable indirect calorimetry monitor (Fick Cosmed C09066-02-99) that analyses the differential partial oxygen pressures of inhaled and exhaled gas using an oxygen sensor. The gas flow was measured via a gas dilution system. The devices were calibrated before each study and the values were time-averaged over at least 6 min. Simultaneous arterial (radial or femoral arterial puncture) and mixed venous blood (distal port of the pulmonary artery catheter) samples were drawn for measurement of arterial oxygen saturation (SaO2) and haemoglobin (Hgb) concentration. Oxygen content was calculated as follows:
Oxygen content = (1.34 × Hgb × SaO2) + (0.003 × PO2).
CO Fick was then calculated by dividing the average oxygen consumption (VO2) value by the difference between the arterial oxygen (CaO2) concentration and mixed venous oxygen content (CvO2):
CO Fick = VO2/CaO2 – CvO2.
Impedance Cardiography
Non-invasive CO measurements were obtained with the PHYSIOFLOW® Enduro PF-0459 device (Manatec Biomedical, France) by the same regular operator who was unaware of the right heart catheterisation results. The skin was prepared with a mildly abrasive gel. Two electrodes (Ref. PF-50), one transmitting and one receiving, were applied to the base of the neck on the left, and two more along the xyphoid area. In addition, two electrodes were positioned to monitor a single electrocardiogram signal.
Impedance variations are related to the change in intrathoracic fluid and thus stroke volume. An equation is used to deduce the systolic ejection volume according to the variation in impedance over time: VES = TEGV [(dz/dt) max/Z0], where TEGV is the ejection time of the left ventricle, K is a constant according to the sex, age, and size of the patient, Z0 is the basic impedance, and dz/dt the variation in impedance over time. The PHYSIOFLOW® system is more precise than other systems of IPc. In fact, basic impedance changes according to the intrathoracic fluids and, in particular, if the patient has a pleural effusion or pulmonary oedema. This system does not use basic impedance and so the measurements are more precise.
We averaged the IPc measurements obtained over a 20-min recording period. We collected data on patients (body mass index, age), disease (PH group, diagnostic or follow-up), and treatment. Laboratory data were also recorded such as NT pro-BNP. The presence of an intracardiac shunt and cardiac arrhythmia was noted. An electrocardiogram was therefore taken before the procedure and all patients underwent a Doppler ultrasound scan before the examination.
Statistics
Data are expressed as mean ± standard deviation (SD). CO measured by IPc, Fick, or TD were correlated by linear regression with the calculation of the Pearson coefficient of correlation. To evaluate accuracy, we calculated the agreement (bias) between methods using the Bland-Altman analysis. Bias is the mean of the differences obtained via the different techniques. Mean bias (accuracy), 95% limits of agreement, and percentage error were calculated. The limits of agreement are defined as mean ± 2 SD. The percentage error between different methods was evaluated using the Critchley and Critchley method []. A bias inferior to 0.3 L/min seems acceptable to consider the IPc as reliable. A percentage inferior or equal to 30% was necessary to confirm the comparability between both techniques. To test differences in CO between the various methods using different factors, we used the Mann-Whitney or the Kruskal-Wallis test, as appropriate. Only significant variables with a p < 0.2 in univariate analyses were used in the multivariate analysis. A p value < 0.05 was considered significant. Excel XLSTAT® statistics software was used.
Results
Selected Patients
A total of 91 consecutive patients were screened and 75 patients were enrolled. Of the 16 patients excluded, the IPc signal could not be interpreted in 10 patients. In these patients, there was a problem when starting the software, so the signal was not recorded correctly. In 6 patients, the recording was performed twice in order to initially study the intra-individual reproducibility of the measurements. The mean recording time was 20 min. The IPc signal was considered stable in 52 patients with a variation of < 1 L/min. In the case of unstable signals, all the values recorded were averaged.
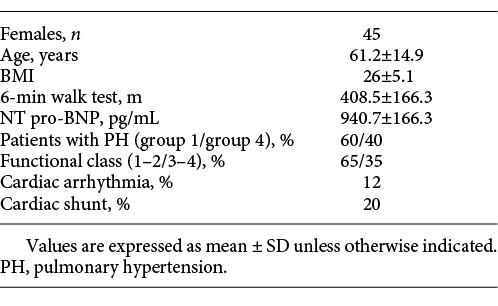
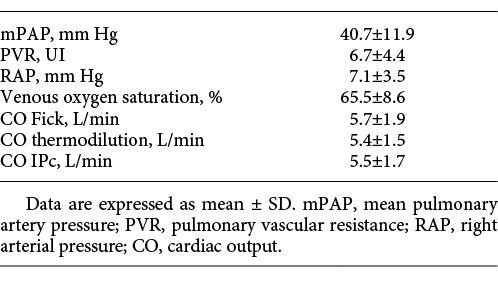
The 75 patients had an average age of 61.2 years (±14.9); 51% of patients were males. A total of 80% of all patients were followed up for PH in group 1 and 20% in group 4. The baseline clinical characteristics of patients and the haemodynamic characteristics of patients obtained by right heart catheterisation are presented in Tables 1 and 2, respectively. The presence of a cardiac shunt was noted in 20% of patients (the signal for these patients was less stable with a variation superior to 1 L/min); 12% of patients had arrhythmia. The mPAP was 40.7 ± 11.9 with a mean sPAP of 67.3 ± 20.1.
Comparison of the Methods
IPc and the Fick Method
The mean CO measurements using IPc and the Fick method were 5.5 ± 1.7 and 5.7 ± 1.9 L/min, respectively. There was no difference between CO measurements using IPc and those using the direct Fick method (p = 0.524).
The regression equation had a slope of 0.36 ± 0.1 and an intercept of 3.4 ± 0.6. These methods were correlated with r = 0.186 (p < 0.001). According to the analysis technique described by Bland and Altman, we found a correlation between CO measurements by IPc and the direct Fick method. Indeed, the bias was 0.149 L/min (95% confidence interval, CI, –0.298 to 0.596) (Fig. 1). The limits of agreement ranged from –3.27 to 3.56 L/min. The percentage error was ±22.5%, and 28% of measurements had a 30% or more difference with the average of these measurements. Measurements with a 30% or more difference with the average had extreme values of CO: < 3 L/min or > 7 L/min of CO.
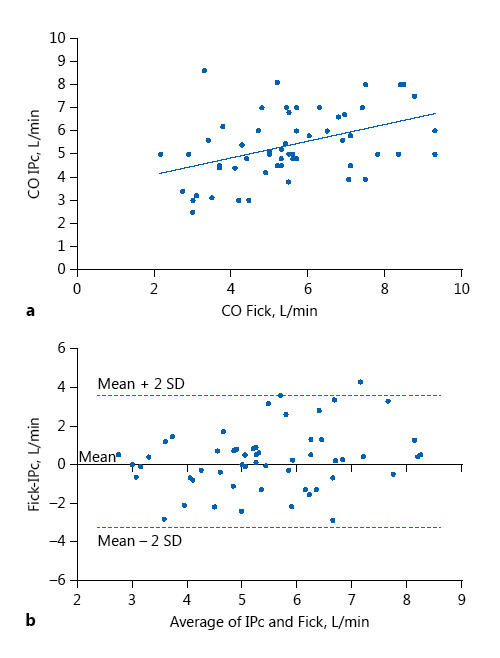
Fig. 1
Relationship between cardiac output (CO) measured by impedance cardiography (IPc) and CO measured by the direct Fick method. a Plot of CO measured by IPc versus the direct Fick method. The regression equation is CO IPc = 3.4 + 0.36 × CO Fick r = 0.186. b Bland-Altman plots of IPc versus the direct Fick method. The solid line represents the mean (0.149 L/min) and the broken lines the 95% limits of agreement (–3.27 to 3.56).
We looked for factors that can influence the correlation between the different methods. In multivariate analysis, the RAP influences the agreement between the Fick method and IPc. An RAP > 7 mm Hg therefore decreases the agreement, and the agreement is improved 3-fold with an RAP < 7 mm Hg (p = 0.08); 81% of patients had an RAP > 3 mm Hg and 28% of patients (n = 21) had an RAP > 7 mm Hg. Moreover, the correlation with the direct Fick method is improved 2-fold when the BMI is > 22 (p = 0.15). In the regression equation, the correlation is better when patients had a BMI > 22 and an RAP < 7 mm Hg (r = 0.263, p = 0.012)
IPc and TD
The mean CO was 5.4 ± 1.5 L/min measured by TD and 5.5 ± 1.7 L/min by impedance. There was no difference between CO measured by IPc and that measured by TD (p = 0.312). The regression equation had a slope of 0.7 ± 0.1 and an intercept of 1.7 ± 0.6. These methods were correlated with r = 0.365 (p < 0.001). Figure 2 shows the agreement between IPc and TD. The agreement was also correct with a bias of –0.153 L/min (95% CI, –0.450 to 0.153). The limits of agreements ranged from –2.7 to 2.4 L/min and the percentage error was ±21% (18% of measurements had a 30% or more difference with the average of these measurements). The measurements which had a 30% difference with the average of the measurements had an average CO of 3.7 ± 1.2 L/min.
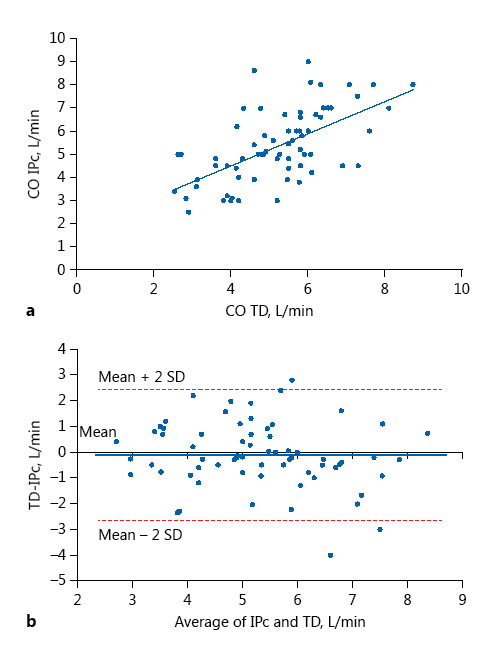
Fig. 2
Relationship between cardiac output (CO) measured by impedance cardiography (IPc) and CO measured by thermodilution (TD). a Plot of CO measured by IPc versus TD. The regression equation is CO IPc = 1.7 + 0.7 × CO TD r = 0.365. b Bland-Altman plots of IPc and TD. The solid line represents the mean (–0.153 L/min) and the broken lines the 95% limits of agreement (–2.7 to 2.4).
Patients monitored for PH in group 4 had a greater agreement between the two techniques in multivariate analysis. This significantly improves the agreement with an odds ratio of 4 (p = 0.04). RAP > 7 mm Hg and cardiac arrhythmia decrease the correlation between the two methods, but these results are not significant (OR = 0.8, 95% CI, 0.25 to 2.6, p = 0.7 and OR = 0.42, 95% CI, 0.04 to 4.4, p = 0.47, respectively). Regardless of the measurement method, the presence of a cardiac shunt or the value of the mean pulmonary pressure did not affect the correlation between the methods.
Discussion
The results of this study show that the measurement of CO by IPc in PH patients is reliable compared to the direct Fick method and TD obtained by right heart catheterisation.
The direct Fick method is the gold standard for measuring CO in PH, but due to several technical constraints in measuring VO2, this method is rarely used. TD is most often employed to determine CO, even if the precision of this method is influenced by several factors: technical factors (temperature and volume of injectate, speed and mode of injection) and factors depending on patients (cardiac shunt, valvular heart diseases, paediatric patients) []. For many years, different methods have been tested to overcome the technical difficulties of these methods. Lador and al. [] tested a non-invasive technique that relies on arterial pulse pressure wave analysis: Modelflow®. This non-invasive technique was compared to TD in 50 patients with group 1 or group 4 PH. Modelflow® was a precise and accurate method for determining CO, with a bias of 0.72 L/min. Nevertheless, this technique had several technical limitations with a possible poor finger pulse wave signal. Other non-invasive techniques with good correlation with the Fick method or TD have already been studied: the acetylene rebreathing technique or the non-invasive CO monitor (bioreactance; NICOM). However, these techniques can fail in certain situations, like when the capillary-alveolar membrane is damaged [, ]. Cardiac magnetic resonance is an emerging technique in the diagnosis of PH. A morphological analysis (right ventricular size and morphology) and a functional analysis (CO) are possible with this technique []. CO can be measured using this technique, and there is a good correlation with CO measured by right heart catheterisation [-]. However, cardiac magnetic resonance is expensive and not widely available. IPc is another technique studied in the literature for measuring CO in PH. IPc is a non-invasive method, which measures blood flow in the chest. This technique highlights the condition of the circulatory system and changes in haemodynamic status. This approach is an easy, fast, and cost-effective technique. Studies evaluating IPc have been realised in a varied patient cohort [, ]. For example, IPc has been used in pulmonary congestion. Ebert et al. [] thus showed a good correlation between changes in central venous pressure and chest impedance. Some studies have evaluated the use of IPc in PH. In a study published in 2000, the difference in CO levels obtained by IPc and TD was 0.31 L/min and the coefficient of correlation was 0.72 (p < 0.001) []. In another study published in 2003 [], the correlation was good (r2 = 0.658, p < 0.001). In the study conducted by Yung and al. [], the bias between IPc and the direct Fick method was –0.24 L/min (95% CI, –1.74 to 1.5), and that between IPc and TD was –0.43 L/min (95% CI, –2.2 to 1.59). The PHYSIOFLOW® system is a new-generation impedancemetry system. Previous studies have focused on the precision of the PHYSIOFLOW® system. Charloux and al. [] showed that the PHYSIOFLOW® system is as precise as the direct Fick method during exercise with a bias of 0.07 L/min (95% CI, –1.1 to 1.3). In a study conducted by Tonelli et al. [], the PHYSIOFLOW® system was compared to TD in 39 patients with PH or suspected PH. The CO measured by IPc was 5.9 ± 2 L/min compared to 5.6 ± 1.5 L/min by TD. An average difference of 0.3 L/min was recorded. Recently, Panagiotou et al. [] found no correlation between IPc and TD, but the number of patients in their study was small. The originality of our study is in comparing CO measurements taken by IPc to those with the direct Fick method in a population already followed for PH. In the previous studies, not all of the patients had PH and there were multiple causes of PH. Our study is the first to study IPc with the PHYSIOFLOW® system in PH groups 1 and 4 to compare with the direct Fick method and TD. Moreover, we investigated factors influencing the correlation.
We found that there is a good correlation between CO measured by IPc and the direct Fick method and between CO measured by IPc and TD. The bias was 0.149 and –0.153 L/min, respectively. This bias can be considered acceptable for this population with PH with a potential CO < 5 L/min. Our study confirms the results showed in the study of Tonelli et al. [] but with a greater correlation thanks to the Bland-Altman method in our selected population. The percentage error was inferior to 30%. Therefore, according to these data, this technique is acceptable []. However, we need to moderate our results. Indeed, the accuracy is low with limits of agreement, which are wide. The dispersion of measurements is important, decreasing the reliability of IPc. So, for the extreme values of CO (< 3 and > 7 L/min) the accuracy is weak and it is necessary to keep a critical eye on the results. Many factors can influence IPc results and explain this dispersion. Valvular disease, arrhythmia, and low CO are all causes of signal instability and so can decrease the precision of the measurements []. In our study, 12% of patients had arrhythmia and 19% had extreme values of CO, which could explain this dispersion. Moreover, tricuspid regurgitation is one piece of data that was not collected. Other causes of this signal instability include the operator’s level of experience and the position of the electrodes. The difficulty in this technique lies in the correct positioning of the electrodes. Incorrect electrode positioning will give a bad signal with a significant variation. In our study, the value of CO by IPc is an average of measurements obtained by a recording time of 20 min, which may call into question the simultaneous nature of the measurements made during our study. The measuring of CO by TD is also an average of many results. The heart is a constantly moving organ, making it difficult to study and to have a stable signal, regardless of the technique used. We investigated other factors influencing the correlation, such as elevated vascular resistance. We know that a low CO decreases the accuracy of the measurements, and RAP is the ratio of mPAP and CO. It therefore makes sense that an elevated RAP decreases the reliability of the IPc measurement. Nevertheless, only a select few patients are affected by this elevated RAP.
Other limits to our study include the lack of values concerning the inter- and intra-individual reproducibility of the measurements. Indeed, the reproducibility of the measurement of CO by IPc in the same patients was not analysed.
However, this new technique does have advantages. It is less invasive, cheaper, and easier to set up. Moreover, IPc is an ambulatory technique. The role of this technique has yet to be defined, but its routine use in consultation should allow treatment to be adapted and PH decompensation to be avoided. It would be interesting to carry out a study comparing the number of cases of decompensation based on the use or not of IPc in the adaptation of the treatment in consultation. However, 20 min are necessary to realise this test, and so, in consultations, its applicability may be difficult. The recording time is the same as that for an echocardiograph. Reducing the number of cases of decompensation will compensate for the limits of this technique, such as its recording time of 20 min.
Conclusion
CO can be measured in patients with arterial pH or chronic thromboembolic PH using IPc. After comparison with the direct Fick method and TD, this technique is reliable, even if many factors decrease the precision of the measurements. Other studies are required to confirm the inter- and intra-individual reproducibility of the measurements and so to use this technique in consultation.
Financial Disclosure and Conflicts of Interest
The company that provided the PHYSIOFLOW® had no role in the design of the trial, the analysis or interpretation of the data, the preparation of the manuscript, and the decision to submit the manuscript for publication.
References
- 1. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al: Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030.
- 2. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al: Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl 25):D34–D41.
- 3. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery J-L, Barbera JA, et al: Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34: 1219–1263.
- 4. Swiston JR, Johnson SR, Granton JT: Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: a systematic review of the literature. Respir Med 2010; 104: 1588–1607.
- 5. Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al: 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.
- 6. Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, et al: Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006; 48: 2546–2552.
- 7. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ: 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133:e694–e711.
- 8. Myers J, Wong M, Adhikarla C, Boga M, Challa S, Abella J, et al: Cardiopulmonary and noninvasive hemodynamic responses to exercise predict outcomes in heart failure. J Card Fail 2013; 19: 101–107.
- 9. Tonelli AR, Alnuaimat H, Li N, Carrie R, Mubarak KK: Value of impedance cardiography in patients studied for pulmonary hypertension. Lung 2011; 189: 369–375.
- 10. Critchley LA, Critchley JA: A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999; 15: 85–91.
- 11. Nishikawa T, Dohi S: Errors in the measurement of cardiac output by thermodilution. Can J Anaesth J Can Anesth 1993; 40: 142–153.
- 12. Lador F, Hervé P, Bringard A, Günther S, Garcia G, Savale L, et al: Non-invasive determination of cardiac output in pre-capillary pulmonary hypertension. PLoS One 2015; 10:e0134221.
- 13. Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, et al: Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 1999; 160: 535–541.
- 14. Rich JD, Archer SL, Rich S: Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J 2013; 42: 125–133.
- 15. Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr: Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1999; 1: 7–21.
- 16. Paz R, Mohiaddin RH, Longmore DB: Magnetic resonance assessment of the pulmonary arterial trunk anatomy, flow, pulsatility and distensibility. Eur Heart J 1993; 14: 1524–1530.
- 17. Robertson MB, Köhler U, Hoskins PR, Marshall I: Quantitative analysis of PC MRI velocity maps: pulsatile flow in cylindrical vessels. Magn Reson Imaging 2001; 19: 685–695.
- 18. Beerbaum P, Körperich H, Barth P, Esdorn H, Gieseke J, Meyer H: Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation 2001; 103: 2476–2482.
- 19. Sageman WS, Riffenburgh RH, Spiess BD: Equivalence of bioimpedance and thermodilution in measuring cardiac index after cardiac surgery. J Cardiothorac Vasc Anesth 2002; 16: 8–14.
- 20. Albert NM, Hail MD, Li J, Young JB: Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care 2004; 13: 469–479.
- 21. Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK: The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med 1986; 57: 49–53.
- 22. Barin E, Haryadi DG, Schookin SI, Westenskow DR, Zubenko VG, Beliaev KR, et al: Evaluation of a thoracic bioimpedance cardiac output monitor during cardiac catheterization. Crit Care Med 2000; 28: 698–702.
- 23. Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML: Impedance cardiography: the next vital sign technology? Chest 2003; 123: 2028–2033.
- 24. Yung GL, Fedullo PF, Kinninger K, Johnson W, Channick RN: Comparison of impedance cardiography to direct Fick and thermodilution cardiac output determination in pulmonary arterial hypertension. Congest Heart Fail 2004; 10(2 suppl 2): 7–10.
- 25. Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M, Mettauer B, et al: A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol 2000; 82: 313–320.
- 26. Panagiotou M, Vogiatzis I, Jayasekera G, Louvaris Z, Mackenzie A, Mcglinchey N, et al: Validation of impedance cardiography in pulmonary arterial hypertension. Clin Physiol Funct Imaging 2017, DOI: 10.1111/cpf.12408.
- 27. Bour J, Kellett J: Impedance cardiography: a rapid and cost-effective screening tool for cardiac disease. Eur J Intern Med 2008; 19: 399–405.
M. Dupuis and E. Noel-Savina contributed equally to this work.