Introduction
Breath-hold (BH) diving is a form of underwater diving that relies on divers’ ability to hold their breath until resurfacing. Some synonyms used are free diving, skin diving, or apneic diving.
BH diving has gained worldwide popularity. Within that community, quite a few athletes compete for depth or BH duration in several disciplines []. Some elite athletes have held their breath for more than 11 min or dived down to depths exceeding 200 m seawater. While these achievements are remarkable in terms of physiology, it should be acknowledged that these extreme exposures incur inherent risks. There have been serious incidents during record attempts, some of which had a fatal outcome []. The statement “breath-hold diving is the most dangerous diving activity” – although already 40 years old – has proven to be true [].
BH divers are exposed to important challenges such as hypoxia, water immersion, and increased ambient pressure []. While these factors affect the body as a whole, the pulmonary system is uniquely challenged by substantial changes in intrapulmonary gas volumes and pressures. In the following, we focus on the effects of BH deep diving on the pulmonary system.
Breath Holding: Easy-Going and Struggle Phase
A voluntary BH can be divided into an initial “easy-going” phase followed by a “struggle” phase (Fig. 1). The “easy-going” phase is the period of entirely voluntary control of respiratory muscle activity. This ends after arterial pCO2 exceeds approximately 45–60 mm Hg, when involuntary inspiratory efforts begin [, ]. These muscular contractions, called the “struggle” phase, produce waves of more negative intrathoracic pressure as those shown in Figure 1.
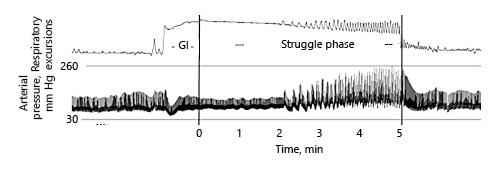
Fig. 1
Easy-going and struggle phase during breath holding. Relatively early, the struggle phase starts with involuntary contractions of the thorax and diaphragm. In parallel, the arterial peak pressure increases up to values of 260 mm Hg. GI: glossopharyngeal insufflation. Reproduced with permission from Heusser et al. [].
As the duration of breath holding increases, air hunger increases []. While arterial pCO2 increases during apnea, involuntary contractions of the thorax and the diaphragm become unavoidable [, ]. These contractions occur at the end of the “easy-going phase” of maximal apneas producing waves of negative intrathoracic pressure [] (Fig. 1). Because the intrathoracic pressure is likely already negative at greater depths, additional negative-pressure waves might well contribute to alveolar hemorrhage damaging the pulmonary capillaries [] in which pressure is considerably increased due to the blood shift (see below).
Submersion and Diving Response
Bradycardia as a physiologic response to submersion is well known to occur in all vertebrates and has been recognized also in humans [, ]. With regard to the respiratory system, bronchoconstriction [] has been described as a component of the diving response. Thus, submersion per se, whether it be while swimming, snorkeling, or BH diving has an effect on the pulmonary system.
Pulmonary Volumes versus Ambient Pressure
If a closed volume of gas undergoes a change in pressure, the ratio of initial to final volumes equals the inverse of the ratio of initial to final pressures. Thus, during apneic deep diving, pulmonary gas volume decreases as ambient pressure increases. In the extreme, total lung capacity (TLC) can be reduced to the residual volume (RV) [] because at RV, alveolar collapse becomes possible [, ]. As the lungs are heterogeneous, alveolar collapse is also possible at volumes above RV. Still, it was concluded that the ratio between TLC and RV would determine the BH diver’s maximum dive depth []. Using typical values (TLC = 6.0 L; RV = 1.2 L), a total pressure of 5 bar (= 40 m) would be tolerable. In the meantime, this 50-year-old conception has proven to be wrong.
Nevertheless, deep depths apparently create intrathoracic pressures lower than the ambient pressure and thus pulmonary barotrauma can develop during the descent. Among BH divers, this barotrauma has been termed lung squeeze, which is not uncommon [] and is accompanied by pulmonary edema and hemorrhage that can be exhibited by ultrasound [, ] and computed tomography [] (Fig. 2).
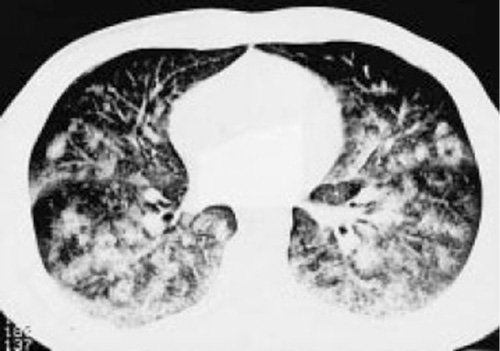
Fig. 2
Thoracic computed tomography. Disseminated alveolar opacification after a BH deep dive. Reproduced with permission from the European Respiratory Society© European Respiratory Journal [].
The results of a more recent study convincingly confirm the pathophysiologic importance of the Boyle-Mariotte law, as the frequency of hemoptysis clearly increased with the personal maximum diving depth; i.e., with sub-RVs. While the frequency of hemoptysis at depths between 12 and 25 m was 7%, at depths between 56 and 76 m it dramatically increased to 88% [].
Hemoptysis may be the visible consequence of lung squeeze, although slight injuries might remain subclinical. On the other hand, regular competitive apnea diving over a few seasons might carry a chronic cardiopulmonary risk leading from early functional changes to the manifestation of pulmonary hypertension [].
Blood Shift
As diving depths attained are considerably greater than the early 40 m, further mechanisms must be invoked to prevent lung squeeze at depth: blood shift is one of these mechanisms. The amount of blood shifted can be divided into two proportions. One is related to immersion-induced buoyancy [, ], and the other is related to the increasing ambient pressure.
After immersion, blood from the periphery is shifted towards the thorax. Such blood volumes for men vary from 0.7 L [] to 1.2 L []. Thus, given a RV of 1.2 L at the surface, shifting 0.7 L blood into the pulmonary capillaries will reduce it to 0.5 L.
Significant pulmonary vascular engorgement due to immersion in combination with physical exercise have been reported to enhance the risk of immersion pulmonary edema [, ]. Extreme exercise will lead to an increase in pulmonary arterial and left atrial pressures, and hypoxia may contribute to higher pulmonary arterial pressure and further increases the likelihood of pulmonary edema. This condition with sudden onset in swimmers, BH divers, and scuba divers is suspected to be aggravated by cold water [].
The other proportion of blood shift is related to the increasing ambient pressure during the dive, which shifts additional blood from the periphery to the chest. The amount of blood shifted is currently unknown but offers an additional depth on the “safe” side.
The above mechanisms will increase the left ventricular (LV) end-diastolic volume and in consequence the LV filling pressure. These changes mean a rightward shift on the end-diastolic pressure-volume relation and thus an increased LV compliance.
Breathing Techniques
Beside blood shift, particular breathing techniques allow for BH diving to increasingly greater depths. During hyperventilation, one of these techniques, the rate or tidal volume of breathing, eliminates more carbon dioxide than the body produces []. Actively hyperventilating lowers the concentration of carbon dioxide dissolved in the blood, and the resulting hypocapna delays the urge to breathe. Hence, hyperventilation has no direct impact on the lungs.
In the following, other breathing techniques are briefly mentioned, including forced expiration, glossopharyngeal insufflation (GI), and glossopharyngeal exsufflation (GE).
Deep Expiration
BH deep divers are healthy and relatively young but thoroughly train the respiratory muscles; i.e., the intracostal muscles, the diaphragm, and the auxiliary respiratory muscles (Fig. 3). Owing to a high thoracic elasticity and using a specific training of the diaphragm, BH athletes can measurably reduce their RV below values attained after normal maximal expiration []. Yet, positive effects of yoga breathing techniques – here pranayama – are described for other individuals to prevent from pulmonary injury []. If yoga breathing techniques are seen as a sort of meditation, then they can reduce sympathetic overactivity, thereby decreasing arterial tone and peripheral resistance resulting in a lowering of the heart rate and diastolic blood pressure []. Even short-term pranayama resulted in a significant decline in the resting heart rate and mean arterial blood pressure []; reduction in the sympathetic drive will decrease oxygen consumption and in turn will prolong breath-holding time.

Fig. 3
Breathing techniques also employ yoga. Pranayama is shown here. Reproduced with permission from Lotta Ericson, Freedive Dahab.
Glossopharyngeal Insufflation
This breathing technique was described almost 70 years ago [] and has been used in the clinic in patients with respiratory failure [] or with neuromuscular diseases []. BH divers employ glossopharyngeal mus cle contractions (= glossopharyngeal pistoning; buccal pumping; lung packing) to increase the volume of air in the lungs above TLC []. They thereby increase the volume of gas available for pressure equalization during descent [, ] and enhance the amount of oxygen available in the lungs [].
GI induces thoracic expansion and downward displacement of the diaphragm. In the regions of the lung where marked hyperexpansion occurs, perfusion is reduced and canapproach zero flow in some regions []. Such thoracic expansion will cease as diving depth increases, and increasing pressure with further diving depth will even deform the thorax to anomalous anatomy.
Data on extensive GI vary considerably. GI may increase the lung gas volume by about 20% [] or up to 2.8 L in individual athletes []. Owing to the low compliance at high volumes, transpulmonary pressures increase drastically with GI. In fact, values up to 80 cmH2O (= 8 kPa) [] or even 100 cmH2O (= 10 kPa) [] have been reported that some healthy individuals apparently can withstand. For comparison, patients with pulmonary insufficiency might be mechanically ventilated in the clinic. There, pressures in pulmonary healthy patients are limited to 40 cmH2O in order not to overinflate the alveoli. Not surprisingly, the high pressures after GI increase the risk of pulmonary barotrauma [, ]; i.e., arterial gas embolism [, ] and/or pneumomediastinum [].
Using CT, pneumomediastinum was found in five out of six competitive BH divers after GI. The sixth participant, who was unable to perform GI, was spared from pulmonary injury []. Based on these results, the authors speculate whether GI effects can accumulate, leading to long-term injury []. It is mentioned that studies of large populations of BH divers are necessary to firmly exclude long-term lung damage induced by BH diving per se []. Because of its adverse effects on the lungs, GI should be used infrequently or avoided altogether [, ]. On the other hand, it has been suggested that GI be used to identify individuals at risk to develop pulmonary barotrauma [].
The increased intrathoracic pressures that follow GI are likely to impede venous return and induce hypotension with consequences varying from dizziness to even fainting just prior to BH diving [, ]. To give but one example: in a healthy female elite apneist, stroke volume was 92 mL and cardiac output was 4.6 L/min during control. After GI and 2-min apnea both stroke volume and cardiac output were considerably decreased to 48 ml and 3.2 L/min, respectively [] (Fig. 4).
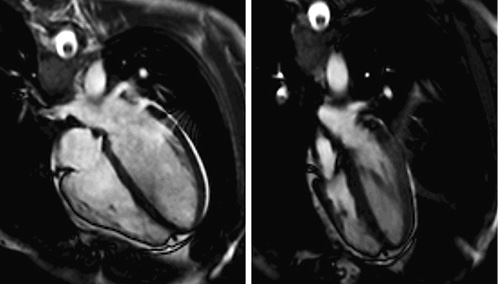
Fig. 4
Glossopharyngeal insufflation (GI). End-diastolic four-chamber view (steady-state free precession MRI). Left: supine control. Right: after GI and 2-min apnea. Reproduced with permission from Schipke et al. [].
The good news: BH diving with performance of GI presumably does not permanently alter pulmonary distensibility or impair static or dynamic measures [].
Glossopharyngeal Exsufflation
Using this procedure, also known as “reverse packing”, the BH diver can draw air from compressed lungs into the pharynx to equalize middle ear pressure [], thereby reducing intrathoracic pressures and RV. If one single GE should bring up 50 mL of air from the lungs and if the maneuver is performed 5 times, GE would reduce RV by 0.25 L. This estimated value is in line with a study in 4 male apneic divers, where RV was reduced by between 0.09 and 0.44 L []. A volume of similar magnitude (= 0.50 L) has been given by Schagatay [].
In a study on two elite BH divers, acute effects of GE were demonstrated using pulmonary Magnetic resonsnace imaging (MRI). After performing GE, and after the divers inhaled small volumes from sub-RVs, MRI demonstrated strikingly inhomogeneous inhalation patterns (Fig. 5), which were interpreted as the result of a reopening of frankly closed airways []. The question whether the airways reopen quickly enough during rapid ascent and prevent from insufficient gas exchange after surfacing remains unanswered.
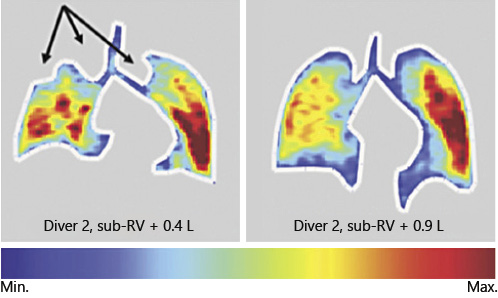
Fig. 5
Inhalation of 0.4 L (left) and 0.9 L (right) from sub-RVs in a diver lead to drastic heterogeneous inhalation patterns. Arrows indicate atelectatic areas. Reproduced with permission from Muradyan et al. [].
Pulmonary Diffusing Capacity
Diffusing capacity is used to evaluate alveolar-capillary membrane integrity []. An increase in this membrane’s resistance may be secondary to (a) a decrease in the effective surface area of the alveolar membrane available for gas exchange; i.e., pulmonary restriction; (b) an increased ventilation/perfusion mismatch; or (c) an alteration in its physical characteristics, such as increased thickness or diffusing distance or reduced permeability []. Two recent studies suggest that mechanical and hypoxic effects of BH diving elicit damage to the alveolar-capillary membrane [, ]. Furthermore, another study reports that pulmonary diffusing capacity for CO is reduced after a single air dive []. To be sure, such injury will depend strongly on the dive profile, but it is not at all uncommon [].
Hypoxia and the Lungs
Because of its great significance, a paragraph is dedicated to decompression sickness (DCS)-like symptoms in BH deep divers and in divers with repetitive BH deep diving profiles [-]. Although the underlying mechanisms of brain damage in BH diving remain to be elucidated, N2 bubbles passing through the heart (= extrapulmonary shunt) or the lungs (= intrapulmonary shunt) so as to become arterialized may be a likely factor contributing to stroke-like clinical presentations in elite BH divers after deep dives []. Indeed, neurological insult has been reported in three competitive deep BH divers after single or repetitive deep dives [].
DCS has been reported to occasionally resolve spontaneously. Similarly, a transient ischemic attack (TIA) is an episode of neurologic dysfunction caused by ischemia that might be induced by an embolus occluding an artery in the brain []. Unlike a stroke, TIA is characterized by an abrupt onset of focal neurological symptoms that resolves within 24 h (Anonymous, 1990). Therefore, the possibility exists that TIA after BH diving may be a clinical presentation of DCS [].
TIAs after BH deep diving seem to be related to intrapulmonary arteriovenous anastomoses (IPAVA). IPAVAs present pathways with 25–50 µm diameter, which allow blood flow to bypass the lung capillaries and provide a route for right-to-left embolus transmission []. In consequence, an embolus passing through such a shunt could reach the heart or the brain. Thus, if present, venous gas bubbles can become arterialized [].
Several factors present during BH dives may facilitate the opening of intrapulmonary shunts, including body positioning and submaximal to maximal exercise []. In particular, hypoxia – an immediate result of BH diving – seemingly contributes to the opening of intrapulmonary shunts. This notion is convincingly supported by a study on healthy adult subjects, in which a 30-min exposure to a gas mixture with a reduced inspired oxygen tension of 10% opened IPAVA in all participants at rest []. Conversely, breathing 100% oxygen prevented arteriovenous shunting during submaximal exercise and dramatically reduced it at maximal exercise [].
Thus, hypoxia may be a key factor in deep BH diving to allow transpulmonary passage of venous gas microbubbles leading to arterial gas embolism and stroke-like phenomena. In fact, hypoxia increases the risk of brain edema [], and hypoxia-induced brain damage after BH diving has been reported [, ].
Conclusion
BH deep divers employ pathophysiological maneuvers that have already led to pulmonary injury and serious accidents. Because of this, the authors recommend that particular breathing maneuvers and extreme depths should be avoided. The rule should be no hyperventilation, no GI, and no GE. We additionally recommend that free divers work on relaxation to slow down breathing and then dive after one deep inspiration. It is hoped that BH deep divers will become better aware of the risks of their extreme leisure sport.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Author Contributions
Jochen D. Schipke, Frederique Lemaitre, Sinclair Cleveland, and Kay Tetzlaff have made substantial contributions to the conception, design, and acquisition of the literature data; drafted the submitted article or revised it critically for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.
- 2. Landsberg PG. South African underwater diving accidents, 1969-1976. S Afr Med J. 1976;50(55):2155–9.
- 3. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284–92.
- 4. Almeling M. Handbuch Tauch- und Hyperbarmedizin. Landsberg/Lech, ecomed, 1998.
- 5. Scholander PF, Hammel HT, Lemessurier H, Hemmingsen E, Garey W. Circulatory adjustment in pearl divers. J Appl Physiol. 1962;17(2):184–90.
- 6. Binks AP, Vovk A, Ferrigno M, Banzett RB. The air hunger response of four elite breath-hold divers. Respir Physiol Neurobiol. 2007;159(2):171–7.
- 7. Schagatay E, Andersson J. Diving response and apneic time in humans. Undersea Hyperb Med. 1998;25(1):13–9.
- 8. Schagatay E, van Kampen M, Emanuelsson S, Holm B. Effects of physical and apnea training on apneic time and the diving response in humans. Eur J Appl Physiol. 2000;82(3):161–9.
- 9. Palada I, Bakovic D, Valic Z, Obad A, Ivancev V, Eterovic D, et al Restoration of hemodynamics in apnea struggle phase in association with involuntary breathing movements. Respir Physiol Neurobiol. 2008;161(2):174–81.
- 10. Kiyan E, Aktas S, Toklu AS. Hemoptysis provoked by voluntary diaphragmatic contractions in breath-hold divers. Chest. 2001;120(6):2098–100.
- 11. Costalat G, Coquart J, Castres I, Tourny C, Lemaitre F. Hemodynamic adjustments during breath-holding in trained divers. Eur J Appl Physiol. 2013;113(10):2523–9.
- 12. Mukhtar MR, Patrick JM. Bronchoconstriction: a component of the ‘diving response’ in man. Eur J Appl Physiol Occup Physiol. 1984;53(2):155–8.
- 13. Schaefer KE, Allison RD, Dougherty JH Jr, Carey CR, Walker R, Yost F, et al Pulmonary and circulatory adjustments determining the limits of depths in breathhold diving. Science. 1968;162(3857):1020–3.
- 14. Fitz-Clarke JR. Mechanics of airway and alveolar collapse in human breath-hold diving. Respir Physiol Neurobiol. 2007;159(2):202–10.
- 15. Steimle KL, Mogensen ML, Karbing DS, Bernardino de la Serna J, Andreassen S. A model of ventilation of the healthy human lung. Comput Methods Programs Biomed. 2011;101(2):144–55.
- 16. Craig AB Jr. Depth limits of breath hold diving (an example of Fennology). Respir Physiol. 1968;5(1):14–22.
- 17. Frassi F, Pingitore A, Cialoni D, Picano E. Chest sonography detects lung water accumulation in healthy elite apnea divers. J Am Soc Echocardiogr. 2008;21(10):1150–5.
- 18. Lambrechts K, Germonpré P, Charbel B, Cialoni D, Musimu P, Sponsiello N, et al Ultrasound lung “comets” increase after breath-hold diving. Eur J Appl Physiol. 2011;111(4):707–13.
- 19. Boussuges A, Pinet C, Thomas P, Bergmann E, Sainty JM, Vervloet D. Haemoptysis after breath-hold diving. Eur Respir J. 1999;13(3):697–9.
- 20. Cialoni D, Sponsiello N, Marabotti C, Marroni A, Pieri M, Maggiorelli F, et al Prevalence of acute respiratory symptoms in breath-hold divers. Undersea Hyperb Med. 2012;39(4):837–44.
- 21. Scherhag A, Pfleger S, Grosselfinger R, Borggrefe M. Does competitive apnea diving have a long-term risk? Cardiopulmonary findings in breath-hold divers. Clin J Sport Med. 2005;15(2):95–7.
- 22. Arborelius M Jr, Balldin UI, Lila B, Lundgren CE. Regional lung function in man during immersion with the head above water. Aerosp Med. 1972;43(7):701–7.
- 23. Risch WD, Koubenec HJ, Beckmann U, Lange S, Gauer OH. The effect of graded immersion on heart volume, central venous pressure, pulmonary blood distribution, and heart rate in man. Pflugers Arch. 1978;374(2):115–8.
- 24. Koehle MS, Lepawsky M, McKenzie DC. Pulmonary oedema of immersion. Sports Med. 2005;35(3):183–90.
- 25. Marabotti C, Cialoni D, Pingitore A. Environment-induced pulmonary oedema in healthy individuals. Lancet Respir Med. 2017;5(5):374–6.
- 26. Cochard G, Arvieux J, Lacour JM, Madouas G, Mongredien H, Arvieux CC. Pulmonary edema in scuba divers: recurrence and fatal outcome. Undersea Hyperb Med. 2005;32(1):39–44.
- 27. Guyton AC, Hall JE. Textbook of medical physiology. 11th ed.Philadelphia: Elsevier Saunders; 2006.
- 28. Muth CM, Ehrmann U, Radermacher P. Physiological and clinical aspects of apnea diving[v.]. Clin Chest Med. 2005;26(3):381–94.
- 29. Abel AN, Lloyd LK, Williams JS. The effects of regular yoga practice on pulmonary function in healthy individuals: a literature review. J Altern Complement Med. 2013;19(3):185–90.
- 30. Vyas R, Dikshit N. Effect of meditation on respiratory system, cardiovascular system and lipid profile. Indian J Physiol Pharmacol. 2002 Oct;46(4):487–91.
- 31. Ankad RB, Herur A, Patil S, Shashikala GV, Chinagudi S. Effect of short-term pranayama and meditation on cardiovascular functions in healthy individuals. Heart Views. 2011 Apr;12(2):58–62.
- 32. Dail CW. “Glossopharyngeal breathing” by paralyzed patients; a preliminary report. Calif Med. 1951;75(3):217–8.
- 33. Westermann EJ, Verweij-van den Oudenrijn LP, Gaytant MA, Kampelmacher MJ. [Lung volume recruitment in impending respiratory failure]. Ned Tijdschr Geneeskd. 2011;155(18):A3371.
- 34. Ambrosino N, Carpenè N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J. 2009;34(2):444–51.
- 35. Eichinger M, Walterspacher S, Scholz T, Tetzlaff K, Röcker K, Muth CM, et alBreath-hold Diving Study Group of Baden-Württemberg. Lung hyperinflation: foe or friend?Eur Respir J. 2008;32(4):1113–6.
- 36. Eichinger M, Walterspacher S, Scholz T, Tetzlaff R, Puderbach M, Tetzlaff K, et al Glossopharyngeal insufflation and pulmonary hemodynamics in elite breath hold divers. Med Sci Sports Exerc. 2010;42(9):1688–95.
- 37. Lindholm P, Nyrén S. Studies on inspiratory and expiratory glossopharyngeal breathing in breath-hold divers employing magnetic resonance imaging and spirometry. Eur J Appl Physiol. 2005;94(5-6):646–51.
- 38. Loring SH, O’Donnell CR, Butler JP, Lindholm P, Jacobson F, Ferrigno M. Transpulmonary pressures and lung mechanics with glossopharyngeal insufflation and exsufflation beyond normal lung volumes in competitive breath-hold divers. J Appl Physiol (1985). 2007;102(3):841–6.
- 39. Seccombe LM, Jenkins CR, Rogers PG, Pearson MA, Peters MJ. Evidence of respiratory system remodelling in a competitive freediver. Eur Respir J. 2013;41(3):760–2.
- 40. Schiffer TA, Lindholm P. Transient ischemic attacks from arterial gas embolism induced by glossopharyngeal insufflation and a possible method to identify individuals at risk. Eur J Appl Physiol. 2013;113(3):803–10.
- 41. Jacobson FL, Loring SH, Ferrigno M. Pneumomediastinum after lung packing. Undersea Hyperb Med. 2006;33(5):313–6.
- 42. Tetzlaff K, Scholz T, Walterspacher S, Muth CM, Metzger J, Roecker K, et al Characteristics of the respiratory mechanical and muscle function of competitive breath-hold divers. Eur J Appl Physiol. 2008;103(4):469–75.
- 43. Chung SC, Seccombe LM, Jenkins CR, Frater CJ, Ridley LJ, Peters MJ. Glossopharyngeal insufflation causes lung injury in trained breath-hold divers. Respirology. 2010;15(5):813–7.
- 44. Linér MH, Andersson JP. Suspected arterial gas embolism after glossopharyngeal insufflation in a breath-hold diver. Aviat Space Environ Med. 2010;81(1):74–6.
- 45. Mijacika T, Dujic Z. Sports-related lung injury during breath-hold diving. Eur Respir Rev. 2016;25(142):506–12.
- 46. Boussuges A, Gavarry O, Bessereau J, Coulange M, Bourc’his M, Rossi P. Glossopharyngeal insufflation and breath-hold diving: the more, the worse?Wilderness Environ Med. 2014;25(4):466–71.
- 47. Novalija J, Lindholm P, Loring SH, Diaz E, Fox JA, Ferrigno M. Cardiovascular aspects of glossopharyngeal insufflation and exsufflation. Undersea Hyperb Med. 2007;34(6):415–23.
- 48. Schipke JD, Kelm M, Siegmund K, Muth T, Sievers B, Steiner S. “Lung packing” in breath hold-diving: an impressive case of pulmo-cardiac interaction. Respir Med Case Rep. 2015;16:120–1.
- 49. Walterspacher S, Scholz T, Tetzlaff K, Sorichter S. Breath-hold diving: respiratory function on the longer term. Med Sci Sports Exerc. 2011;43(7):1214–9.
- 50. Schagatay E. Predicting performance in competitive apnea diving. Part III: deep diving. Diving Hyperb Med. 2011;41(4):216–28.
- 51. Muradyan I, Loring SH, Ferrigno M, Lindholm P, Topulos GP, Patz S, et al Inhalation heterogeneity from subresidual volumes in elite divers. J Appl Physiol (1985). 2010;109(6):1969–73.
- 52. Roughton FJ, Forster RE, Cander L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol. 1957;11(2):269–76.
- 53. Puri S, Baker BL, Oakley CM, Hughes JM, Cleland JG. Increased alveolar/capillary membrane resistance to gas transfer in patients with chronic heart failure. Br Heart J. 1994;72(2):140–4.
- 54. Garbella E, Piarulli A, Fornai E, Pingitore A, Prediletto R. Preliminary observations on the effect of hypoxic and hyperbaric stress on pulmonary gas exchange in breath-hold divers. Diving Hyperb Med. 2011;41(2):97–100.
- 55. Prediletto R, Fornai E, Catapano G, Carli C, Garbella E, Passera M, et al Time course of carbon monoxide transfer factor after breath-hold diving. Undersea Hyperb Med. 2009;36(2):93–101.
- 56. Dujić Z, Eterović D, Denoble P, Krstacić G, Tocilj J, Gosović S. Effect of a single air dive on pulmonary diffusing capacity in professional divers. J Appl Physiol (1985). 1993;74(1):55–61.
- 57. Thorsen E, Segadal K, Myrseth E, Påsche A, Gulsvik A. Pulmonary mechanical function and diffusion capacity after deep saturation dives. Br J Ind Med. 1990;47(4):242–7.
- 58. Gempp E, Blatteau JE. Neurological disorders after repetitive breath-hold diving. Aviat Space Environ Med. 2006;77(9):971–3.
- 59. Kohshi K, Wong RM, Abe H, Katoh T, Okudera T, Mano Y. Neurological manifestations in Japanese Ama divers. Undersea Hyperb Med. 2005;32(1):11–20.
- 60. Lemaître F, Kohshi K, Tamaki H, Nakayasu K, Harada M, Okayama M, et al Doppler detection in Ama divers of Japan. Wilderness Environ Med. 2014;25(3):258–62.
- 61. Kohshi K, Tamaki H, Lemaître F, Okudera T, Ishitake T, Denoble PJ. Brain damage in commercial breath-hold divers. PLoS One. 2014;9(8):e105006.
- 62. Tetzlaff K, Schöppenthau H, Schipke JD. Risk of Neurological Insult in Competitive Deep Breath-Hold Diving. Int J Sports Physiol Perform. 2017;12(2):268–71.
- 63. Ferro JM, Falcão I, Rodrigues G, Canhão P, Melo TP, Oliveira V, et al Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27(12):2225–9.
- 64. Schipke JD, Tetzlaff K. Why predominantly neurological decompression sickness in breath-hold divers?J Appl Physiol (1985). 2016;120(12):1474–7.
- 65. Bates ML, Jacobson JE, Eldridge MW. Transient intrapulmonary shunting in a patient treated with β2-adrenergic agonists for status asthmaticus. Pediatrics. 2014;133(4):e1087–91.
- 66. Madden D, Ljubkovic M, Dujic Z. Intrapulmonary shunt and SCUBA diving: another risk factor?Echocardiography. 2015;32Suppl 3:S205–10.
- 67. Lovering AT, Stickland MK, Eldridge MW. Intrapulmonary shunt during normoxic and hypoxic exercise in healthy humans. Adv Exp Med Biol. 2006;588:31–45.
- 68. Laurie SS, Yang X, Elliott JE, Beasley KM, Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol (1985). 2010;109(4):1072–9.
- 69. Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol (1985). 2008;104(5):1418–25.
- 70. Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8(2):175–91.
- 71. Andersson JP, Linér MH, Jönsson H. Increased serum levels of the brain damage marker S100B after apnea in trained breath-hold divers: a study including respiratory and cardiovascular observations. J Appl Physiol (1985). 2009;107(3):809–15.
- 72. Havnes MB, Hjelde A, Brubakk AO, Møllerløkken A. S100B and its relation to intravascular bubbles following decompression. Diving Hyperb Med. 2010;40(4):210–2.
- 73. Heusser K, Dzamonja G, Breskovic T, Steinback CD, Diedrich A, Tank J, et al Sympathetic and cardiovascular responses to glossopharyngeal insufflation in trained apnea divers. J Appl Physiol (1985). 2010;109(6):1728–35.
Prof. Sinclair Cleveland passed away at the end of 2018. We lost an outstanding friend, teacher, colleague and reliable scientist.
