Introduction
Endoscopic valve treatment has been established as interventional treatment approach in patients with advanced emphysema and absent interlobar collateral ventilation []. In a precisely selected patient group, valve implantation leads to hyperinflation reduction and improvement of exercise capacity and quality of life [-]. Furthermore, retrospective trials suggest that valve therapy may prolong survival of emphysema patients who develop complete lobar atelectasis following valve therapy [-]. Although valve implantation is a minimally invasive treatment approach, it can be associated with risks, whereby the pneumothorax with a rate up to 34% is the most common complication []. Further adverse events include COPD exacerbations, pneumonia, or respiratory failure. The latter complication occurred in up to 2.7% in the randomized controlled trials [, ] and was reported as the cause of death in end-stage COPD patients in 1 patient (4%) in the BeLieVeR-HIFi study [] and in 1 patient (3%) in the STELVIO study []. To minimize the risk of respiratory failure following valve treatment, hypercapnia with partial pressure of carbon dioxide (pCO2) >50 mm Hg or >60 mm Hg was an exclusion criterion in the randomized controlled trials LIBERATE, EMPROVE, or STELVIO, respectively [, , ]. Patients enrolled in these trials presented a mean pCO2 of 38 ± 6 mm Hg (STELVIO), 40 ± 5 mm Hg (LIBERATE), and 40 ± 6 mm Hg (EMPROVE).
However, it is known that hyperinflation in patients with severe emphysema correlates significantly with hypercapnia []. This suggests that therapeutic interventions aiming at reduction of hyperinflation may reduce hypercapnia. There are some encouraging case reports describing a successful valve therapy in patients who required mechanical ventilation due to hypercapnic respiratory failure [, ]. Therefore, the aim of this study was to evaluate the efficacy of valve treatment in emphysema patients with chronic respiratory failure and particularly its impact on hypercapnia.
Methods
All emphysema patients with mild to severe hypercapnia who underwent consecutively endoscopic valve therapy were enrolled in this retrospective trial. All data were taken from a database maintained for patients receiving endoscopic lung volume reduction from 2005 to 2017 in Thoraxklinik, University of Heidelberg, Germany. All patients gave general consent for scientific use of the data. The Local Ethics Committee of the Medical University of Heidelberg approved the protocol of this trial (S-202/2017).
Subjects and Intervention
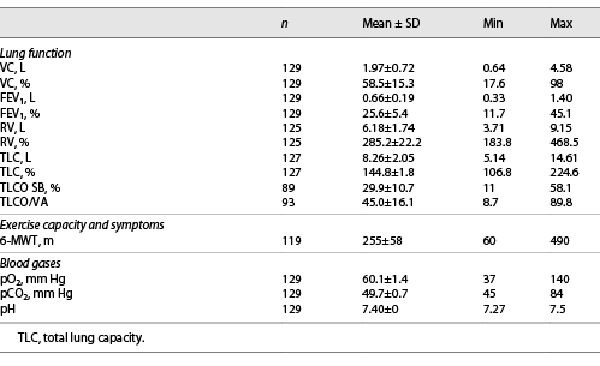
All emphysema patients with hypercapnia defined as pCO2 ≥45 mm Hg who were treated by endoscopic valve therapy for heterogeneous or homogeneous emphysema at the Thoraxklinik, University of Heidelberg were enrolled in this retrospective analysis. Prior to valve treatment, demographic data (age, gender, need of long-term oxygen therapy and/or noninvasive ventilation [NIV]), blood gas analysis (pO2, pCO2, pH), lung function parameter (vital capacity, forced expiratory volume in 1 s [FEV1], residual volume [RV], total lung capacity), diffusion capacity (TLCO SB [transfer factor for carbon monoxide, single breath], TLCO/VA [transfer factor for carbon monoxide, per unit alveolar volume]) and 6-min-walk test (6-MWT) were obtained. Furthermore, patients underwent a multidetector computed tomography scan and perfusions scan for identifying the target lobe for valve implantation and for fissure analysis. As this retrospective analysis covers the period from 2005 to 2017, also emphysema patients with incomplete fissures are included, as the impact of absent interlobar collateral ventilation became known in 2010.
For valve implantation, patients underwent a rigid bronchoscopy with high frequency jet ventilation. The most destroyed lung lobe was occluded by one-way valves (EBV, Pulmonx, Inc., Palo Alto, CA, US; IBV, Olympus, Tokyo, Japan). Following procedure, patients were monitored in the hospital for a minimum of 48 h. At 3 and 6 months, lung function test, blood gas analysis, and 6-MWT were assessed. Radiological follow-up was performed to evaluate whether the patient experienced a lobar atelectasis. Based on the result of the multidetector computed tomography scan at 3-month follow-up (FU), patients were assigned to subgroups (“complete atelectasis” defined as 100% lobar volume loss vs. “incomplete atelectasis”).
Patients with changes in oxygen therapy at the different time points of sampling blood gas analysis were excluded as oxygen delivery may influence pCO2. Furthermore, patients in whom NIV was started simultaneously to valve therapy (n = 9) were excluded from analysis to avoid bias (NIV started immediately prior to valve placement in 7 patients, following valve placement in 2 patients).
Statistical Analysis
Descriptive statistics and comparisons between groups were performed by R (Version R-3.6.1, R Core Team). Data are presented as mean ± standard deviation, minimum and maximum, and absolute numbers and percentages. Changes in lung function parameters from baseline to 3- and 6-month follow-up were expressed by descriptive statistics (mean ± standard deviation). Statistical comparison for the baseline examinations versus follow-up examinations was made using the McNemar test dichotomous paired variables and a paired two-tailed Student’s t test for the continuous variables. p values <0.05 were considered statistically significant. Responder rates were calculated by counting the number of patients who met the minimal important difference of >15% improvement in FEV1 [] and ≥25 m improvement in 6-MWT. A subanalysis was performed to compare the patients who developed complete atelectasis following valve treatment with patients with incomplete atelectasis. For the categorical variables the χ2 test was used and to identify differences between the subgroups means, an unpaired two-tailed Student’s test was used. A p value <0.05 was considered statistically significant.
Results
Patient Characteristics
In this analysis, 129 COPD patients (43% male, mean age 64 ± 7 years) were enrolled with a mean FEV1 of 0.66 L (25.6% of predicted) and a mean RV of 6.18 L (285.2% of predicted). Laboratory tests revealed alpha-1 antitrypsin deficiency in 3 out of the 129 patients. Blood gases at baseline revealed a mean pCO2 of 49.7 ± 0.7 mm Hg (range 45 mm Hg–84 mm Hg). 79% of the patients received long-term oxygen therapy and 24% of the patients NIV. One patient presented a severe hypercapnia with pCO2 of 84 mm Hg while receiving intermittent NIV. Computed tomography revealed severe heterogeneous emphysema so that valve therapy as potential but high-risk intervention was discussed in detail with this patient who gave informed consent. Characteristics of all patients are presented in Table 1. During combined rigid and flexible bronchoscopy, valves were implanted in the right upper lobe in 6 patients, right lower lobe in 28 patients, left upper lobe in 35 patients, and left lower lobe in 59 patients. One patient was treated sequentially in the right upper lobe and middle lobe.
Changes in Blood Gases
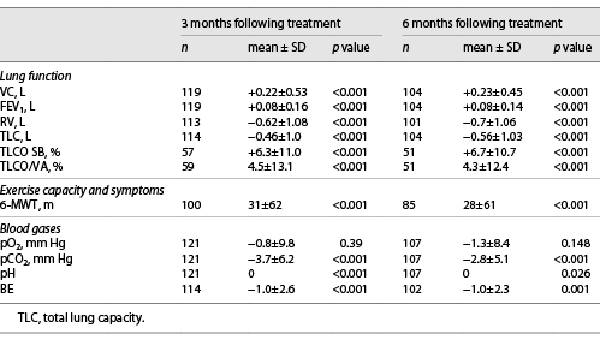
Three and 6 months following valve placement, mean pCO2 was significantly improved (p < 0.001). Base excess also decreased significantly (p = 0.001). These results are presented in Table 2. At 3-month and 6-month FU, 40% of the patients (49/121 and 42/106) were normocapnic (Fig. 1,2). During follow-up, pO2 did not change significantly from 60.1 mm Hg to 58.9 mm Hg at 3 months (−0.8 mm Hg) and 58.5 mm Hg at 6 months (−2.8 mm Hg, p 0.148).
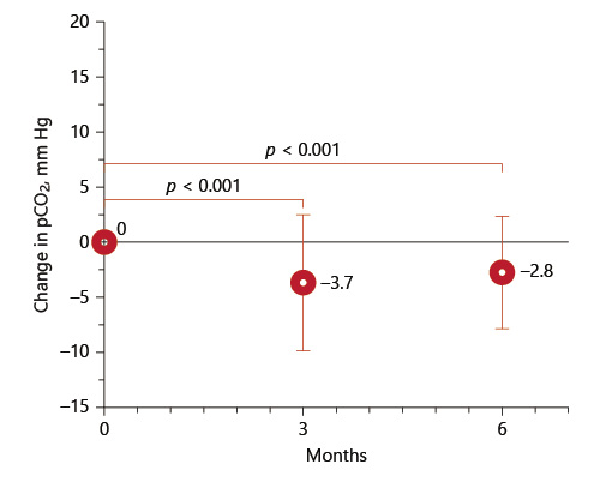
Fig. 1
Change of pCO2 at 3 and 6 months.
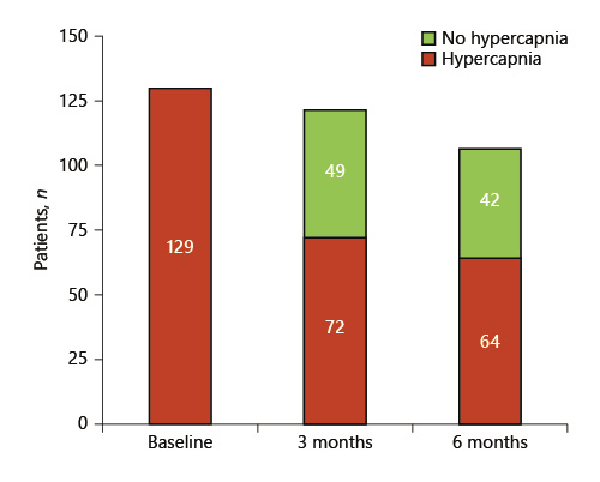
Fig. 2
Number of patients with normocapnia at 3 and 6 months from baseline.
Changes of Lung Function, Diffusion Capacity, and 6-MWT
All lung function parameters, TLCO SB, TLCO/VA, and 6-MWT were significantly improved at 3- and 6-month FU (Table 2). At 3-month and 6-month FU, 40% and 38% of the patients met the efficacy threshold of greater than 15% improvement in FEV1, respectively. 54% and 52% of the patients experienced a greater than 25 m improvement on the 6-MWT 3 and 6 months following valve treatment, respectively.
Subgroup Analysis Based on Radiological Outcome
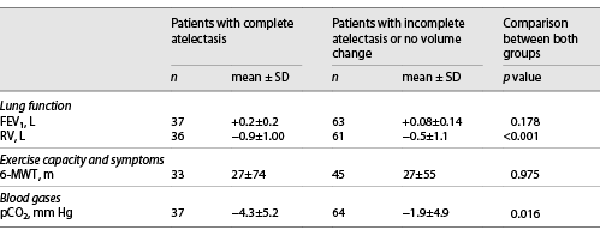
Patients who developed a complete lobar atelectasis (n = 40) following the valve therapy experienced a superior hyperinflation reduction compared to patients with incomplete atelectasis (RV −0.92 L ± 1.01 L vs. −0.50 ± 1.1, p < 0.001). There was no significant difference in change of FEV1 (+0.1 L ± 0.1 L vs. +0.2 L ± 0.2 L, p = 0.178) or 6-MWT (+27 m ± 74 m vs. 27 m + 55 m, p = 0.975). In the subgroup of patients who developed a complete lobar atelectasis and thus a statistically significant RV reduction, a more pronounced pCO2 was found (pCO2 −4.3 mm Hg ± 5.2 mm Hg vs. −1.9 ± 4.9 mm Hg, p 0.016). Results are presented in Table 3.
Pneumothorax and Respiratory Failure
Out of the 129 patients, 21 patients (16%) developed a pneumothorax as anticipated complication of valve therapy. 95% of the patients who experienced pneumothorax received chest tube insertion. After drainage, all patients recovered from pneumothorax and no further intervention was required to seal the fistula.
As mentioned above, 9 patients in whom NIV was started immediately prior to valve therapy (n = 7) or following valve placement (n = 2) was excluded to avoid bias as NIV influence hypercapnia. However, transient deterioration of hypercapnia following valve placement that required NIV immediately after intervention in 2 patients have to be considered as complication: 1 patient experienced pneumothorax immediately after vale placement and required NIV due to worsening of preexistent hypercapnia at time point of pneumothorax. NIV was continued after recovering from pneumothorax. In another patient, NIV was started 1 month following valve placement as an increase of pCO2 was observed.
Discussion
Hypercapnia is one complication of advanced COPD and emphysema. Chronic respiratory failure in COPD patients is associated with dyspnoea, daytime tiredness, disturbed sleep, and oedema and is a signal of end-of-life stage. Long-term NIV provides the control of pCO2 that leads to benefits in COPD patients and improves the survival [].
The pathophysiological explanation for the development of chronic hypercapnia is failure of the respiratory muscles. Due to increased residual volume, change of geometric factors, i.e., muscle shortening that accompanies hyperinflation, reduces the muscle capacity to generate pressure to overcome the load [] and thus leads to chronic hypercapnia. A retrospective analysis demonstrated that lung hyperinflation correlates significantly with hypercapnia []. Based on these pathophysiological considerations, it can be assumed that a reduction of hyperinflation will lead to a decrease of pCO2.
Valve therapy has emerged as therapeutic approach in patients with advanced emphysema and absent interlobar collateral ventilation []. Although valve implantation is a minimally invasive treatment, it can be associated with adverse events whereby postprocedural pneumothorax is the most common complication. Moreover, randomized controlled trials reported the advent of respiratory failure in almost 3% of the patients []. As patients with chronic hypercapnia and thus limited respiratory reserve may have higher rates for periprocedural and postprocedural complications, e.g., in the case of pneumothorax, patients with pCO2 >50 mm Hg or >60 mm Hg were excluded from the majority of the clinical trials [, , ] and thus counted as a contraindication []. There are only some case reports describing encouraging results of valve therapy in single patients with acute hypercapnic respiratory failure [, ]. This retrospective study evaluated the impact of valve therapy on chronic hypercapnia in emphysema patients for the first time.
The results of this analysis demonstrate a significant reduction of hypercapnia induced by endoscopic valve therapy at 3 months and 6 months of 3.7 mm Hg ± 6.1 and −2.8 mm Hg ± 5.1 (p < 0.001) (Fig. 1), respectively, after valve placement compared to baseline assessment. In total 40% of the patients showed normocapnia at the times of follow-up (Fig. 2). Patients who developed a complete lobar atelectasis and thus a target lobe volume reduction of 100% experienced greatest pCO2 reduction. This group of patients had a pCO2 change of −4.3 mm Hg ± 5.2 compared to −1.9 mm Hg ± 4.9 (p = 0.016) in patients with incomplete atelectasis from baseline. However, also patients who did not experience complete lobar atelectasis benefit from valve treatment as a lobar volume loss of ∼50% is associated with clinical improvement [].
In contrast to pCO2 reduction, there were no significant changes of pO2. The main reason for this finding may be the reduction of the gas exchange surface due to the development of atelectasis resulting in reduced pO2 uptake. Hypercapnia in COPD patients however is mainly caused by respiratory muscle failure. Valve therapy reduces hyperinflation and thus respiratory muscle workload but has no to mild negative impact on the gas exchange surface. To minimize the reduction on gas exchange surface, we recommend performing a perfusion scan prior to valve implantation that assists in identifying the lung area with the lowest perfusion as the target area.
Furthermore, improvements of lung function parameters and exercise capacity could be observed, which confirmed the results of earlier trials []. Especially the RV decreased by −0.62 L ± 1.08 and −0.70 L ± 1.06 and the FEV1 increased by 0.08 L ± 0.16 and 0.08 L ± 0.14 (each p < 0.001) at the follow-ups as a sign of a more effective respiratory mechanic. As well, a significant part of patients even met the efficacy threshold for FEV1 of 15% improvement (40% and 38% at 3- and 6-month follow-up) and exercise capacity of more than 25 m additional walking distance (54% and 52% respectively). As an anticipated complication pneumothorax occurred in 21 cases (16%) which is nearly comparable to the rates of earlier studies [-].
Nine emphysema patients with hypercapnia who were treated by valves were excluded from this analysis, as NIV was started prior to or immediate following endoscopic valve treatment and thus may influence the impact of valve therapy on hypercapnia. Although this study demonstrates the benefits of valve therapy on hypercapnia, in some patients who are considered to be at high risk for an acute on chronic respiratory failure due to invasive treatment, NIV should be started prior to intervention to avoid such a complication. As there is no pCO2 threshold up to which a valve therapy can be safely performed, clinical aspects, e.g., symptoms of hypercapnia or muscle wasting, must be taken into account.
The limitation of this study is its retrospective character. Several patients were lost to follow-up, particularly at 6-month FU (e.g., as they lived far away from the hospital, refused further FU) that may influence the results. Therefore, the encouraging findings of this study must be evaluated in a bigger prospective trial.
Conclusion
In summary, endoscopic valve therapy can reduce hypercapnia in patients with severe emphysema by reduction of hyperinflation. Valve therapy will not replace NIV in hypercapnia therapy, but patients with stable hypercapnic patients should not be excluded from this therapeutic approach.
Statement of Ethics
All patients gave general consent for scientific use of the data. The Local Ethics Committee of the Medical University of Heidelberg approved the protocol of this trial (S-202/2017). For this retrospective analysis, no further written informed was required.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Sources
There are no funding regarding this study.
Author Contributions
Matthias Roetting: data acquisition, analysis, and interpretation; Katharina Kriegsmann: statistical analysis; Markus Polke and Nilab Polke: data acquisition; Konstantina Kontogianni: data acquisition; Ralf Eberhardt: substantial revision and providing infrastructure; Felix J.F. Herth: substantial revision and providing infrastructure; Daniela Gompelmann: conception/design of the work and supervision.
Data Availibility Statement
All data generated and analysed during this study are included in this article. Further enquiries regarding the generated data can be directed to the corresponding author.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD 2020 [cited 2021 May]. Available from: https://goldcopd.org/gold-reports/.
- 2. Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386(9998):1066–73. https://doi.org/10.1016/s0140-6736(15)60001-0.
- 3. Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–35. https://doi.org/10.1056/nejmoa1507807.
- 4. Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med. 2017;196(12):1535–43. https://doi.org/10.1164/rccm.201707-1327oc.
- 5. Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE).. Am J Respir Crit Care Med. 2018;198(9):1151–64. https://doi.org/10.1164/rccm.201803-0590OC.
- 6. Criner GJ, Delage A, Voelker K, Hogarth DK, Majid A, Zgoda M, et al. Improving lung function in severe heterogenous emphysema with the spiration valve system (EMPROVE). A multicenter, open-label randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(11):1354–62. https://doi.org/10.1164/rccm.201902-0383oc.
- 7. Hopkinson NS, Kemp SV, Toma TP, Hansell DM, Geddes DM, Shah PL, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J. 2011;37(6):1346–51. https://doi.org/10.1183/09031936.00100110.
- 8. Gompelmann D, Benjamin N, Bischoff E, Kontogianni K, Schuhmann M, Hoffmann H, et al. Survival after endoscopic valve therapy in patients with severe emphysema. Respiration. 2019;97(2):145–52. https://doi.org/10.1159/000492274.
- 9. Klooster K, Hartman JE, ten Hacken NHT, Slebos DJ. Improved predictors of survival after endobronchial valve treatment in patients with severe emphysema. Am J Respir Crit Care Med. 2017 May;195(9):1272–4. https://doi.org/10.1164/rccm.201610-1993le.
- 10. Garner J, Kemp SV, Toma TP, Hansell DM, Polkey MI, Shah PL, et al. Survival after endobronchial valve placement for emphysema: a 10-year follow – up study. Am J Respir Crit Care Med. 2016 Aug;194(4):519–21. https://doi.org/10.1164/rccm.201604-0852le.
- 11. Rötting M, Polke M, Sarmand N, Herth FJF, Gompelmann D. Korrelation von pCO2 und pO2 mit Lungenfunktion und weiteren physiologischen Markern bei Patienten mit fortgeschrittener COPD. Pneumologie. 2018;72(S 01):S64. https://doi.org/10.1055/s-0037-1619286.
- 12. Tsujino K, Sasada S, Kodama M, Ishihara H, Kawase I. Severe bullous emphysema and hypercapnia successfully treated by bronchoscopic lung volume reduction. Respirology. 2009 Aug;14(6):907–9. https://doi.org/10.1111/j.1440-1843.2009.01581.x.
- 13. Sexton P, Garrett JE, Rankin N, Anderson G. Endoscopic lung volume reduction effectively treats acute respiratory failure secondary to bullous emphysema. Respirology. 2010 Oct;15(7):1141–5. https://doi.org/10.1111/j.1440-1843.2010.01824.x.
- 14. Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012 jun;39(6):1334–42. https://doi.org/10.1183/09031936.00161611.
- 15. Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. https://doi.org/10.1016/s2213-2600(14)70153-5.
- 16. Calverley PMA. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;47:26S–30S. https://doi.org/10.1183/09031936.03.00030103.
- 17. Polke M, Rötting M, Sarmand N, Krisam J, Eberhardt R, Herth FJF, et al. Interventional therapy in patients with severe emphysema: evaluation of contraindications and their incidence. Ther Adv Respir Dis. 2019 Jan–Dec;13:175346661983549. https://doi.org/10.1177/1753466619835494.
- 18. Gompelmann D, Kontogianni K, Schuhmann M, Eberhardt R, Heussel CP, Herth FJ. The minimal important difference for target lobe volume reduction after endoscopic valve therapy. Int J Chron Obstruct Pulmon Dis. 2018 Feb 1;13:465–72. https://doi.org/10.2147/copd.s152029.