Introduction
Chronic respiratory diseases were the most common noncommunicable diseases in the world. The main risk factor for chronic respiratory diseases is the toxic environmental, occupational, and behavioral inhalation exposure []. Asthma, chronic obstructive pulmonary disease (COPD), and bronchiectasis are major respiratory diseases. Nearly 545 million people in the world suffered from chronic airway diseases in 2017, an increase of 39.8% since 1990 []. Systemic corticosteroid therapy was used in the treatment of exacerbations of asthma and COPD, and in stage 5 of severe asthma. Inhaled corticosteroids (ICSs) were the cornerstone of stable asthma treatment. In COPD, although ICSs have been progressively reduced, they were generally prescribed at higher doses due to the reduced responsiveness of the majority of patients []. Noncystic fibrosis bronchiectasis (“bronchiectasis”) is a chronic, slowly progressing, inflammatory pulmonary disease that is characterized by airway inflammation and excess sputum production. A high rate of ICS use in bronchiectasis patients was observed. As ICS can be delivered directly into the small airways and exhibits anti-inflammatory effects when compared to systemic corticosteroid, it can reduce the risk of unwanted systemic effects [-]. However, in cross-sectional studies, long-term treatment with ICS resulted in systemic side effects including easy bruising [], adrenal suppression [], decreased bone mineral density [], and mycobacterial infection [].
Nontuberculous mycobacteria (NTM) are all mycobacteria except M. tuberculosis complex and M. leprae. NTM, which are environmental organisms that can cause progressive lung disease associated with increased morbidity and mortality []. Analysis of a 5% sample of Medicare Part B beneficiaries calculated a prevalence of 47 cases per 100,000 population in the USA, which increased by 8.2% annually from 1997 to 2007 []. Similar prevalence and trend have been reported in Canada and outside North America []. In addition, epidemiological studies have reported that the incidence of NTM pulmonary disease is on the rise globally [, -] and that accurate estimates are limited by the heterogeneity of case definitions and the variability of diagnostic reports. Current available data show an association between ICS therapy and an increased risk of reactivation of NTM. For example, Andréjak and colleagues found that ICS use was associated with increased odds of NTM infection in a national registry of patients with chronic respiratory disease in Denmark []. Brode and colleagues also found that ICS use was associated with increased odds of NTM infection in chronic airway diseases [].
Tuberculosis (TB), caused by Mycobacterium tuberculosis, was one of the top 10 causes of death globally [, ]. Today, despite the goals achieved with the “Global Plan to Stop TB” (2006–2015), TB remains the leading cause of death from a single infectious agent, surpassing HIV/AIDS and malaria. The World Health Organization thus launched a new program called “The End TB Strategy” with the aim of ending the global TB epidemic by 2035 through a reduction in deaths by 95% and in incidence by 90% compared with levels in 2015 [-]. There have been several reports showing the association between ICS use and risk of TB infection [-].
In summary, multiple studies [, -] have reported the association between ICS and mycobacteria. However, there is no clear evidence that ICS use was associated with an increased risk of mycobacterial infection, as the sample size was relatively modest, and the results were inconclusive. To investigate the association between ICS and mycobacteria, we performed this meta-analysis.
Materials and Methods
Search Strategy
We searched [#1(inhaled corticosteroid); #2(ICS); #3(tuberculosis); #4(TB); #5(non-tuberculous mycobacteria); #6(NTM); #7(lung); #8(pulmonary)] in PubMed (MEDLINE), Cochrane, Web of Science, and EMBASE databases for articles that were published during and before 2021 with the search type: (#1 OR #2) AND (#7 OR #8) AND [(#3 OR #4) OR (#5 OR #6)]. Literature search was conducted by three authors (Y.Y., Y.N., and G.S.).
Inclusion and Exclusion Criteria
This meta-analysis included studies that met the following selection criteria. (1) All patients in the study were diagnosed with a chronic airway disease, such as asthma, COPD, and bronchiectasis according to the International Classification of Diseases (ICD), 9th (ICD-9) or 10th (ICD-10). (2) Chronic airway disease patients with NTM or TB infection were defined using microbiological criteria: two or more sputa culture positive or one bronchoscopy or lung biopsy or pleural specimen culture positive. (3) All studies evaluated ICS use and mycobacterium risk. (4) Using a case-control or cohort design or retrospective cohort study. (5) ICS use; dose of ICS use; type of ICS use; oral corticosteroids’ (OCS) use; patients with prior pulmonary TB, bronchiectasis, asthma, and COPD were included. Preclinical studies, editorials, review articles, commentaries, conference abstracts, and book chapters were excluded.
Data Extraction
All articles identified in the initial database search were filtered by title, abstract, and full-text to confirm eligibility and avoid data overlap. Titles and abstracts were screened by 3 authors (Y.Y., Y.N., and G.S.), and studies unrelated to the topic were excluded. Relevant data were extracted from eligible publications: first author name, year of publication, number of patients analyzed, baseline characteristics, etc. Disagreement about the study judgment was discussed by three authors (Y.Y., Y.N., and G.S.).
Statistical Analysis
We evaluated the risk of NTM infection in patients who used ICS because of asthma, COPD, bronchiectasis. Subgroup analysis evaluated the risk of NTM infection according to ICS dose and type of ICS. We also evaluated the risk of TB infection in patients who use ICS and OCS because of asthma, COPD, and bronchiectasis. Continuous variables were presented as standardized mean differences with 95% confidence intervals (CIs). Pooled standardized mean difference with 95% CI was calculated, and p < 0.05 was accepted with statistical significance. Heterogeneity of the study was determined by I2 statistics using Cochrane Review Manager 5.3. I2 ≥ 25%, 50%, and 75% were considered mild, moderate, and high heterogeneity, respectively. For I2 > 50%, a random-effects model was used to evaluate the overall effect of a given comparison. The classified data were presented as odds ratios (ORs) with 95% CI. Random-effects model was used for meta-analysis.
Quality Score and the Risk of Bias Assessment
To assess the risk of bias assessments for the included studies, we made a funnel diagram through Rev-man5 software, and to assess the quality, three reviewers independently scored the studies according to the Newcastle Ottawa Scale (NOS), which evaluates cohort and case-control studies in terms of selection of participants (source and selection of case and control) and comparability of case and control to exposure (determination of exposure and no-response rates). The 9-point NOS contains 3 items: selection (0–4), comparability (0–2), and exposure (0–3). Research with an NOS score of more than 7 was considered high quality. Disagreement among the three judges was discussed.
Results
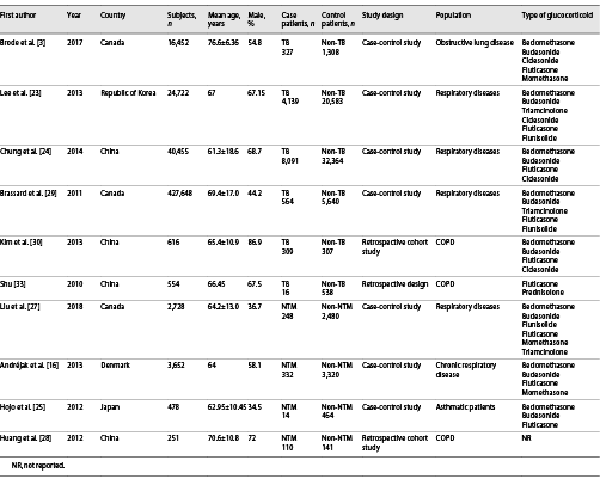
A total of 312 potentially relevant articles were obtained to determine further eligibility. Of these, 302 articles did not meet our inclusion criteria and were excluded. This meta-analysis included 10 publications published between 2010 and 2018 that satisfied our inclusion criteria. According to the PRISMA claim shown in Figure 1, the average age of the subjects was 58.22 years, and the proportion of male subjects ranged from 34.5% to 86.9%. Studies were conducted in the Republic of Korea, Canada, Denmark, Japan, and China. The main characteristics of the studies included in the meta-analysis are shown in Table 1, and the PRISMA checklist is shown in the online supplementary material (for all online suppl. material, see http://www.karger.com/doi/10.1159/000525980).
Fig. 1
Flow chart of literature retrieval and studies’ selection.
In the NTM pooled analyses, ICS use increased odds of NTM infection in patients with chronic airway diseases (OR = 3.93, 95% CI 2.12–7.27) (Fig. 2). Within the subgroup analysis, we found that high-dose ICS increased the NTM infection risk in patients with chronic respiratory disease (OR = 2.27, 95% CI 2.08–2.48) and that low-dose ICS did not increase the NTM infection risk (OR = 0.55, 95% CI 0.24–1.22) (Fig. 3). In addition, compared to budesonide (OR = 0.71, 95% CI 0.32–1.60), fluticasone use (OR = 2.42, 95% CI 2.23–2.63) was more likely to induce the NTM infection (Fig. 4).
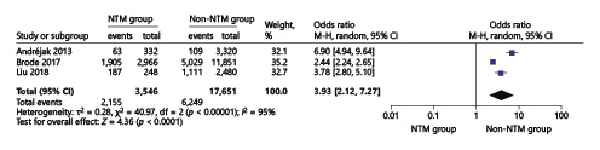
Fig. 2
Forest plots of ICS use increases odds of NTM infection in chronic respiratory diseases. OR, odds ratio; CI, confidence interval.
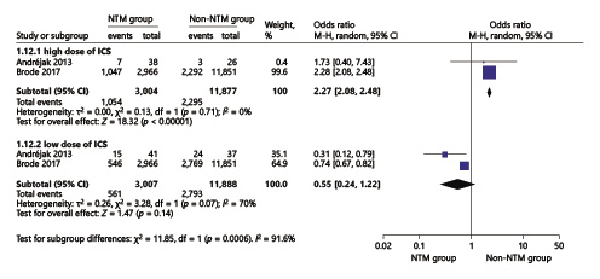
Fig. 3
Forest plots of low- and high-dose ICS use increases odds of NTM infection in chronic respiratory diseases. OR, odds ratio; CI, confidence interval.
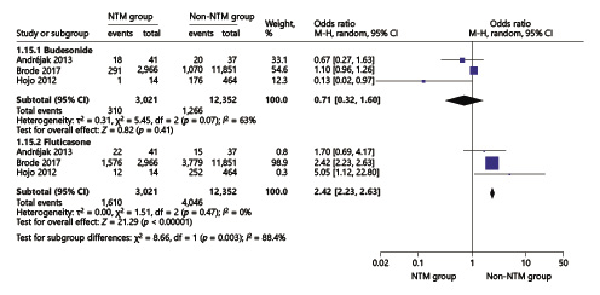
Fig. 4
Forest plots of budesonide and fluticasone use with the risk of NTM infection in chronic respiratory diseases. OR, odds ratio; CI, confidence interval.
The TB pooled analyses showed that ICS use increased odds of TB infection in patients with chronic respiratory diseases (OR = 2.01, 95% CI 1.23–3.29) (Fig. 5). In the chronic respiratory diseases subgroup analysis, we found that in COPD patients, ICS use increased odds of TB infection (OR = 1.45, 95% CI 1.29–1.63) (Fig. 6) in high-dose ICS use (OR = 1.70, 95% CI 1.56–1.86) but did not increase odds of TB infection in medium-dose ICS use (OR = 1.08, 95% CI 0.85–1.36) and low-dose ICS use (OR = 1.03, 95% CI 0.68–1.56) (Fig. 7).
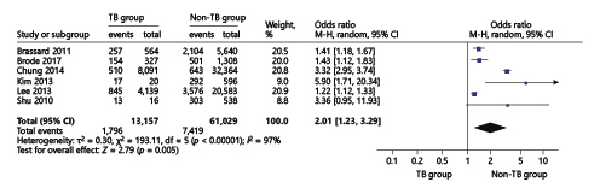
Fig. 5
Forest plots of ICS use increases odds of TB infection in chronic respiratory diseases. OR, odds ratio; CI, confidence interval.
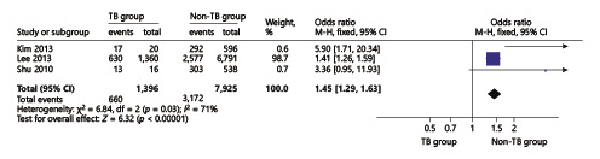
Fig. 6
Forest plots of ICS use increases odds of TB infection in COPD patients. OR, odds ratio; CI, confidence interval.
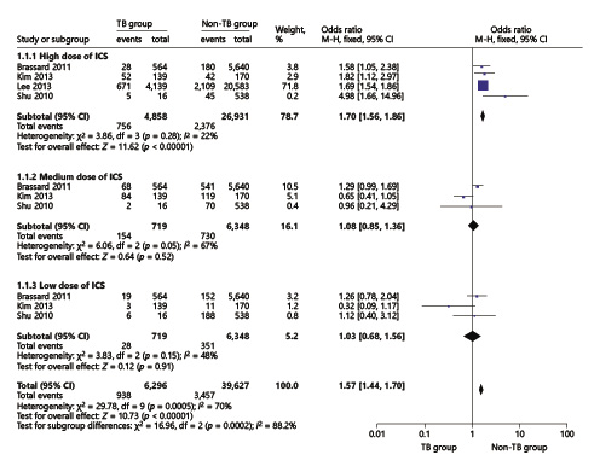
Fig. 7
Forest plots of low-, medium-, and high-dose ICS use increases odds of TB infection in chronic respiratory diseases. OR, odds ratio; CI, confidence interval.
In this meta-analysis, ten studies evaluated by NOS score averaged 4–7 out of 9. The risk of bias assessment of the included studies is presented in online supplementary Figures 1, 2. The absolute risk and risk difference are presented in online supplementary Tables 1, 2. Some results are highly heterogeneous, such as ICS use in the risk of NTM infection (I2 = 97%) and TB infection (I2 = 98%) in patients with chronic respiratory diseases. Therefore, we conducted sensitivity analysis. We omitted one study per round; however, the results did not change much. The heterogeneity of NTM infection fluctuated between 87% and 97% (online suppl. Table 3), and the results of TB infection fluctuated between 65% and 98% (online suppl. Table 5). For the high heterogeneity after sensitivity analyses, we have done subgroup analysis of age, sex, population region, chronic respiratory disease patients with comorbidities, but there were no meaningful results. In the sensitivity analyses of budesonide use with NTM infection, when we omitted the study by Hojo et al. [], the heterogeneity deceased (I2 = 15%). After we reread the study, we found that the time of ICS use was much longer than in other studies, which maybe influence the heterogeneity (online suppl. Table 4). The sensitivity analyses of ICS use with TB infection in COPD patients show high heterogeneity (I2 = 80%), when we omitted the study by Lee et al. The forest plots have no heterogeneity (I2 = 0%). When we reread the study, we found that in COPD patients, the definition of dose of ICS is higher than in other studies, which maybe influence the heterogeneity (online suppl. Table 6).
Discussion
The results of our meta-analysis showed that there was a positive association between ICS use and mycobacterial infection in chronic respiratory disease patients. A high dose of ICS increased the odds of NTM infection; and compared with budesonide, fluticasone was more likely to increase the odds of NTM infection. In addition, ICS use increased the odds of TB infection in COPD patients, and this association was also found in high-dose ICS use.
The study by Ni et al. [] has revealed the relationship between ICS use and the risk of mycobacterial infection in patients with chronic airway diseases. Our conclusions were partially consistent with them, such as ICS use did not increase odds of TB infection among OCS users, and ICS use increased odds of TB infection in the patients with past TB history. However, in addition to the above conclusions, our meta-analysis obtained more results, such as a high dose of ICS increased the odds of NTM infection, fluticasone was more likely to increase the odds of NTM infection, and ICS use increased the odds of TB infection in COPD patients, and this association was also found in high-dose ICS use.
ICS Use with NTM Infection in Chronic Respiratory Diseases Patients
ICSs were commonly used to treat chronic airway diseases, particularly asthma and COPD. Several studies in chronic airway diseases patients with NTM infection have reported the ICS use increased odds of NTM infection []; Liu et al. [] showed that corticosteroid was an important factor for NTM infection, their crude OR was 3.88 (2.87–5.26); Andréjak et al. [] showed that chronic respiratory disease, treated with ICS, is a strong risk factor for NTM pulmonary disease, and their adjusted OR was 15.6 (8.9–27.5). Our results demonstrate once again the ICS use increases the odds of NTM infection in chronic airway disease patients.
Hojo et al. [] performed a single-institution nested case-control study of asthma patients, in which NTM-infected patients received a higher daily dose of ICS than controls (p < 0.01), and Andréjak et al. [] showed that when ICS was further subdivided by ICS dose and type, the NTM infection risk in COPD patients increased from 28.1 for doses lower than 800 μg/day (low dose) to 47.5 for doses higher than 800 μg/day (high dose); our pooled result (2.28; 2.08–2.48) also proved that high doses of ICS increased odds of NTM infection. In addition, our result showed that NTM infection risk in fluticasone was higher than in budesonide. The OR 2.42 (2.23–2.63) was consistent with the study by Brode et al. []; their crude OR for fluticasone was 2.09 (1.80–2.43), and for this result, the study by Brode et al. [] ran two post hoc-adjusted analyses to explore whether there was a differential risk between fluticasone and budesonide. (1) They changed the reference group to current budesonide users and found that current fluticasone use was associated with a significantly increased risk of NTM-PD compared with current budesonide use (aOR 1.76, 95% CI 1.50–2.07; p < 0.001). (2) They divided current users of fluticasone and budesonide into those exposed to low, moderate, or high mean daily dose (based on beclomethasone equivalents), in order to account for the higher potency of fluticasone. Finally, with high-dose budesonide users as the reference group, they found that current use of low-, moderate-, or high-dose fluticasone was associated with increased risk of NTM-PD. However, in our study, the dose-response relationship was not found.
ICS Use with TB Infection in Chronic Respiratory Disease Patients
TB was the leading cause of death worldwide by an identifiable infectious agent [], systemic corticosteroid therapy would increase risk of reactivation of LTBI, and numerous studies have reported that ICS use increased odds of TB infection []. The study by Lee et al. [] showed that ICS use increased the odds of TB infection in patients with COPD and asthma, their adjusted OR was 1.20 (1.08–1.34). Chung et al. [] found that there was a significant association between ICS use and the risk of TB infection in patients with chronic airway disease. In the study by Brode et al. [], their crude OR for ICS use with TB infection was 1.47 (1.10–1.96), which was consistent with our pooled analysis, and this proved that ICS use increased odds of TB infection. In addition, there were studies concerned about the relationship between ICS use and TB infection in COPD patients [-]; the study by Lee et al. [] reported that their unadjusted OR was 1.47 (1.3–1.66), our pooled result OR was 1.41 (1.26–1.59), which means that ICS use would increase odds of TB infection in COPD patients, and our results were also consistent with a meta-analysis by Ni et al. []. As the dose of ICS use, studies have reported that high-dose ICS use increased the odds of TB infection; Brassard et al. [] reported their adjusted RR result was 1.55 (0.99–2.44), and our result was 1.58 (1.05–2.28) which proves that high doses of the ICS would increase TB infection.
Overall, ICS use was common in chronic airway diseases but the mechanism of the association between ICS use and increased odds of mycobacteria infection remains unclear, animal data showed that ICS alter cellular immune functions that were essential for host response and resistance to pathogens []. For example, ICS reduced mucosal-associated invariant T cells in bronchial tissue of patients with COPD, and mucosal-associated invariant T cells cells help prevent mycobacterial infection. However, airway remodeling and mucus gland impaction were not weakened by ICS [].
We should thus strengthen the standardized management of chronic airway disease, clarify the indication of treatment containing ICS, reduce the exposure of ICS, and reduce the risk of mycobacterial infection. Previous studies [, ] have revealed the relationship between ICS use and TB infection, but few studies revealed the relationship between ICS use and NTM infection. Our current study was the first meta-analysis to investigate the association between ICS use and risk of NTM and TB infection in patients with chronic respiratory diseases. Our meta-analysis revealed that in routine clinical assessment, when using ICS, clinicians should pay attention not only to TB infection but also to NTM infection, and pay attention to the types and dose of ICS use.
However, this systematic overview has some limitations based on the predefined data abstraction form. First, a few minor changes were made to facilitate data pooling. Second, the lack of data on some of the outcomes reduced the number of eligible studies. Third, the low and high dose of ICS results included only two articles, and because the number of people included in the study by Andréjak et al. [] was too small compared to Brode et al. [], it may require careful interpretation and further study. In addition, our study involving 517,556 patients was assessed to be of moderate to high quality, and among our study’s limitations, the most important was the high heterogeneity between included studies about ICS use and mycobacterial infection risk in chronic respiratory diseases. In order to explain this, we performed sensitivity analysis. We omitted one study per round, but the fluctuation range of heterogeneity results was small, then we have done subgroup analysis of age, sex, population region, comorbidities, but there were no meaningful results to explain the heterogeneity. When we reread all included studies, we knew that the actual relationship was complicated, such as HIV, malnutrition, alcohol misuse, smoking, or diabetes, which were consistently considered risk factors for mycobacterial infection, and another reason maybe was that in some studies, ICS use increased higher risk, and in other studies, ICS use has no risk of mycobacterial infection, but the whole effect showed that ICS use increases odds of mycobacterial infection. So, for a better explanation of heterogeneity, more studies need to be analyzed in the future. Although our results indicated that ICS use increased odds of mycobacterial infection, but the impact of this risk factor on the epidemiology of NTM or TB was limited; our work here has not been able to fully explain the heterogeneity, so we should pay more attention to this question in future studies. In addition, a burden of chronic respiratory diseases was reported high in the Asian region, we thus believed that our results may provide useful data on the epidemiology of ICS use with NTM or TB infection in Asia. However, no study performed in countries other than Canada and Denmark was found, so any inference could be highly biased.
Conclusions
The results of our meta-analysis indicated that ICS use may increase the odds of mycobacterial infection in chronic respiratory disease patients, and this conclusion is more applicable to patients with high dose of ICS or fluticasone in NTM infection subgroups. In addition, high-dose ICS use may have higher risk of TB infection in patients with chronic respiratory diseases, especially COPD. Therefore, we should be vigilant about the application of ICS use in chronic respiratory diseases to avoid infection.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on the published literature.
Conflict of Interest Statement
The three authors reported no conflict of interest in the work.
Funding Sources
The National Natural Science Foundation of China (NSFC): 81970020; 8217003. The Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases: (20dz2261100).
Author Contributions
Guochao Shi: manuscript conception, data extraction, data analysis, risk of bias assessment, manuscript redaction, and final approval and is the guarantor for the entire manuscript. Yajie You and Yingmeng Ni: literature search, study inclusion, data extraction, data analysis, risk of bias assessment, manuscript redaction, and final approval.
Data Availability Statement
The data supporting the results of this study are openly available from the reference listed in Table 1. The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/ [, , -, -, ].
References
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
- 2. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–96. https://doi.org/10.1016/S2213-2600(20)30105-3.
- 3. Brode SK, Campitelli MA, Kwong JC, Lu H, Marchand-Austin A, Gershon AS, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50(3):1700037. https://doi.org/10.1183/13993003.00037-2017.
- 4. Man SFP, Sin DD. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a clinical benefit?Drugs. 2005;65(5):579–91. https://doi.org/10.2165/00003495-200565050-00001.
- 5. Gartlehner G, Hansen RA, Carson SS, Lohr KN. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med. 2006;4(3):253–62. https://doi.org/10.1370/afm.517.
- 6. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–78. https://doi.org/10.1183/09031936.00138707.
- 7. Mak VH, Melchor R, Spiro SG. Easy bruising as a side-effect of inhaled corticosteroids. Eur Respir J. 1992;5(9):1068–74.
- 8. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941–55. https://doi.org/10.1001/archinte.159.9.941.
- 9. Connett J, Scanlon P, Scanlon P, Skeans M, Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902–9. https://doi.org/10.1056/NEJM200012283432601.
- 10. Ni S, Fu Z, Zhao J, Liu H. Inhaled corticosteroids (ICS) and risk of mycobacterium in patients with chronic respiratory diseases: a meta-analysis. J Thorac Dis. 2014;6(7):971–8. https://doi.org/10.3978/j.issn.2072-1439.2014.07.03.
- 11. McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148(6):1517–27. https://doi.org/10.1378/chest.15-0458.
- 12. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–6. https://doi.org/10.1164/rccm.201111-2016OC.
- 13. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–9. https://doi.org/10.1086/648443.
- 14. Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–6. https://doi.org/10.1164/rccm.201002-0310OC.
- 15. Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–82. https://doi.org/10.1164/rccm.201003-0503OC.
- 16. Andréjak C, Nielsen R, Thomsen VØ, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–62. https://doi.org/10.1136/thoraxjnl-2012-201772.
- 17. Wallis RS, Johnson JL. Adult tuberculosis in the 21st century: pathogenesis, clinical features, and management. Curr Opin Pulm Med. 2001;7(3):124–32. https://doi.org/10.1097/00063198-200105000-00003.
- 18. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68(12):1105–13. https://doi.org/10.1136/thoraxjnl-2012-203175.
- 19. Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320(9):545–50. https://doi.org/10.1056/NEJM198903023200901.
- 20. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. https://doi.org/10.1371/journal.pmed.1002152.
- 21. Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the end TB era. Lancet Respir Med. 2018;6(4):299–314. https://doi.org/10.1016/S2213-2600(18)30057-2.
- 22. Bahçeciler NN, Nuhoglu Y, Nursoy MA, Kodalli N, Barlan IB, Başaran MM. Inhaled corticosteroid therapy is safe in tuberculin-positive asthmatic children. Pediatr Infect Dis J. 2000;19(3):215–8. https://doi.org/10.1097/00006454-200003000-00008.
- 23. Lee CH, Lee MC, Shu CC, Lim CS, Wang JY, Lee LN, et al. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis. 2013;13(194):194. https://doi.org/10.1186/1471-2334-13-194.
- 24. Chung WS, Chen YF, Hsu JC, Yang WT, Chen SC, Chiang JY. Inhaled corticosteroids and the increased risk of pulmonary tuberculosis: a population-based case-control study. Int J Clin Pract. 2014;68(10):1193–9. https://doi.org/10.1111/ijcp.12459.
- 25. Hojo M, Iikura M, Hirano S, Sugiyama H, Kobayashi N, Kudo K. Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology. 2012;17(1):185–90. https://doi.org/10.1111/j.1440-1843.2011.02076.x.
- 26. Henkle E, Aksamit TR, Barker AF, Curtis JR, Daley CL, Anne Daniels ML, et al. Pharmacotherapy for non-cystic fibrosis bronchiectasis: results from an NTM info & research patient survey and the bronchiectasis and NTM Research Registry. Chest. 2017;152(6):1120–7. https://doi.org/10.1016/j.chest.2017.04.167.
- 27. Liu VX, Winthrop KL, Lu Y, Sharifi H, Nasiri HU, Ruoss SJ. Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann Am Thorac Soc. 2018;15(10):1169–76. https://doi.org/10.1513/AnnalsATS.201804-245OC.
- 28. Huang CT, Tsai YJ, Wu HD, Wang JY, Yu CJ, Lee LN, et al. Impact of non-tuberculous mycobacteria on pulmonary function decline in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2012;16(4):539–45. https://doi.org/10.5588/ijtld.11.0412.
- 29. Brassard P, Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011;183(5):675–8. https://doi.org/10.1164/rccm.201007-1099OC.
- 30. Kim JH, Park JS, Kim KH, Jeong HC, Kim EK, Lee JH. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013;143(4):1018–24. https://doi.org/10.1378/chest.12-1225.
- 31. Lee CM, Heo J, Han SS, Moon KW, Lee SH, Kim YJ, et al. Inhaled corticosteroid-related tuberculosis in the real world among patients with asthma and COPD: a 10-year nationwide population-based study. J Allergy Clin Immunol Pract. 2019;7(4):1197–1206.e3. https://doi.org/10.1016/j.jaip.2018.10.007.
- 32. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19–26. https://doi.org/10.1002/art.21705.
- 33. Shu CC, Wu HD, Yu MC, Wang JT, Lee CH, Wang HC, et al. Use of high-dose inhaled corticosteroids is associated with pulmonary tuberculosis in patients with chronic obstructive pulmonary disease. Medicine. 2010;89(1):53–61. https://doi.org/10.1097/MD.0b013e3181cafcd3.
- 34. Patterson CM, Morrison RL, D’Souza A, Teng XS, Happel KI. Inhaled fluticasone propionate impairs pulmonary clearance of Klebsiella pneumoniae in mice. Respir Res. 2012;13(1):40. https://doi.org/10.1186/1465-9921-13-40.
- 35. Hogg JC, Chu FSF, Tan WC, Sin DD, Patel SA, Pare PD, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454–9. https://doi.org/10.1164/rccm.200612-1772OC.
- 36. Castellana G, Castellana M, Castellana C, Castellana G, Resta E, Carone M, et al. Inhaled corticosteroids and risk of tuberculosis in patients with obstructive lung diseases: a systematic review and meta-analysis of non-randomized studies. Int J Chron Obstruct Pulmon Dis. 2019;14:2219–27. https://doi.org/10.2147/COPD.S209273.
Yajie You and Yingmeng Ni contributed equally to this work.