PsA patients with enthesitis had greater disease activity, pain and psoriasis than those without enthesitis.
Secukinumab and adalimumab led to similar achievement of enthesitis resolution overall and at individual sites.
Patients taking either drug displayed similar time to enthesitis resolution, irrespective of baseline enthesitis severity.
Introduction
Enthesitis is an early and characteristic musculoskeletal manifestation of PsA that is associated with greater disease activity and reduced quality of life [, ]. Defined as inflammation of the entheses—where a tendon or ligament inserts into bone—enthesitis has been proposed to be the primary manifestation of PsA [, ]. Clinical enthesitis occurs in around 35–50% of patients with PsA [, ]; these patients experience significantly higher disease burden and greater erosive damage than patients without enthesitis [, ]. Resolution of enthesitis is an important clinical outcome used to assess treatment response among patients with PsA and has been associated with improvements in other clinical outcomes [, ].
The European Alliance of Associations for Rheumatology (EULAR; formerly the European League Against Rheumatism) guidelines recommend biologic DMARDs (bDMARDs) for patients with enthesitis and an inadequate response to or intolerance of NSAIDs. However, no guidance on specific bDMARDs is offered []. Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has shown early and sustained efficacy and safety in treating PsA [] and has specifically been shown to reduce enthesitis in patients with PsA in a pooled analysis of four phase 3 trials []. Adalimumab, a TNF inhibitor, is approved and widely used for the treatment of PsA and remains a mainstay of PsA treatment [, ].
In the EXCEED head-to-head, double-blind study (NCT02745080) comparing secukinumab with adalimumab in patients with PsA and active psoriasis, the primary endpoint of superiority of American College of Rheumatology 20% improvement criteria (ACR20) response at week 52 for secukinumab vs adalimumab was not met, although similar efficacy was observed across a range of musculoskeletal endpoints, including resolution of enthesitis at week 52 []. However, a detailed analysis of enthesitis was not conducted. This study aims to provide detail on enthesitis treatment response, including temporal response and site-specific enthesitis data along with additional enthesitis assessments, in patients with PsA treated with secukinumab or adalimumab over 52 weeks in EXCEED.
Methods
Study design and patient data
EXCEED was a multicentre, randomized, double-blind, active-controlled, parallel-group, phase 3 trial that evaluated the efficacy and safety of secukinumab vs adalimumab in treating patients with PsA []. Eligible patients had a diagnosis of PsA per the Classification Criteria for Psoriatic Arthritis (CASPAR) and active plaque psoriasis or nail changes consistent with psoriasis and were naïve to bDMARDs. Patients were randomized 1:1 to secukinumab 300 mg or adalimumab 40 mg per the label; to maintain blinding, both groups received placebo injections to ensure that a consistent number of injections were administered per visit. The EXCEED study was conducted at 187 centres in 26 countries and was approved by the institutional review board or independent ethics committee at each participating institution and conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent before starting any study-related procedures.
In this post hoc analysis, patient data from EXCEED were grouped by presence or absence of baseline enthesitis based on the Leeds Enthesitis Index (LEI) and the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC). All enrolled patients were included in this post hoc analysis. The study was approved by the central institutional review board Chesapeake IRB (now Advarra; IRB no. 00023362).
Outcomes and assessments
Demographics and baseline disease characteristics of all four enthesitis groups (LEI/SPARCC enthesitis or no enthesitis) were summarized. The efficacy of secukinumab vs adalimumab through week 52 was evaluated among the enthesitis subset according to several outcome measures of enthesitis response, including median time to resolution of LEI/SPARCC enthesitis score in patients receiving secukinumab or adalimumab; LEI and SPARCC mean total score by visit; LEI and SPARCC mean change from baseline at weeks 24 and 52; resolution of enthesitis as measured by LEI (LEI = 0) or SPARCC (SPARCC = 0) at weeks 24 and 52; and relapse of clinical enthesitis after a first resolution of enthesitis at weeks 24 and 52. Prevention of enthesitis among patients with no enthesitis at baseline (LEI = 0 or SPARCC = 0) was determined by the proportion of patients with no new LEI or SPARCC findings through week 52.
Resolution of LEI or SPARCC enthesitis at week 52 and time to resolution of enthesitis was also assessed by number of involved entheses at baseline. For these analyses, LEI severity was based on the following cutoffs []: LEI >0 to <2, LEI 2 to ≤3, and LEI >3 to ≤6; SPARCC severity levels were defined as SPARCC >0 to <3, SPARCC 3 to ≤6, and SPARCC >6 to ≤16. Site-level enthesitis distribution at baseline and resolution at weeks 12, 24 and 52 were evaluated for all SPARCC and LEI enthesitis sites among patients with baseline enthesitis as measured by SPARCC or LEI.
Improvement in quality of life was determined by the achievement of patient-reported outcome (PRO) responses per week-52 enthesitis resolution status (among patients with and without enthesitis at week 52). Health-related quality of life (HRQOL) improvements were assessed by the achievement of a minimal clinically important difference (MCID) of ≥2.5 in the raw 36-Item Short Form Health Survey physical component summary (SF-36 PCS; scale, 0–100) as established in rheumatoid arthritis []. Physical function was evaluated by achievement of the MCID of ≥0.35 in the Health Assessment Questionnaire–Disability Index (HAQ-DI; scale, 0–3) [].
Statistical analysis
Time to resolution of enthesitis, both overall and by number of enthesitis-affected joints at baseline, was assessed using Kaplan–Meier analysis. Non-responder imputation was used to assess achievement of enthesitis resolution. Descriptive statistics were provided for other endpoints using an observed-case approach. No statistical hypothesis tests for superiority or equivalence with respect to enthesitis response were planned in the EXCEED study protocol, and none were performed in this exploratory, hypothesis-generating, secondary analysis.
Results
Demographics and baseline characteristics
This post hoc analysis included all patients from the EXCEED study who completed 52 weeks of treatment (n = 853). Of 851 total patients assessed by LEI, 498 (58.5%) had enthesitis at baseline, and 353 (41.5%) did not; of 853 total patients assessed by SPARCC, 632 (74.1%) had enthesitis at baseline, and 221 (25.9%) did not.
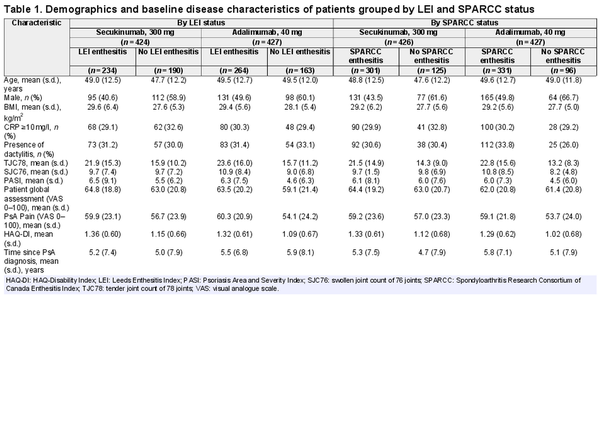
Demographics and baseline disease characteristics, including the proportion of patients with baseline CRP ≥10 mg/l, were balanced in the LEI/SPARCC enthesitis subsets, although a higher proportion of all patients with enthesitis were women vs those with no enthesitis (Table 1). The proportion of female and male patients was similar in both the secukinumab and adalimumab treatment groups overall. Patients with baseline enthesitis tended to present with greater disease activity, including higher tender joint counts, higher Psoriasis Area and Severity Index score, higher PsA pain visual analogue scale scores and worse physical function, vs patients without enthesitis. Patients with enthesitis had higher body mass index than those without, although the distribution of body mass index was similar across treatment groups. The distribution of enthesitis severity at baseline was similar between groups of patients receiving either secukinumab or adalimumab; each treatment group contained similar proportions of patients grouped by level of baseline enthesitis site involvement (Supplementary Table S1, available at Rheumatology online).
Efficacy among LEI and SPARCC subsets
Both secukinumab and adalimumab resulted in similar improvements in enthesitis as measured by the mean change from baseline in LEI and SPARCC enthesitis counts at weeks 24 and 52 (Fig. 1A). At week 24, mean (S.D.) change from baseline in LEI and SPARCC enthesitis counts for patients receiving secukinumab was −1.6 (1.6) and −3.3 (3.5), respectively; LEI and SPARCC improvement from baseline for patients receiving adalimumab was −1.6 (1.6) and −3.1 (3.5), respectively. These improvements were sustained through week 52, when mean (S.D.) change from baseline in LEI and SPARCC enthesitis counts for patients receiving secukinumab was −1.8 (1.6) and −3.6 (3.2), respectively; LEI and SPARCC improvement for patients receiving adalimumab was −2.1 (1.7) and −3.9 (3.8), respectively. Similar proportions of patients in both treatment groups achieved resolution of LEI and SPARCC at weeks 24 and 52 (Fig. 1B). By week 24, 49.6% and 45.8% of patients receiving secukinumab and 43.6% and 43.5% of patients receiving adalimumab achieved resolution of LEI and SPARCC enthesitis, respectively. These results were extended through week 52, when 60.7% and 53.2% of patients receiving secukinumab and 55.3% and 51.4% of patients receiving adalimumab achieved resolution of LEI and SPARCC enthesitis, respectively. Additionally, change from baseline in number of affected entheses by visit followed similar trajectories for patients receiving either drug (Supplementary Fig. S1, available at Rheumatology online).
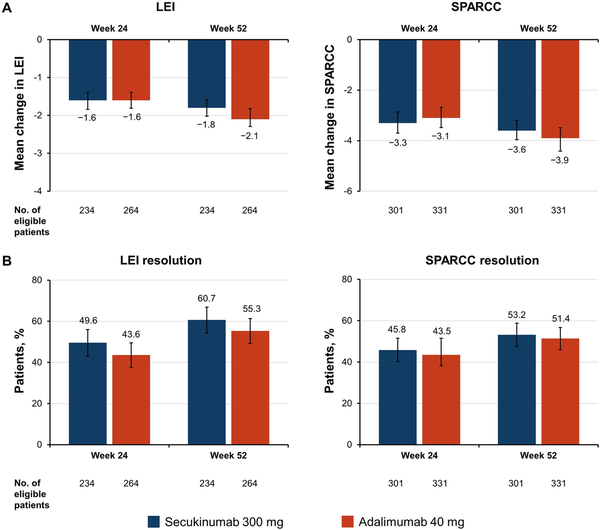
Figure 1
Enthesitis improvements at weeks 12 and 24 as determined by (A) mean change from baseline in LEI and SPARCC enthesitis counts and (B) the proportion of patients achieving LEI or SPARCC resolution (non-responder imputation). Error bars represent 95% CIs. LEI: Leeds Enthesitis Index; SPARCC: Spondyloarthritis Research Consortium of Canada Enthesitis Index
The time to enthesitis resolution was comparable for both drugs. Similar median (95% CI) times to resolution of enthesitis were observed between groups of patients receiving secukinumab and adalimumab, with overlapping 95% CIs (Fig. 2, Supplementary Fig. S2, available at Rheumatology online). Time to LEI resolution was 85 (57–113) days for patients receiving secukinumab and 85 (57–86) days for patients receiving adalimumab. Time to SPARCC resolution was 113 (85–169) days for patients receiving secukinumab and 88 (85–114) days for patients receiving adalimumab. Additionally, achievement of enthesitis resolution was similar between treatment groups irrespective of disease severity, as defined above by number of LEI or SPARCC sites involved (Fig. 3). However, patients in either treatment group with a greater number of involved joints at baseline experienced the longest time to response among all subgroups.
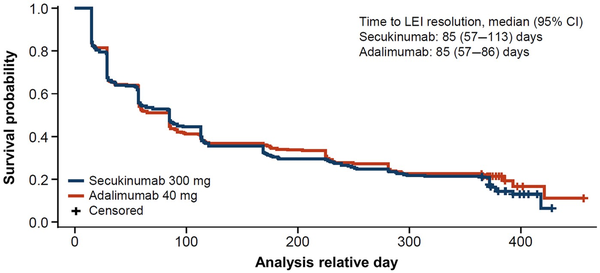
Figure 2
Kaplan–Meier estimate of time to first resolution of LEI enthesitis up to week 52. LEI: Leeds Enthesitis Index
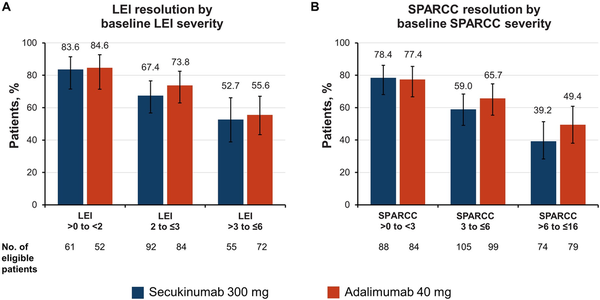
Figure 3
Proportion of patients achieving resolution of enthesitis at week 52 by baseline (A) LEI and (B) SPARCC enthesitis severity. Error bars represent 95% CIs. LEI: Leeds Enthesitis Index; SPARCC: Spondyloarthritis Research Consortium of Canada Enthesitis Index
The proportion of patients who experienced relapse after achieving initial resolution of enthesitis was low across both treatments (Supplementary Table S2, available at Rheumatology online). Similarly, both drugs prevented the development of enthesitis through week 52 among patients who had no enthesitis at baseline (Supplementary Fig. S3, available at Rheumatology online).
Site-specific enthesitis distribution and treatment response
Distribution of enthesitis sites at baseline was well balanced among patients across the two treatment groups and by upper and lower extremity involvement (Supplementary Fig. S4, available at Rheumatology online). Patients randomized to secukinumab most frequently experienced baseline enthesitis of the lateral epicondyle (33.0%) and greater trochanter (32.8%); those randomized to adalimumab most frequently had baseline enthesitis of the lateral epicondyle (39.3%) and the Achilles tendon insertion into the calcaneum (38.6%).
By week 12, a high proportion of patients in both treatment groups achieved similar resolution of enthesitis at the level of individual entheses across all SPARCC sites (Fig. 4), with sustained improvement through week 52. Secukinumab and adalimumab resulted in similar improvements in enthesitis of the medial femoral condyle through week 52, which is measured by LEI but not by SPARCC.
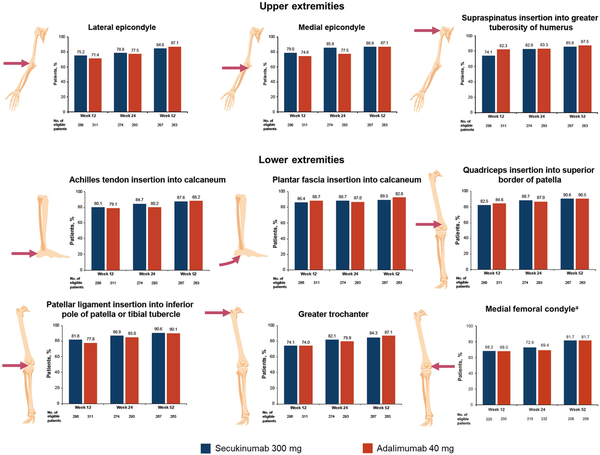
Figure 4
Patients achieving enthesitis resolution at SPARCC sites in the upper and lower extremities at weeks 12, 24, and 52. aMedial femoral condyle is measured by LEI and not SPARCC; results are presented for patients with enthesitis at baseline as measured by LEI. LEI: Leeds Enthesitis Index; SPARCC: Spondyloarthritis Research Consortium of Canada Enthesitis Index
Improvements in HRQOL by enthesitis status at week 52
Finally, we assessed the efficacy of secukinumab and adalimumab treatment on HRQOL and physical function by enthesitis status (enthesitis resolution or no enthesitis resolution as measured by LEI and SPARCC) at week 52 (Fig. 5). Secukinumab and adalimumab resulted in similar achievement of SF-36 PCS improvement ≥2.5 and HAQ-DI improvement ≥0.35 at week 52 within enthesitis status groups. For both drugs, the greatest improvements occurred among patients who achieved enthesitis resolution.
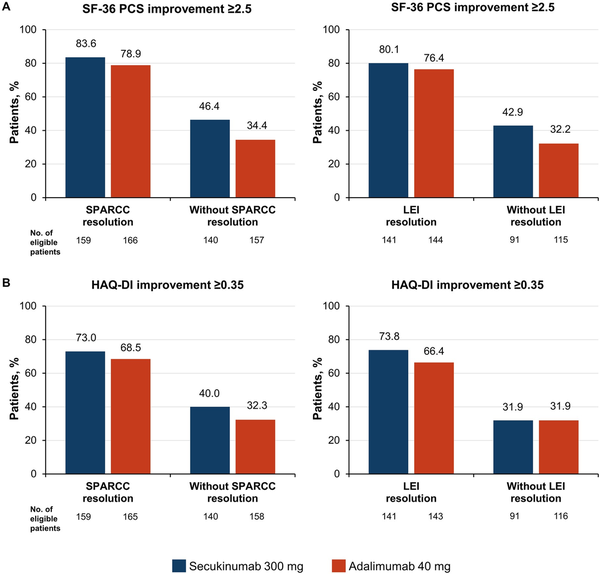
Figure 5
Achievement of (A) SF-36 PCS improvement ≥2.5 and (B) HAQ-DI improvement ≥0.35 by enthesitis status at week 52. HAQ-DI, HAQ-Disability Index; LEI: Leeds Enthesitis Index; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey; SPARCC, Spondyloarthritis Research Consortium of Canada Enthesitis Index
Discussion
In this post hoc analysis of the EXCEED study, secukinumab and adalimumab performed similarly in patients with enthesitis at baseline, as measured by LEI >0 or SPARCC >0. Enthesitis was evenly distributed across both treatment groups, although patients with baseline enthesitis presented with greater disease activity and pain than those without, consistent with the patient population enrolled in the previous FUTURE trials []. Additionally, enthesitis was not evenly distributed by sex; a greater proportion of patients in either treatment group who had enthesitis at baseline were women compared with patients without enthesitis. This observation potentially reflects the greater disease burden, presentation of peripheral arthritis and limitations in function among women vs men with PsA observed in the real world []. In a previous post hoc analysis of EXCEED, women appeared to achieve numerically higher musculoskeletal responses with secukinumab than with adalimumab, while men had similar musculoskeletal responses with both drugs []. Although both treatment groups here had a similar proportion of female patients, no subgroup analysis of enthesitis resolution by sex was performed in this study. Future analyses of the effects of sex on enthesitis treatment response are needed.
Although the overall primary outcome of superiority of ACR20 response for secukinumab vs adalimumab was not met in EXCEED [], this post hoc analysis indicates that secukinumab and adalimumab resulted in similar improvements in enthesitis. Both drugs showed similar time to response with respect to resolution of enthesitis, both overall and by severity at baseline. Efficacy according to number of affected entheses at baseline was similar for both drugs, although achievement of resolution with either drug tended to be lower among patients with greater baseline severity of enthesitis.
One potential confounder with assessing enthesitis severity by number of sites, especially among patients with large numbers of affected entheses, is the possible concomitant or differential diagnosis of fibromyalgia [], which may influence the enthesitis count and limit the apparent treatment response. More broadly, differential diagnoses such as fibromyalgia, chronic widespread pain, osteoarthritis, mechanically triggered pain and obesity contribute to the challenge of clinical assessment of enthesitis, as clinical assessment of tenderness does not discriminate by cause [, ]. Using fibromyalgia or chronic widespread pain as exclusion criteria could mitigate this issue in clinical trials; however, real-world implications remain for patients. As musculoskeletal ultrasound or magnetic resonance imaging could provide a more sensitive and specific measure of enthesitis and other PsA-related inflammatory changes compared with clinical assessment alone, such imaging tools represent an excellent complement in the diagnosis and monitoring of enthesitis and other PsA signs [, ]. The lack of an imaging endpoint for enthesitis can be viewed as a limitation of the EXCEED study.
We observed that enthesitis relapse after initial resolution was infrequent for both the secukinumab and adalimumab groups. Additionally, most patients without enthesitis at baseline experienced no new enthesitis through 52 weeks of treatment with either drug, suggesting that inhibition of IL-17 or TNF signalling could provide some protection from enthesitis progression among patients with PsA.
The anatomical distribution of enthesitis at baseline was mostly balanced between the upper and lower extremities in both secukinumab and adalimumab groups in a pattern consistent with previous reports []. These analyses also highlight the early and sustained resolution of enthesitis through 52 weeks at site level with both drugs. Little difference was observed in treatment response among load-bearing joints of the lower extremities compared with non-load-bearing joints of the upper extremities.
Improvements in PROs measuring HRQOL and disability seen with both drugs only partially reflect the consequence of improvement or resolution of enthesitis. Both the SF-36 PCS and HAQ-DI PROs indirectly capture impacts on enthesitis features and disease activity beyond those measured by LEI and SPARCC, such as axial enthesitis and other measures of PsA disease activity that consider the whole patient. For example, the EXCEED primary study demonstrated that patients receiving secukinumab experienced greater improvements in psoriasis vs those receiving adalimumab []. This difference in psoriasis response, for example, could partially underlie any small numerical improvements in PROs observed here among patients receiving secukinumab vs adalimumab.
bDMARDs are widely used in the treatment of PsA, although few studies have directly compared treatment response with different bDMARDs in a head-to-head manner. The open-label, blinded-assessor SPIRIT-H2H study comparing the IL-17 inhibitor ixekizumab with adalimumab found significantly greater resolution of SPARCC enthesitis for patients receiving ixekizumab vs adalimumab at week 24 (56.6% vs 45.0%; P = 0.019), although no significant differences in LEI resolution were observed at this time (59.7% vs 55.1%; P = 0.432) []. Although a greater proportion of patients receiving ixekizumab had SPARCC enthesitis at baseline compared with those receiving adalimumab (67% vs 60%), mean (S.D.) baseline SPARCC scores were better for patients receiving ixekizumab than those receiving adalimumab (4.9 [3.5] vs 5.7 [3.8]) []. The entheseal sites assessed by SPARCC and LEI largely overlap; however, the sites are not identical, and the greater number of entheses assessed by SPARCC vs LEI could lead to increased sensitivity for detecting presence of enthesitis and response to treatment. By week 52 of SPIRIT-H2H, no differences in enthesitis resolution were observed with either drug as measured by LEI or SPARCC []. However, the open-label design of this study limits the interpretation of these results.
In the ECLIPSA study comparing the IL-12/23 inhibitor ustekinumab with adalimumab in patients with PsA and enthesitis, 73.9% of patients receiving ustekinumab achieved the primary endpoint of SPARCC resolution at week 24 compared with 41.7% of those receiving adalimumab (P = 0.007) []. Interpretation of these results is limited by both the open-label study design and by the small study size (47 total enrolled patients).
In conclusion, this post hoc analysis of EXCEED is the first blinded, head-to-head study investigating detailed enthesitis response between two bDMARDs. Overall, the findings from this study indicate that secukinumab and adalimumab improve enthesitis among patients with PsA to a similar extent in terms of achievement of enthesitis resolution, timing of response, site-specific responses and occurrence of relapse after first resolution. Our findings suggest that inhibition of IL-17A with secukinumab can result in improvements over 52 weeks in enthesitis responses comparable to those achieved with adalimumab among patients with PsA.
Acknowledgements
Medical writing support was provided by Richard Karpowicz, PhD, CMPP, of Health Interactions, Inc., and was funded by Novartis Pharmaceuticals Corporation. This publication was developed in accordance with Good Publication Practice (GPP3) guidelines. Authors had full control of the content and made the final decision on all aspects of this publication. P.G.C. and D.M. are supported in part by the UK National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- 1. Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum2018;48:35–43.
- 2. Schett G, Rahman P, Ritchlin C et al Psoriatic arthritis from a mechanistic perspective. Nat Rev Rheumatol2022;18:311–25.
- 3. Bakewell C, Aydin SZ, Ranganath VK, Eder L, Kaeley GS. Imaging techniques: options for the diagnosis and monitoring of treatment of enthesitis in psoriatic arthritis. J Rheumatol2020;47:973–82.
- 4. McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum1999;42:1080–6.
- 5. Polachek A, Li S, Chandran V, Gladman DD. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res (Hoboken)2017;69:1685–91.
- 6. Orbai AM, Birt JA, Holdsworth EA et al Impact of enthesitis on psoriatic arthritis patient-reported outcomes and physician satisfaction with treatment: data from a multinational patient and physician survey. Rheumatol Ther2020;7:937–48.
- 7. Macía-Villa C, Cruz Valenciano A, De Miguel E. Enthesis lesions are associated with X-ray progression in psoriatic arthritis. Int J Rheum Dis2021;24:828–33.
- 8. McGonagle D, McInnes IB, Deodhar A et al Resolution of enthesitis by guselkumab and relationships to disease burden: 1-year results of two phase 3 psoriatic arthritis studies. Rheumatology (Oxford)2021;60:5337–50.
- 9. Gladman DD, Orbai AM, Klitz U et al Ixekizumab and complete resolution of enthesitis and dactylitis: integrated analysis of two phase 3 randomized trials in psoriatic arthritis. Arthritis Res Ther2019;21:38.
- 10. Gossec L, Baraliakos X, Kerschbaumer A et al EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis2020;79:700–12.
- 11. Baeten D, Sieper J, Braun J et al; MEASURE 1 Study Group; MEASURE 2 Study Group. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med2015;373:2534–48.
- 12. Schett G, Baraliakos X, Van den Bosch F et al Secukinumab efficacy on enthesitis in patients with ankylosing spondylitis: pooled analysis of four pivotal phase III studies. J Rheumatol2021;48:1251–8.
- 13. Mease PJ, Gladman DD, Ritchlin CT et al; Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum2005;52:3279–89.
- 14. McInnes IB, Behrens F, Mease PJ et al; EXCEED Study Group. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet2020;395:1496–505.
- 15. Coates LC, Wallman JK, McGonagle D et al Secukinumab efficacy on resolution of enthesitis in psoriatic arthritis: pooled analysis of two phase 3 studies. Arthritis Res Ther2019;21:266.
- 16. Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE Jr. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum2000;43:1478–87.
- 17. Mease PJ, Woolley JM, Bitman B et al Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol2011;38:2461–5.
- 18. Passia E, Vis M, Coates LC et al Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther2022;24:22.
- 19. Nas K, Capkin E, Dagli AZ et al; Anatolian Group for the Assessment in Rheumatic Diseases (ANGARD). Gender specific differences in patients with psoriatic arthritis. Mod Rheumatol2017;27:345–9.
- 20. Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis2013;72:578–82.
- 21. Wright G, Nash P, Coates L et al Comparison of secukinumab versus adalimumab efficacy by sex in psoriatic arthritis from a phase 3b, double-blinded, randomized, active-controlled study. Arthritis Rheumatol2020;72(Suppl 10):0507.
- 22. Zhao SS, Duffield SJ, Goodson NJ. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best Pract Res Clin Rheumatol2019;33:101423.
- 23. Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol2017;29:304–10.
- 24. Mease P. Enthesitis in psoriatic arthritis (part 3): clinical assessment and management. Rheumatology (Oxford)2020;59:i21–8.
- 25. Kaeley GS, Bakewell C, Deodhar A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: a narrative review. Arthritis Res Ther2020;22:1.
- 26. Mathew AJ, Krabbe S, Eshed I et al The OMERACT MRI in Enthesitis Initiative: definitions of key pathologies, suggested MRI sequences, and a novel heel enthesitis scoring system. J Rheumatol2019;46:1232–8.
- 27. D'Agostino MA, Schett G, López-Rdz A et al Response to secukinumab on synovitis using power Doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatology (Oxford)2022;61:1867–76.
- 28. Mease PJ, Liu M, Rebello S et al Disease characteristics, quality of life, and work productivity by enthesitis site: real-world data from the US Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol2021;48:367–75.
- 29. Mease PJ, Smolen JS, Behrens F et al; SPIRIT H2H Study Group. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis2020;79:123–31.
- 30. Smolen JS, Mease P, Tahir H et al Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis2020;79:1310–9.
- 31. Araujo EG, Englbrecht M, Hoepken S et al Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum2019;48:632–7.