Introduction
In an era of waning paternalism and shared decision-making, patient preference is increasingly emphasized. In practice, this preference often includes requests to reduce or stop antipsychotic medication from people with psychotic disorders. Indeed, patients often do stop their medication abruptly, and they may be more likely to do so when their preferences are not considered by their physicians. Abruptly stopping antipsychotic medication is the method most likely to induce relapse and withdrawal symptoms.,
These concepts are germane to the broader context of the practice of deprescribing in medicine, as part of high-quality prescribing practice, aiming for an optimal balance of benefits and harms in the use of medication. The practice originally derives from geriatric medicine, with its concerns around polypharmacy and the uncertain balance between risks and benefits in drugs prescribed over many years; some of these concerns are relevant to psychiatric practice.
Although evidence for the benefits of antipsychotic medication in short-term treatment is established, there is an ongoing debate about the need for and benefit of prophylactic long-term antipsychotics in every person with schizophrenia., In the context of adverse effects of long-term antipsychotic medication (movement disorders, such as tardive dyskinesia (TD), metabolic effects, and effects on brain structure),, and, importantly, patient preference, it may be reasonable to attempt reduction or cessation of antipsychotics in people with nonaffective psychotic illnesses who have remitted after treatment, guided by psychiatrists. There is currently significant uncertainty about what proportion of patients might be able to stay well without antipsychotics, with numerous antipsychotic discontinuation studies in progress, but some suggest it may be up to 40%., It has been proposed that patients on long-term antipsychotics could have them carefully reduced to minimum effective doses, which for some might be zero, without negatively affecting clinical outcome and potentially improving social functioning in some patients., Importantly, when asked, patients often prioritize social functioning over symptom reduction. Here, we explore what is known about reducing and discontinuing antipsychotics, including withdrawal syndromes, and put forward some principles for reducing and discontinuing antipsychotics in a manner that minimizes the risk of relapse.
Withdrawal Syndromes
Withdrawal syndromes occur with many medications,, including all classes of psychotropics. Indeed, withdrawal syndromes are so common that it has been said that drug discontinuation effects are a predictable aspect of the pharmacology of any drug that is eliminated more quickly than the time taken for established adaptations to the drug to resolve.
Receptor antagonists reduce the activation of target receptors and, as a result, receptors may be upregulated (increased in sensitivity and/or number). When the antagonist is abruptly withdrawn, physiological levels of the receptor’s ligand can cause overstimulation of the sensitized receptors, leading to withdrawal or rebound symptoms., For example, abrupt removal of beta-blockers can cause adrenergic rebound, characterized by increased blood pressure and heart rate, anxiety, headache, and even myocardial infarction.
Antipsychotic Withdrawal Syndrome
Although not widely recognized, withdrawal symptoms can occur on the cessation of both first- and second-generation antipsychotics (FGAs and SGAs).,, These symptoms can be grouped into somatic symptoms (nausea, sweating, etc.), motor symptoms, and psychological symptoms (including psychosis; figure 1).,,
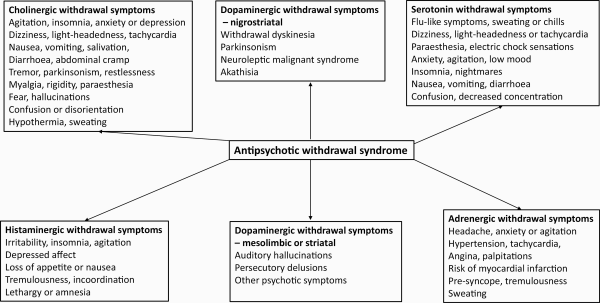
Fig. 1
Symptoms of the antipsychotic withdrawal syndrome, adapted from Chouinard et al.
Autonomic and somatic symptoms that occur on cessation generally start within days of dose reduction and resolve within weeks.,, They are attributed to the abrupt removal of the antagonistic cholinergic, adrenergic, serotonergic, and histaminergic effects of antipsychotics., For example, after only 4 weeks of clozapine treatment, 13% of patients stopping the drug abruptly experienced moderate to severe symptoms of nausea, vomiting, and diarrhea, with 40% experiencing mild symptoms. These symptoms have been attributed to cholinergic rebound following the induction of cholinergic hypersensitivity during clozapine treatment. Notably, the cholinergic rebound can also be characterized by agitation, fear, and hallucinations, which may be mistaken for psychotic relapse.
Motor symptoms occur most commonly with the withdrawal of FGAs but also with SGAs, and consist of dyskinesia, parkinsonism, and neuroleptic malignant syndrome (NMS). Symptoms develop over weeks following withdrawal and can persist for months or longer., Motor withdrawal symptoms have a reported incidence of 31%–50% after abrupt FGA withdrawal, following long-term treatment. SGAs, including risperidone and aripiprazole, can also be associated with dyskinesia upon cessation, but no formal studies looking at incidence rates have been conducted. NMS has been noted to occur on the abrupt withdrawal of antipsychotics, particularly clozapine, although no systematic study of incidence has been performed.
Neurobiology of Antipsychotic Withdrawal Syndrome
Motor withdrawal effects have been attributed to dopaminergic hypersensitivity arising in nigrostriatal pathways caused by antipsychotic treatment., When antipsychotics are abruptly withdrawn, sensitized dopamine receptors are exposed to physiological levels of dopamine, which could cause increased striatal dopaminergic activity., Withdrawal dyskinesia has been interpreted as evidence of an intermediate form of dopaminergic hypersensitivity, unmasked by the removal of dopaminergic antagonists, as compared with TD where hypersensitivity is present to a degree evident even during treatment.
In animals, dopaminergic blockade leads to dopaminergic hypersensitivity., In one study, the administration of antipsychotics for 2 weeks gave rise to dopaminergic hypersensitivity in rats, as evidenced by the waning ability of antipsychotics to suppress amphetamine-induced behavioral effects. D2 receptor number also increased 20%–40%, alongside a 2- to 3-fold increase in highly sensitive dopamine receptors. In another study in rats, 9 months of haloperidol led to a 2- to 3-fold increase in D2 receptors. Receptor numbers stayed high (and even increased) over the 2 months following haloperidol withdrawal. This time period in rats has been suggested to be equivalent to more than a year for humans.
There is also evidence that dopaminergic hypersensitivity occurs in humans. Meta-analysis of molecular imaging studies in schizophrenia found increased D2/D3 receptor availability only in those subjects who had been exposed to antipsychotic medication but not in antipsychotic-naïve patients. One study quantified this increase as 30% relative to antipsychotic-naïve patients. Longitudinal studies confirm this effect: one patient demonstrated a 10% increase in D2/D3 receptor availability over 2 months when treated with very large doses of chlorpromazine; another study demonstrated an increase in D2/D3 receptor availability in some brain regions after 2–3 weeks of treatment with low-dose antipsychotics.
Withdrawal-Associated Psychosis
Dopaminergic hypersensitivity has been proposed to contribute to early relapse into psychosis after antipsychotic cessation. Dopaminergic hypersensitivity observed in nigrostriatal pathways may also be present in other dopaminergic pathways,, including the associative striatal pathways, now centrally implicated in schizophrenia.
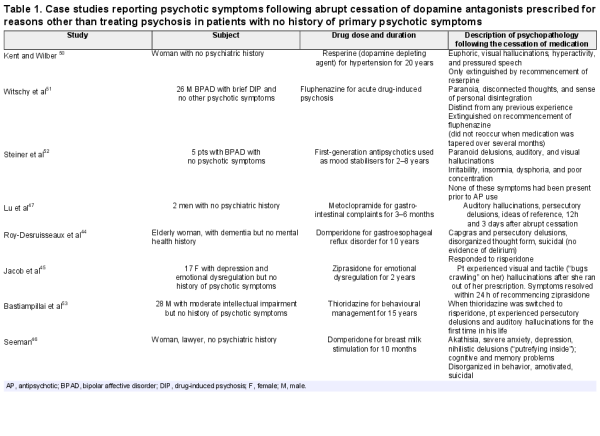
People with no history of psychosis who develop psychotic symptoms on abrupt cessation of dopamine antagonists used in a non-psychiatric context (eg, breast milk stimulation or treatment of nausea) provide support for this hypothesis (table 1). Eight case reports describe psychotic symptoms arising in 13 patients with no history of psychosis after the abrupt withdrawal of dopamine antagonists (table 1). These cases manifested cardinal symptoms of psychosis, such as auditory hallucinations, persecutory, nihilistic, and Capgras delusions, and, in some instances, were only terminated by the reintroduction of a dopamine antagonist., In one case, the dopamine antagonist was not recommenced and symptoms persisted for 10 months: the same duration as the initial treatment course. These cases have been attributed to dopaminergic hypersensitivity, although, of course, they represent a tiny fraction of those non-psychotic? individuals treated with dopamine antagonists. However, Seeman suggests that such reactions may be underreported. These observations are consistent with reports that the severity of psychotic psychopathology following clozapine withdrawal exceeded that in the illness before clozapine treatment.,
Schizophrenia is attributed to increased presynaptic dopaminergic synthesizing capacity (DSC); a recent study found that treatment with antipsychotics does not appear to alter DSC. However, the effects of D2 blockade may result in what starts as a presynaptic disorder being complicated and amplified by antipsychotic-induced postsynaptic effects. Upon the removal of blockade, persistently increased baseline presynaptic DSC in patients with schizophrenia may interact with elevated postsynaptic sensitivity to increase the likelihood of relapse.,
Withdrawal-Associated Psychosis in Patients With Schizophrenia
Distinguishing withdrawal-associated relapse from the endogenous relapse of a psychotic illness is difficult. Withdrawal-associated relapse has been thought to be characterized by early onset following the elimination of medication, and association with other evidence of withdrawal, including motor symptoms.,,
The strongest evidence for the existence of withdrawal-associated relapse is the preponderance of relapses that occur in the weeks and months following cessation., That is, relapses are not distributed evenly across time but tend to occur predominantly around the point of drug cessation. This is distinct from the natural history of the disease in which relapses in people with schizophrenia are evenly distributed across time, as seen in the early Northwick Park studies, which examined people with schizophrenia treated with placebo (thereby revealing the natural history of the disease), in which a similar proportion of patients relapsed each month. This pattern is understandable because there should be no systematic correlation between the times at which different patients relapse.
Some have suggested that the reason that relapse rates are higher in the first few months following drug cessation, and then gradually reduce, is because there are fewer patients “available” to relapse as time goes on because they have already relapsed. However, the marked increase in relapse rates that occur following drug discontinuation cannot be explained by simple attrition of patients over time: the clustering of relapses around the point of discontinuation is of a much larger magnitude. For example, in the 2 studies with more than 2 years follow-up after treatment discontinuation, one found 43% of the original cohort of patients relapsed in the first year, 21.5% in the second year, and just 3.7% a year in each of the 5 years following,; the other found that 56.5% of patients relapsed in the first year (41.3% in the first 6 months), 8.7% in the second year, and 2.2% per year thereafter. This rebound pattern is evident in other discontinuation trials,, and is not consistent with simply attrition over time.
Meta-analyses also support this apparent “rebound” effect.,, One reported that 48% of relapses occur in the first 12 months after antipsychotic discontinuation (40% in the first 6 months), with only 2% per year after this. Meta-regression found that relapse rates for maintained and discontinued patients converge at 3 years after discontinuation, though higher in discontinued patients before that point. It appears that relapse rates are raised in the discontinuation group for 1–3 years following a switch to placebo, before converging with relapse rates of medicated patients thereafter.,
This period of increased relapse following antipsychotic cessation may be due in part to the effect of withdrawal-associated relapse. This temporary increase in relapse rate has been referred to as a “rebound” effect and is consistent with the concept of neuroadaptations that take months or years to resolve following discontinuation; these may increase the susceptibility to relapse during this period by exaggerating the increased sensitivity to life events and other triggers, hypothesized as the pathogenic mechanism for psychotic illness., Persisting neuroadaptation may lower the threshold for psychosis but only give rise to relapse in the context of relevant triggers, explaining why not all relapses occur straight after drug discontinuation but may be increased for the period of time for which the neuroadaptations persist. This time period of 1–3 years is consistent with the period of time for which TD can persist, as outlined below.
This pattern of early relapse, consistent with withdrawal-related effects from discontinuation, is not restricted to antipsychotics but also evident for antidepressants in anxiety, as well as lithium and other mood stabilizers in bipolar affective disorder (BPAD),, also persisting for months.,, On the cessation of lithium in BPAD, in patients with a mean cycle length (average period of euthymia between episodes) before treatment of 11.6 months, the time to a new episode following the abrupt cessation of lithium therapy was just 1.7 months.
Pharmacological Characteristics of Antipsychotic Withdrawal
There is evidence that patients with longer exposure to antipsychotics are more likely to have psychosis on withdrawal. One study, using a nationwide cohort, found a striking relationship between the length of antipsychotic treatment and the risk of relapse on discontinuation. While patients who discontinued antipsychotic treatment soon after their first discharge had a small increased risk of relapse, this risk increased with the length of time patients had taken antipsychotics: the risk doubled after 1–2 years of exposure, tripled after 2–5 years of exposure, and increased 7 times after 8 years of antipsychotic exposure. It has been suggested that this finding might be explained by the patients with more severe conditions being treated for longer—however, there is no clear consensus on what constitutes a reliable measure of “severity,” and, certainly, symptom scores do not predict the risk of relapse. However, another paper found that longer treatment was associated with a smaller risk of relapse, after controlling for a wide variety of demographic and clinical variables, although the relapse rates of 2%–5% per year in these patients were unusually low, making extrapolation to other populations difficult. Furthermore, patients who demonstrate greater evidence of tolerance to antipsychotics (increasing dose requirements, the development of treatment resistance, or TD) are more likely to have withdrawal psychosis.
Tapering Antipsychotics
Standard guidelines do not mention antipsychotic deprescribing, or tapering, although some current guidelines encourage reduction to minimum effective doses without specifying how to do so., The principal means to mitigate withdrawal symptoms is to reduce the rate at which the equilibrium is disturbed, so allowing time for the reversal of underlying neuroadaptations to return to baseline.,, Gradual tapering of antipsychotics, when cessation is the goal, is sometimes advised on this principle.,, Tapering may reduce the likelihood and intensity of withdrawal symptoms, including, potentially, the risk of withdrawal psychosis.,
The persistence of TD, the most visible manifestation of dopaminergic hypersensitivity, for a considerable time following cessation of antipsychotics, provides evidence that neuro-adaptations to antipsychotics persist for many years and supports the need for long tapering. An early review of studies found that it took 2–5 years for 60%–90% of symptoms of TD to resolve following antipsychotic cessation (supplementary table S1). Another study found that 92.8% of patients achieved a 50% reduction in TD symptoms 46 weeks after discontinuing on average 10 years of antipsychotic treatment. A more recent study examined patients with 1 year of TD in whom dopamine antagonists (including metoclopramide) were ceased after 5 years of exposure: in 13% of the patients, symptoms resolved completely (taking 2–4 years, average 2.3), and the number defined as moderate or severe decreased from 63% to less than 20%. This suggests that TD (along with, presumably, dopaminergic hypersensitivity) can resolve when antipsychotics are discontinued but this process might, in some cases, take years, and may be irreversible in some patients.
Tapering periods probably need to be similarly prolonged to minimize the risk of psychotic relapse in people who have been on these medications long term. This is consistent with the finding that relapse rates for discontinued patients tend to match those of maintained patients but only after 1–3 years.,, Prolonged tapering is not unreasonable given the duration of antipsychotic treatment in many patients, the long persistence of adaptations to antipsychotics in animals to antipsychotics,, and the evidence that withdrawal from other medications like selective serotonin reuptake inhibitors (SSRIs) can be prolonged, lasting for months or years, with months-long tapering most effective in minimizing withdrawal symptoms in some people.
Evidence for Prolonged Tapering
There is evidence to support the notion that longer tapering periods may lead to lower relapse rates compared with more rapid tapering periods.,,,,, A meta-analysis of studies examining relapse comparing abrupt discontinuation with “gradual” discontinuation (average period of 4 weeks) found no significant benefits for this “gradual” discontinuation over abrupt cessation. However, 4 weeks is a brief period and a more recent meta-analysis finds an inverse dose response between the duration of discontinuation and the rate of relapse over the next year: abrupt stopping led to relapse in 77% of patients; stopping over 1–2 weeks to relapse in 57%; 3–10 weeks to 47%; and stopping over longer than 10 weeks led to a relapse rate of 31%.
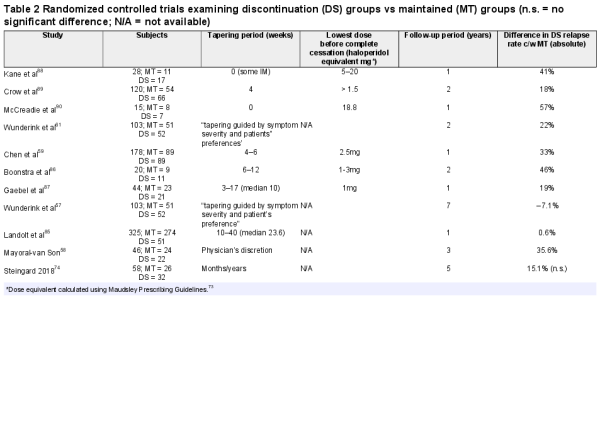
Recent systematic reviews, have included 10 tapering studies (n = 1040) with maintenance arms in people with first-episode psychosis (FEP), with one further study looking at a group with longer-term illness (table 2). The discontinuation group showed more relapses than the maintenance group, in all but 3 studies examined.,, These 3 studies were distinguished by either the longer length of their tapering process or the longer length of their follow-up period, or both (however, this last study was not randomized, meaning selection bias is possible).
One of these studies was in people with FEP with a tapering period of 10–40 weeks and found no significant difference in relapse rates between withdrawn patients and patients who were maintained on antipsychotics. Tapering over months and years (by 25%–30% reductions of original dose every 3 months) also demonstrated no significant difference in relapse rates between patients who continued or discontinued antipsychotics.
The final dose of medication before complete cessation may also be a predictor of relapse because it might represent a large “step down” in dopaminergic (or other target) blockade (table 2). However, this is a difficult value to establish, with most authors reporting that they used either the smallest available tablet or half that value, or that this value was not recorded.,
Dose-Response Studies of Antipsychotics
The relationship between antipsychotic dose and effect at receptor targets may also be informative for tapering. Positron emission tomography (PET) studies demonstrate hyperbolic relationships between doses (or plasma level) of antipsychotic and D2 receptor occupancy (figure 2)., This relationship is typical of bimolecular interactions (one receptor and one ligand) that follow the law of mass action and is widely applicable to many medications and their receptor targets. The nature of this relationship is often obscured by the habit of plotting dose-response curves on semi-logarithmic axes, giving the appearance of a linear relationship at intermediate doses. Systems obeying these dynamics are described by Emax equations of the form y = maximal occupancy × x/(x + ED50) (y = percentage receptor occupancy, x = dose, ED50 = dose required to achieve 50% of maximum occupancy of receptor target), which are used as lines of best fit in the graphs displayed and allow the calculation of receptor occupancy for given dosage.
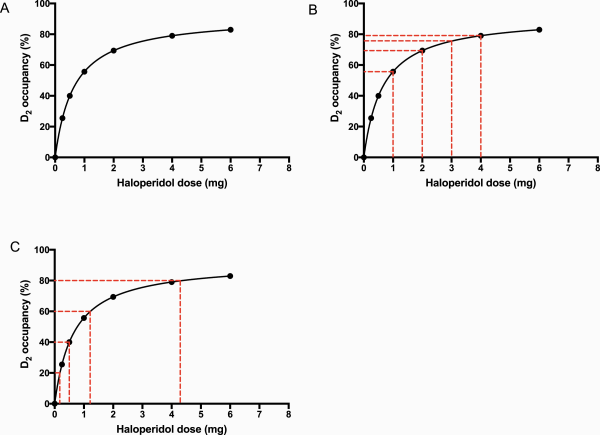
Fig. 2
The effect of linear or hyperbolic reductions of dose of antipsychotic on D2 receptor occupancy. (A) Relationship between haloperidol dose and D2 dopaminergic receptor occupancy (%) on PET, adapted from the equation for the line of best fit in Lako et al. (B) Linear dose reductions of haloperidol produce hyperbolically increasing changes in D2 occupancy, with the largest decrease of 55.7 percentage points of D2 occupancy occurring when the dose is reduced from 1 mg to 0. (C) Hyperbolically decreasing doses of haloperidol correspond to linear reductions in D2 dopaminergic occupancy (in this case, intervals of 20 percentage points of D2 occupancy). The doses in this case correspond to 4.4 mg (80% D2 occupancy), 1.2 mg (60% D2 occupancy), 0.50 mg (40% D2 occupancy), and 0.18 mg (20% D2 occupancy).
Taking the example of haloperidol (figure 2 and table 3a), it is notable that linear dose reductions from therapeutic doses of 4 mg produce increasingly large reductions in percentage points of D2 dopamine antagonism: 3.5 percentage points (4 to 3 mg), 6.1 percentage points (3 to 2 mg), 13.7 percentage points (2 to 1 mg), and 55.7 percentage points (1 to 0 mg; figure 2b and table 3a). It is, therefore, likely that discontinuation studies employing linear dose reductions,,,,, as recommended in some older guidelines, will be more likely to induce withdrawal reactions (including, potentially, withdrawal psychosis) because reductions in D2 antagonism become increasingly large, causing greater likelihood of dopaminergic rebound.
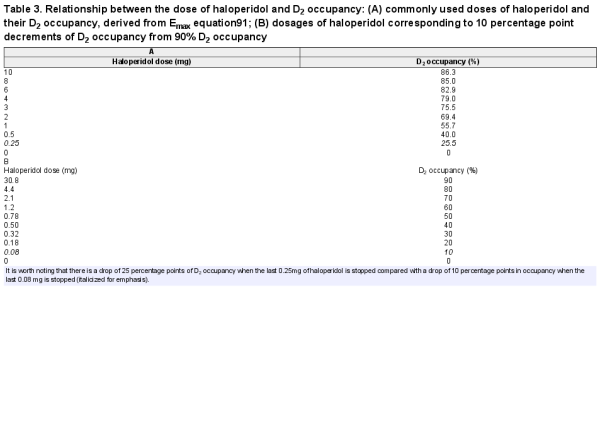
Table 3. Relationship between the dose of haloperidol and D2 occupancy: (A) commonly used doses of haloperidol and their D2 occupancy, derived from Emax equation; (B) dosages of haloperidol corresponding to 10 percentage point decrements of D2 occupancy from 90% D2 occupancy
Indeed, even reductions from 0.5 mg of haloperidol (the smallest available tablet) to 0 mg will produce a reduction in D2 antagonism of 40.0 percentage points, and reduction from 0.25 mg (half the smallest tablet) to 0 mg will produce a 25.5 percentage point reduction (larger than the change from 20 to 2 mg [19.6 percentage points]); this may account for the relative ease of reductions at higher doses of antipsychotic and the difficulties in tapering at lower doses.,, In one recent study, there was no significant increase in relapse rate when patients reduced their dose by 40% compared with the significant chance of early relapse when patients discontinue their entire dose. When interpreting PET data, it should be noted that there is a degree of individual variability in D2 receptor binding and response across studies.
In this context, it is notable that a handful of studies have suggested that low doses of medication are effective in maintaining patients with psychotic disorders. For example, very low doses of depot antipsychotic (2.5–10 mg of fluphenazine decanoate every 2 weeks) were as effective in preventing a relapse as the standard recommended doses, 5–10 times that amount,; in another study, 1 mg of haloperidol or less was effective in symptom reduction in FEP in the majority of patients.
Application to Tapering Antipsychotics
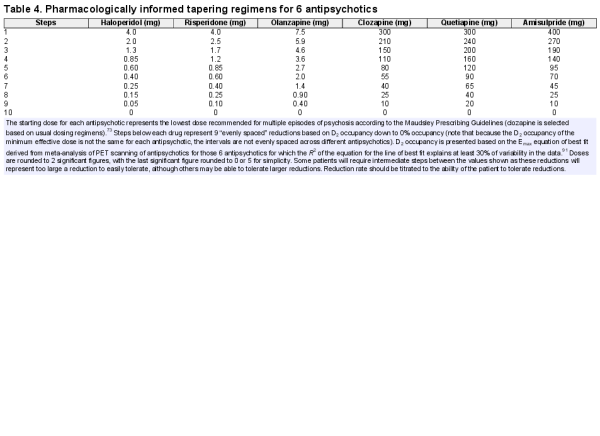
Given that reduced antagonism of D2 dopaminergic receptors has been implicated in many of the withdrawal phenomena attributed to antipsychotics, including psychotic symptoms,,, we suggest that tapering regimes should aim to reduce D2 receptor antagonism in a linear fashion with adequate time provided in between dose reductions to allow adaptation to lower doses of the drug, as this may produce more “evenly spread” perturbations to the system, which may minimize withdrawal-associated effects (figure 3)., Linear decrements of, eg, 10 percentage point D2 receptor occupancy from 90% for haloperidol require hyperbolic dose reductions (table 3b; decrements of 20 percentage points in figure 2c), with further medications shown in table 4 and supplementary tables S3–S11.
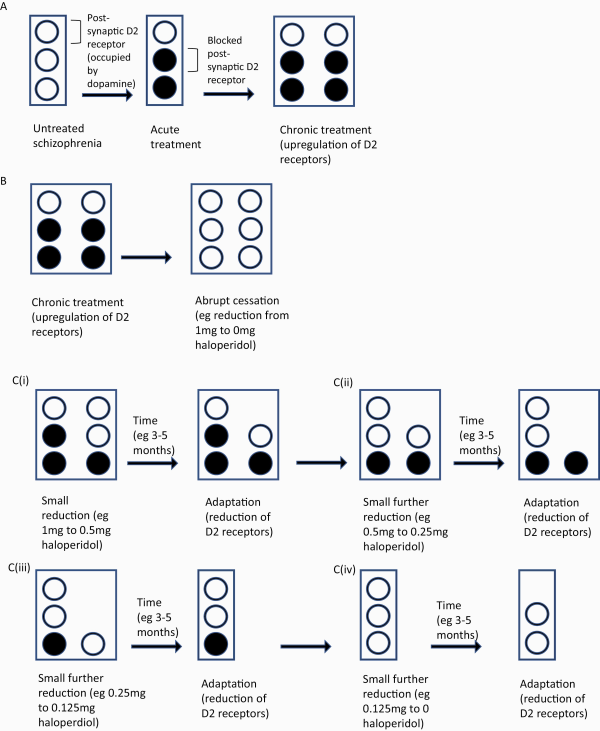
Fig. 3
Theoretical representation of abrupt vs gradual reduction of antipsychotic dose. (A) Untreated schizophrenia is represented by postsynaptic D2 receptors (open circles). Antipsychotics cause blockade of D2 receptors (filled in circles). Chronic treatment is hypothesized to cause the upregulation of D2 receptors. (B) Abrupt cessation of antipsychotic causes a large increase in dopaminergic transmission in sensitized D2 receptors, possibly associated with an increased risk of relapse. (C) (i) Gradual reduction in antipsychotic dose leads to incrementally increased dopaminergic transmission, possibly associated with a smaller increase in the risk of relapse or temporary increase in symptoms. Over time (likely months or years) D2 upregulation starts to resolve. (ii)–(iv) Gradual antipsychotic dose reduction is repeated. Please note the time frames indicated here are theoretical, based on few small studies.,
In the absence of direct data in humans of the time taken for dopamine D2 receptor adaptations to resolve, we can be guided by observations that TD improves over 2–3 years, and this is the same time period over which relapse rates of patients discontinued from medication takes to converge with relapse rates of patients maintained on medication, suggesting that this may be a reasonable time period over which to taper patients who have received long-term antipsychotics.
There is some support for this time period for tapering in small studies examining tolerable rates of discontinuation. One study has investigated reducing antipsychotics in patients with remitted psychosis by 25% of the most recent dose every 6 months (a logarithmic pattern that closely approximates hyperbolic dose reductions), with 3 quarters of patients able to achieve between 25% and 71.4% dose reduction without relapse in the first year of the study. Another pilot study found that patients with chronic schizophrenia who, on average, achieved a 42% reduction in antipsychotic dose over 6 months demonstrated no difference in relapse rates from patients maintained on antipsychotics. This suggests that many patients may tolerate dose reductions of 25%–50% of the most recent dose (corresponding approximately to 5–10 percentage point decrements of D2 occupancy) every 3–6 months. Smaller reductions (such as 10% of the most recent dose) made every month may be more tolerable in the aim of producing more “evenly spread” perturbation to the equilibrium.
There is likely to be considerable interindividual variability in this process, with patients able to tolerate greatly varying rates of reduction: given the data on time for TD to resolve, some long-term treated patients may need longer periods, and some first-episode patients or those treated for briefer periods may tolerate shorter tapering periods.,,, However, we suggest that, even in quicker tapering protocols, tapering should follow a hyperbolic course to “evenly spread” change at receptors. If the reason for discontinuation is pressing, such as a severe adverse reaction, such schedules may need to be much quicker, acknowledging the increased risk of withdrawal symptoms. In patients with poor compliance, such prolonged tapers will be challenging, although a reduced adverse effect burden and the possibility of discontinuation may enhance engagement with the process.
The tapering process should be conceptualized as finding a new minimum effective dose., The process could be individualized by observing patients for 3–6 months following an initial reduction of 5–10 percentage points of D2 receptor blockade, and gauging response, to determine a tolerable rate of decrease thereafter. As outlined recently, it is possible that there would be an increase in symptoms following a dose reduction, but it might be expected that these symptoms would resolve over time, as underlying adaptations resolve (though this can take weeks or even months); increased psychosocial support may be necessary to manage this period if risks are manageable.,
An example tapering regime for haloperidol, taking into account ease of administration and D2 occupancy, might be a reduction in dose from 4 to 3 to 2 to 1.5 to 1 to 0.75 to 0.5 to 0.375 to 0.25 to 0.125 to 0.06 to 0 mg, with tablet splitting and liquid formulations required for the lower doses (figures 2c and tables 3 and 4). The size of reductions may be decreased or the time between reductions increased if patients experience a significant increase in symptoms. Holding the dose for a prolonged period or increasing to a previously tolerated dose may be advisable if the patient experiences significant symptoms; some may be able to tolerate slower or more gradual reductions subsequently. Similar regimes for other antipsychotics are shown in table 4 (with further detail in the supplementary material). One of the major barriers to the pharmacologically informed tapering proposed is achieving doses intermediate between commonly available tablet formulations and the very small doses suggested at the end of the taper: tablet cutters can be helpful, some antipsychotics are available in liquid formulations, and “tapering strips” developed in Holland are another option, providing small formulations of tablets that can be combined to facilitate a wide variety of doses for incremental tapers.
Depot medications provide a useful option in tapering as their extended half-lives represent a form of “in-built” tapering (supplementary table S3). For example, 3-monthly paliperidone depot takes 52 weeks to reach a steady state and may be tapered over 3 years by yearly dose reductions equivalent to approximately 30% D2 occupancy (equivalent to reducing by 10 percentage points of D2 occupancy every 4 months), with the time taken to reach a steady state providing time for neural adaptation. Very small doses of the depot would be required for final dosing (eg, 90 mg, equivalent to 30% D2 occupancy). For drugs with short half-lives or “fast off” characteristics, like clozapine or quetiapine (supplementary table S12), with reputations for quicker onset of psychosis following withdrawal, more caution may be required and it may be necessary to reduce doses by 2.5–5 percentage points of D2 (or cholinergic or histaminergic) occupancy every 6–12 weeks, depending on individual responses (see supplementary material). There is recent evidence that drugs like aripiprazole that are partial agonists at the D2 receptor are less likely to induce dopaminergic hypersensitivity (as evidenced by very low rates of TD) and, therefore, may be less likely to cause a relapse on discontinuation as supported by animal data, but this has not yet been examined in clinical studies.
Limitations
There are potential limitations to the interpretation of the dose-response curves from PET studies. First, individual variation may not have been captured by the relatively small sample sizes. However, the shape of the dose-activity curve (ie, hyperbolic) should be the same for each individual, suggesting that hyperbolic dose reduction regimes should be universally applicable. It should also be noted that there is some heterogeneity in the meta-analysis of PET studies indicating the likelihood of interindividual differences in receptor occupancy for a given dose, emphasizing the importance of individualized tapering guided by the patient’s response to reductions.
Second, it is difficult to determine whether D2 occupancy will linearly correspond to withdrawal effects. There is a body of evidence that suggests that a minimum threshold of D2 antagonism is required before a clinical effect or adverse effects are seen; this may also apply to withdrawal effects. However, a recent meta-analysis has found a continuous hyperbolic relationship between antipsychotic dose and clinical response, mirroring the relationship between dose and D2 occupancy. One meta-analysis found evidence of a positive linear relationship between clinical improvement and D2 receptor occupancy (when patients taking quetiapine and clozapine, thought to act through mechanisms other than D2 antagonism, and outliers with very high dopaminergic blockade were excluded). Evidence of a linear relationship between D2 antagonism and therapeutic effects may extend to withdrawal effects, but this requires further investigation.
Third, although the principal therapeutic effects of antipsychotics are attributed to D2 antagonism, other receptor targets, including 5HT2 and 5HT1A, have also been thought relevant, and these receptors, and others, such as cholinergic receptors, may also determine withdrawal effects. Therefore, tapering according to the binding affinity of receptor subtypes other than D2 receptors may be indicated with some antipsychotics. This limitation may apply particularly to clozapine because it acts partly through non-dopaminergic? pathways, and it demonstrates the lowest correlation between plasma levels and D2 occupancy of commonly used antipsychotics., However, dose reduction schedules using alternative receptor dose-response curves follow similar hyperbolically reducing dosing schedules because these relationships also follow the law of mass action.
Future Directions
This paper offers some pharmacological principles that may aid in withdrawing from antipsychotics. We anticipate that this regime might reduce relapse during and after discontinuation. At a minimum, it should be recognized that tapering periods of weeks down to minimum or half-minimum therapeutic doses of medication are likely to be inadequate to avoid withdrawal symptoms, including early relapse. In those who have received antipsychotics over prolonged periods, tapering regimes over months and years down to small portions of drug doses are more likely to be effective. The hypothesis put forward in this paper should be tested in further tapering trials of antipsychotics, including slower tapering down to lower final doses before complete cessation, including the use of depot preparations. Establishment of formal guidelines for tapering antipsychotics is required.
Acknowledgments
M.A.H. conceived the manuscript idea, wrote the manuscript, and drew the figures. D.T. helped develop the idea and revised and edited the manuscript. R.M., S.N., and S.J. helped to substantially revise and edit the manuscript.
References
- 1. Morant N, Azam K, Johnson S, Moncrieff J. The least worst option: user experiences of antipsychotic medication and lack of involvement in medication decisions in a UK community sample. J Ment Health.2018;27(4):322–328.
- 2. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry.2013;12(3):216–226.
- 3. Viguera AC, Baldessarini RJ, Hegarty JD, van Kammen DP, Tohen M. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry.1997;54(1):49–55.
- 4. Bogers JPAM, Hambarian G, Michiels M, Vermeulen J, de Haan L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia: a systematic review and meta-analysis. Schizophr Bull Open2020; 1(1). doi:10.1093/schizbullopen/sgaa002.
- 5. Leucht S, Cipriani A, Spineli L, et al Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet.2013;382(9896):951–962.
- 6. Murray RM, Quattrone D, Natesan S, et al Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Br J Psychiatry. 2016;209(5):361–365.
- 7. Moncrieff J. Antipsychotic maintenance treatment: time to rethink? PLoS Med.2015;12(8):e1001861.
- 8. Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? a meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry.2015;78(6):403–412.
- 9. Voineskos AN, Mulsant BH, Dickie EW, et al Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry2020; doi:10.1001/jamapsychiatry.2020.0036.
- 10. Murray RM, Di Forti M. Increasing expectations and knowledge require a more subtle use of prophylactic antipsychotics. World Psychiatry.2018;17(2):161–162.
- 11. McGorry P, Alvarez-Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first-episode psychosis: less is more. JAMA Psychiatry.2013;70(9):898–900.
- 12. McGorry P, Alvarez-Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first-episode psychosis: less is more. JAMA Psychiatry.2013;70(9):898–900.
- 13. Gunnmo P, Bergman HF. What do individuals with schizophrenia need to increase their well-being. Int J Qual Stud Health Well-being2011; 6: 1–11.
- 14. Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc.2008;56(10):1946–1952.
- 15. Reidenberg MM. Drug discontinuation effects are part of the pharmacology of a drug. J Pharmacol Exp Ther.2011;339(2):324–328.
- 16. Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry.2005;18(3):249–255.
- 17. Howland RH. Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv.2010;48(9):11–14.
- 18. Franks M, Macritchie KA, Mahmood T, Young AH. Bouncing back: is the bipolar rebound phenomenon peculiar to lithium? A retrospective naturalistic study. J Psychopharmacol.2008;22(4):452–456.
- 19. Howland RH. Potential adverse effects of discontinuing psychotropic drugs: part 2: antidepressant drugs. J Psychosoc Nurs Ment Health Serv.2010; 48: 9–12.
- 20. Cerovecki A, Musil R, Klimke A, et al Withdrawal symptoms and rebound syndromes associated with switching and discontinuing atypical antipsychotics: theoretical background and practical recommendations. CNS Drugs.2013;27(7):545–572.
- 21. Littleton J. Receptor regulation as a unitary mechanism for drug tolerance and physical dependence—not quite as simple as it seemed!Addiction.2001;96(1):87–101.
- 22. Turton S, Lingford-Hughes A. Neurobiology and principles of addiction and tolerance. Medicine (Baltim).2016; 7–10.
- 23. Chouinard G, Samaha AN, Chouinard VA, et al Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom.2017;86(4):189–219.
- 24. Houston MC, Hodge R. Beta-adrenergic blocker withdrawal syndromes in hypertension and other cardiovascular diseases. Am Heart J.1988;116(2 Pt 1):515–523.
- 25. Amore M, Zazzeri N. Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry.1995;19(8):1323–1334.
- 26. Tranter R, Healy D. Neuroleptic discontinuation syndromes. J Psychopharmacol.1998;12(4):401–406.
- 27. Shiovitz TM, Welke TL, Tigel PD, et al Cholinergic rebound and rapid onset psychosis following abrupt clozapine withdrawal. Schizophr Bull.1996;22(4):591–595.
- 28. Dixon L, Thaker G, Conley R, Ross D, Cascella N, Tamminga C. Changes in psychopathology and dyskinesia after neuroleptic withdrawal in a double-blind design. Schizophr Res.1993;10(3):267–271.
- 29. Schultz SK, Miller DD, Arndt S, Ziebell S, Gupta S, Andreasen NC. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry.1995;38(11):713–719.
- 30. Kane JM, Woerner M, Lieberman J. Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol.1988;8(4 Suppl):52S–56S.
- 31. Perényi A, Frecska E, Bagdy G, Révai K. Changes in mental condition, hyperkinesias and biochemical parameters after withdrawal of chronic neuroleptic treatment. Acta Psychiatr Scand.1985;72(5):430–435.
- 32. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol.2007;27(6):705–707.
- 33. Kurien R, Vattakatuchery JJ. Psychotropic discontinuation leading to an NMS-like condition who developed NMS-like symptoms following abrupt discontinuation of clozapine. Prog Neurol Psychiatry2013;17(5):8–9.
- 34. Chouinard G, Chouinard VA. Atypical antipsychotics: CATIE study, drug-induced movement disorder and resulting iatrogenic psychiatric-like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother Psychosom.2008;77(2):69–77.
- 35. Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci.2007;27(11):2979–2986.
- 36. Joyce J. D2 but not D3 receptors are elevated after 9 or 11 months chronic haloperidol treatment: influence of withdrawal period. Synapse2001;40:137–144.
- 37. Quinn R. Comparing rat’s to human’s age: how old is my rat in people years?Nutrition.2005;21(6):775–777.
- 38. Howes OD, Kambeitz J, Kim E, et al The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry.2012;69(8):776–786.
- 39. Silvestri S, Seeman MV, Negrete JC, et al Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl).2000;152(2):174–180.
- 40. Konig P, Benzer MK, Fritzsche H. SPECT - Atechnique for visualization of cerebral dopamine D2 receptors. Biol Psychiatry.1991;148:1607–1608.
- 41. Mizrahi R, Agid O, Borlido C, et al Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res.2011;131(1–3):63–68.
- 42. Moncrieff J. Does antipsychotic withdrawal provoke psychosis? review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse. Acta Psychiatr Scand.2006;114(1):3–13.
- 43. McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci.2019;42(3):205–220.
- 44. Roy-Desruisseaux J, Landry J, Bocti C, Tessier D, Hottin P, Trudel JF. Domperidone-induced tardive dyskinesia and withdrawal psychosis in an elderly woman with dementia. Ann Pharmacother.2011;45(9):e51.
- 45. Jacob MK, Ash P, Craighead WE. Adolescent female with withdrawal psychosis following abrupt termination of ziprasidone. Eur Child Adolesc Psychiatry.2012;21(3):165–168.
- 46.
- 47. Lu ML, Pan JJ, Teng HW, Su KP, Shen WW. Metoclopramide-induced supersensitivity psychosis. Ann Pharmacother.2002;36(9):1387–1390.
- 48. Borison RL, Diamond BI, Sinha D, Gupta RP, Ajiboye PA. Clozapine withdrawal rebound psychosis. Psychopharmacol Bull.1988;24(2):260–263.
- 49. Meltzer HY, Lee MA, Ranjan R, Mason EA, Cola PA. Relapse following clozapine withdrawal: effect of neuroleptic drugs and cyproheptadine. Psychopharmacology (Berl).1996;124(1–2):176–187.
- 50. Kent TA, Wilber RD. Reserpine withdrawal psychosis: the possible role of denervation supersensitivity of receptors. J Nerv Ment Dis.1982;170(8):502–504.
- 51. Witschy JK, Malone GL, Holden LD. Psychosis after neuroleptic withdrawal in a manic-depressive patient. Am J Psychiatry.1984;141(1):105–106.
- 52. Steiner W, Laporta M, Chouinard G. Neuroleptic-induced supersensitivity psychosis in patients with bipolar affective disorder. Acta Psychiatr Scand.1990;81(5):437–440.
- 53. Bastiampillai T, Fantasia R, Nelson A. Letters. Aust N Z J Psychiatry.2014; 48: 585–586.
- 54. McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull.2018;44(6):1301–1311.
- 55. Jauhar S, Veronese M, Nour MM, et al The effects of antipsychotic treatment on presynaptic dopamine synthesis capacity in first-episode psychosis: a positron emission tomography study. Biol Psychiatry.2019;85(1):79–87.
- 56. Leucht S, Tardy M, Komossa K, et al Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet.2012;379(9831):2063–2071.
- 57. Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry.2013;70(9):913–920.
- 58. Mayoral-van Son J, de la Foz VO, Martinez-Garcia O, et al Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: a 3-year naturalistic follow-up study. J Clin Psychiatry.. 2016;77(4):492–500.
- 59. Chen EY, Hui CL, Lam MM, et al Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ.2010;341:c4024.
- 60. Johnstone EC, Geddes J. How high is the relapse rate in schizophrenia?Acta Psychiatr Scand Suppl.1994;382:6–10.
- 61. Wunderink L, Nienhuis FJ, Sytema S, Slooff CJ, Knegtering R, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry.2007;68(5):654–661.
- 62. Emsley R, Fleischhacker WW. Relapse after antipsychotic discontinuation in schizophrenia as a withdrawal phenomenon vs illness recurrence: a post hoc analysis of a randomized placebo-controlled study. J Clin Psychiatry.2018; 79: 52–59.
- 63. Weiden PJ, Kim E, Bermak J, Turkoz I, Gopal S, Berwaerts J. Does half-life matter after antipsychotic discontinuation? A relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry.2017;78(7):e813–e820.
- 64. Thompson A, Winsper C, Marwaha S, et al Maintenance antipsychotic treatment versus discontinuation strategies following remission from first episode psychosis: systematic review. BJPsych Open.2018;4(4):215–225.
- 65. Bockting CLH, Klein NS, Elgersma HJ, et al Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised controlled trial. Lancet Psychiatry.2018;5(5):401–410.
- 66. Suppes T, Baldessarini RJ, Faedda GL, Tohen M. Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry.1991;48(12):1082–1088.
- 67. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry.2018;175(8):765–773.
- 68. Robinson D, Woerner MG, Alvir JM, et al Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry.1999;56(3):241–247.
- 69. Hayes JF, Osborn DP, Lundin A, Dalman C. Psychiatric hospitalization following antipsychotic medication cessation in first episode psychosis. J Psychopharmacol.2019;33(4):532–534.
- 70. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Adv2018; 24: 295–302.
- 71. Takeuchi H, Suzuki T, Uchida H, Watanabe K, Mimura M. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res.2012;134(2–3):219–225.
- 72.
- 73. Taylor D, Barnes T, Young A. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Hoboken, New Jersey: Wiley Blackwell; 2018.
- 74. Steingard S. Five year outcomes of tapering antipsychotic drug doses in a community mental health center. Comm Ment Health J. 2018;54(8):1097–1100. doi:10.1007/s10597-018-0313-1.
- 75. Caroff SN, Ungvari GS, Cunningham Owens DG. Historical perspectives on tardive dyskinesia. J Neurol Sci.2018;389:4–9.
- 76. Marsden CD. Is tardive dyskinesia a unique disorder? In: Casey DE, Chase TN, Christensen AV, Gerlach J, eds. Dyskinesia: Research and Treatment. Berlin Heidelberg, Germany: Springer-Verlag; 1985:64–71.
- 77. Glazer WM, Morgenstern H, Schooler N, Berkman CS, Moore DC. Predictors of improvement in tardive dyskinesia following discontinuation of neuroleptic medication. Br J Psychiatry.1990;157:585–592.
- 78. Zutshi D, Cloud LJ, Factor SA. Tardive syndromes are rarely reversible after discontinuing dopamine receptor blocking agents: experience from a university-based movement disorder clinic. Tremor Other Hyperkinet Mov (N Y).2014;4:266.
- 79. Muller P, Seeman P. Dopaminergic supersensitivity after neuroleptics: time-course and specificity. Psychopharmacology (Berl).1978;60(1):1–11.
- 80. Stockmann T, Odegbaro D, Timimi S, Moncrieff J. ‘SSRI and SNRI withdrawal symptoms reported on an internet forum’, Int J Risk Safety Med. 2018;29(3–4):175–180. doi:10.3233/JRS-180018.
- 81. Guy A, Brown M, Lewis S, Horowitz M. The “patient voice”: patients who experience antidepressant withdrawal symptoms are often dismissed, or misdiagnosed with relapse, or a new medical condition. Ther Adv Psychopharmacol.2020;10:2045125320967183.
- 82. Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry.2019;6(6):538–546. https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(19)30219-6/fulltext
- 83. Alvarez-Jimenez M, O’Donoghue B, Thompson A, et al Beyond clinical remission in first episode psychosis: thoughts on antipsychotic maintenance vs. guided discontinuation in the functional recovery era. CNS Drugs.2016;30(5):357–368.
- 84. Bowtell M, Ratheesh A, McGorry P, Killackey E, O’Donoghue B. Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. a systematic review. Schizophr Res.2018;197:9–18.
- 85. Landolt K, Rössler W, Ajdacic-gross V, et al Predictors of discontinuation of antipsychotic medication and subsequent outcomes in the European First Episode Schizophrenia Trial (EUFEST). Schizophr Res.2016; 1–7.
- 86. Boonstra G, Burger H, Grobbee DE, Kahn RS. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. Int J Psychiatry Clin Pract.2011;15(2):128–134.
- 87. Gaebel W, Riesbeck M, Wölwer W, et al; German Study Group on First-Episode Schizophrenia. Relapse prevention in first-episode schizophrenia—maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry.2011;72(2):205–218.
- 88. Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos-Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry.1982;39(1):70–73.
- 89. Crow TJ, MacMillan JF, Johnson AL, Johnstone EC. A randomised controlled trial of prophylactic neuroleptic treatment. Br J Psychiatry.1986;148:120–127.
- 90. McCreadie RG, Wiles D, Grant S, et al The Scottish first episode schizophrenia study. VII. Two-year follow-up. Acta Psychiatr. Scand. 1989; 80: 597–602.
- 91. Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol.2013;33(5):675–681.
- 92. Kapur S, Zipursky RB, Remington G, et al 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry.1998;155(7):921–928.
- 93. Holford N. Pharmacodynamic principles and the time course of delayed and cumulative drug effects. Transl Clin Pharmacol.2018;26(2):56–59.
- 94. Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull.1998;24(1):1–10.
- 95. Huhn M, Leucht C, Rothe P, et al Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorder: a randomized controlled pilot trial. Eur Arch Psychiatry Clin Neurosci.2020. doi:10.1007/s00406-020-01109-y.
- 96. Jauhar S, Howes OD. Understanding and predicting variability in response to treatment in psychotic disorders: in vivo findings. Clin Pharmacol Ther.2019;105(5):1079–1081.
- 97. Lehman AF, Lieberman JA, Dixon LB, et al Practice guideline for the treatment of partients with schizophrenia. Am J Psychiatry. 2004;161(2 SUPPL):1–56.
- 98. Oosthuizen P, Emsley RA, Turner J, Keyter N. Determining the optimal dose of haloperidol in first-episode psychosis. J Psychopharmacol.2001;15(4):251–255.
- 99. Horowitz MA, Murray RM, Taylor D. Tapering antipsychotic treatment. JAMA Psychiatry. 2021;78(2):125–126. doi:10.1001/jamapsychiatry.2020.2166.
- 100. Liu CC, Takeuchi H. Achieving the lowest effective antipsychotic dose for patients with remitted psychosis: a proposed guided dose-reduction algorithm. CNS Drugs.2020;34(2):117–126.
- 101. Quitkin F, Rifkin A, Gochfeld L, Klein DF. Tardive dyskinesia: are first signs reversible?Am J Psychiatry.1977;134(1):84–87.
- 102. Cooper RE, Hanratty É, Morant N, Moncrieff J. Mental health professionals’ views and experiences of antipsychotic reduction and discontinuation. PLoS One.2019;14(6):e0218711.
- 103. Moncrieff J, Gupta S, Horowitz MA. Barriers to stopping neuroleptic (antipsychotic) treatment in people with schizophrenia, psychosis or bipolar disorder. Ther Adv Psychopharmacol.2020;10:2045125320937910.
- 104. Groot PC, van Os J. Outcome of antidepressant drug discontinuation with taperingstrips after 1-5 years. Ther Adv Psychopharmacol.2020;10:2045125320954609.
- 105. Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry.2001;158:360–369.
- 106. Carbon M, Kane JM, Leucht S, Correll CU. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330–340.
- 107. Tadokoro S, Okamura N, Sekine Y, Kanahara N, Hashimoto K, Iyo M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull.2012;38(5):1012–1020.
- 108. Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry.2000;157(4):514–520.
- 109. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry.2020;177(4):342–353.
- 110. Yilmaz Z, Zai CC, Hwang R, et al Antipsychotics, dopamine D2 receptor occupancy and clinical improvement in schizophrenia: a meta-analysis. Schizophr Res.2012;140(1-3):214–220.
- 111. Mauri MC, Paletta S, Maffini M, et al Clinical pharmacology of atypical antipsychotics: an update. EXCLI J.2014;13:1163–1191.
- 112. Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Predicting dopamine D2 receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol.2011;31(3):318–325.