Introduction
As aerobic organisms, humans require oxygen and the brain is one of the most oxygen-dependent organs []. Low environmental oxygen availability (hypoxia), as occurs with increasing altitude can therefore be a challenge for the brain. Although this would suggest a vulnerability in people with neuropsychiatric diseases and possibly a risk of developing mental symptoms even in healthy people, these aspects of altitude related health research are surprisingly poorly understood []. In this narrative review, we aim to summarize major responses and adaptations of the brain to hypoxia. These responses may be more pronounced, when people engage in physical activity (e.g., different types of mountain sports), which is commonly the case during or after translocation to high altitude. We put those consequences of altitude exposure in the context of cerebral forms of high-altitude illnesses, as well as of changes in other parameters of mental health, in particular such related to selected psychiatric diseases. On this basis, we attempt to identify the most pressing research questions to improve the safety of high and extreme altitude exposures for individuals with mental vulnerabilities, and to suggest new approaches to improve cerebral resilience and mental health with targeted hypoxia interventions. We focus on non-athlete population of healthy and mentally diseased people that are exposed to high altitude and often perform physical activity there. Due to a lack of literature, we only discuss athletes who train or compete at altitude to a very limited extent, but emphasise that this is also an important topic for future research.
Methods
In this narrative review we aim to first provide a broad overview of common brain-related altitude illnesses. We then attempt to better demarcate the poorly defined research field of high-altitude effects on mental health from a highly interdisciplinary perspective. To this end the authors present their perception of the state of the art on these topics from the view of their diverse backgrounds, including sports and mountain medicine, sports psychiatry, sports psychology, sports physiology and neurobiology. Their contributions are based on their own literature collections supplemented with searches for recent advances in Pubmed, Google Scholar, Web of Science, Psycinfo and Scopus. Since for this narrative review no systematic review of the literature has been conducted, potential biases cannot be excluded.
High altitude adaptation of the cerebrovascular system
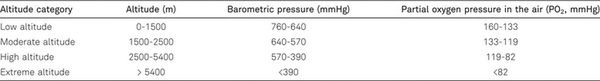
The exposure to high or extreme altitude is often associated with different environmental and behavioral challenges. The defining environmental alteration with increasing altitude is the decreasing barometric pressure and therefore a dropping partial pressure of oxygen in the air (Table 1). Increasing altitudes are also characterized by on average lower temperatures (as compared to the respective climatic region, about -6.5°C per 1000 m altitude), which can have complex effects on hypoxia adaptations []. If the altitude exposure is combined with physical challenges (e.g., during mountaineering), additional important interactions of the physiological responses to hypoxia with the increased oxygen demand especially of working muscles, respiratory and cardiovascular systems have to be expected.
A low partial pressure of oxygen in the air leads to decreased oxygen availability and manifests in lower arterial oxygen partial pressure (PaO2), frequently non-invasively assessed as peripheral oxygen saturation (SpO2), in humans. Consequently, physical and mental capacities transiently decrease, especially in non-acclimatized individuals, in a “hypoxic-dose”-dependent manner (i.e., greater effects are expected at higher altitudes) [, ]. To manage the risks of hypoxia the human body responds on different levels, from molecular to systemic, in order to compensate for lower oxygen availability and/or initiate physiological processes to improve hypoxia resilience or repair hypoxic damage []. The brain, as a greatly oxygen-dependent organ and a principal consumer of the inspired oxygen, is particularly sensitive to hypoxia []. In the following, we discuss hypoxia-induced responses and adaptations specifically in the brain and in other tissues, if relevant for the brain and for mental health.
Decreasing oxygen availability is associated with reductions in PaO2 and the induced hyperventilation reduces the arterial partial pressure of CO2 (PaCO2). The changes in these blood gas levels are sensed by a number of central and peripheral chemosensors, which relay this information and induce specific responses. While the involvement of central chemosensors in the major systemic hypoxia responses to reductions in PaO2 is still debated [, ], peripheral chemosensors, and particularly the carotid bodies in the carotid bifurcation in the neck, appear to be the most important components. Glomus cells in the carotid bodies induce the hypoxic ventilatory response and activate the sympathetic nervous system, hereby contributing to cardiovascular responses []. Specifically, this results in a fast (within seconds to minutes) increase in minute ventilation, heart rate, cardiac output and elevated blood brain flow. Thereby, these responses contribute to increased oxygen supply and notably overlap with the acute mental stress response that facilitates the fight or flight response by improving oxygen availability and, therefore, the energetic status in relevant tissues []. This suggests a link between the hypoxia response and mental stress, the mechanistic basis of which is currently poorly understood. The ventilatory response (hyperventilation) will result in a reduction of CO2 (hypocapnia), to which central chemoreceptors are very sensitive. Central chemoreceptors are considered brain cells that respond to changes in blood pH to regulate breathing in order to keep PaCO2 in a narrow range [].
While sympathetic activation would promote vasoconstriction in normoxia, in hypoxia these effects are counteracted by local dilator mechanisms e.g., the release of nitric oxide (NO) [], resulting in a complex pattern of blood vessel regulation, which in acute hypoxia usually leads to vasodilation in most vascular beds, except for those in the lungs []. Moreover, increasing altitude exposure results in higher cerebral blood flow and brain parenchyma volume, which are compensated by cerebrospinal fluid reduction [, ].
Less rapid systemic effects include the upregulation of erythropoiesis (the production of red blood cells), hemoconcentration and associated plasma volume reductions. Eventually, usually after weeks of high-altitude exposure, elevated levels of total hemoglobin and vascular remodeling and the formation of new blood vessels can be observed, effects that improve the efficiency of oxygen transport (Figure 1).
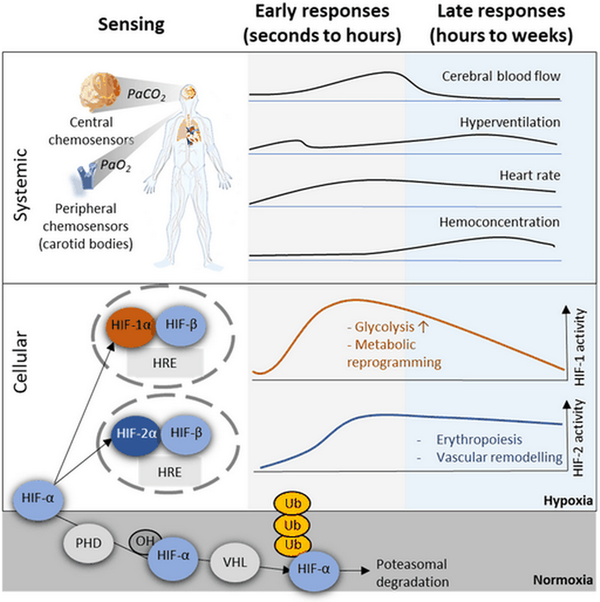
1. From cellular and systemic hypoxia sensing to hypoxia responses and adaptations. On the systemic levels reduced partial pressures of oxygen (PaO2) and associated reduced partial pressures of CO2 (PaO2) are sensed by specialized chemosensors. Of these, the carotid bodies likely are the most important components for the induction of the major systemic hypoxia responses. Among the many cellular mechanisms involved in hypoxia sensing, hypoxia-inducible factors (HIF) are especially prominent. While the α subunits of both HIF-1 and HIF-2 are degraded in normoxia via hydroxylation (OH) through prolyl dehydroxylases (PHD), von Hippel-Lindau protein (VHL) recognition and poly-ubiquitination (Ub), in hypoxia they stabilize and regulate genes via hypoxia response elements (HRE).
Next to the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis (HPA) plays an important role in the human mental stress response. Hypoxia also activates the HPA and thereby affects cortisol-signaling; the induction of the HPA probably contributes to metabolic and mental effects of hypoxia exposure [].
Systemic hypoxia responses depend on molecular mechanisms, such as hypoxia-inducible factor (HIF) pathways [], functional electron-transport in mitochondria [] and the inactivation of oxygen-sensitive potassium channels [], for example in glomus cells. However, cellular hypoxia-responses do not only occur in chemosensitive tissues but virtually in all cells. HIFs are the best-known molecular coordinators of cellular responses to hypoxia but they are complemented by numerous other biochemical, transcriptional and post-transcriptional mechanisms that contribute to cellular oxygen sensing and hypoxia responses [].
HIFs are dimeric transcription factors. In hypoxia, HIF-α and β subunits dimerize and – depending on co-factors such as CREB binding protein (CBP) and p300 – bind hypoxia response elements (HRE), thereby regulating the expression of hundreds of genes. The ubiquitously expressed HIF-1 is particularly important in the regulation of responses to acute hypoxia and its activity increases during the first hours of hypoxia exposure but declines again thereafter [, ]. Conversely, HIF-2 (the expression of which is restricted to fewer cell types) activity remains elevated also in prolonged hypoxia. This temporal regulation of HIF-1 and HIF-2 has been termed the “HIF-switch” []. HIF activity is regulated by many factors, transcriptionally and post-transcriptionally []. The sensitivity of HIFs to oxygen, however, is primarily owed to a mechanism of degradation of the HIF-α subunits, which works similarly for HIF-1 and HIF-2. As long as sufficient oxygen is available, HIF-α gets hydroxylated by prolyl hydroxylases (PHDs) [, ], allowing recognition by the von Hippel-Lindau tumor suppressor protein, which will result in proteasomal degradation of HIF-α. Alternatively, hydroxylation of HIF-α asparagine residues by factor inhibiting HIF (FIH) can downregulate HIF-activity by interfering with the interactions of HIF with its co-activators [] (Figure 1).
HIF-1 and HIF-2 exert distinct, interdependent and sometimes antagonizing effects. This is possible due to differential DNA binding, recruitment of distinct co-activators and specific temporal and regional activity of HIF-1 and HIF-2 []. HIF-1 primarily regulates a switch from aerobic to anaerobic energy metabolism, e.g., by increasing the transcription and translation of glucose transporters and glycolytic enzymes [, ]. It also upregulates pyruvate dehydrogenase kinase 1 (PDK1) [, ] which results in reduced tricarboxylic acid cycle – and therefore respirational – activity in mitochondria, and modulates the activity of mitochondrial respiratory complexes, in particular of complexes I and IV, as recently reviewed []. HIF-2’s distinct functions include vessel remodeling and the control of blood oxygen transport via the regulation of erythropoiesis []. For many hypoxia responses, HIF-1 and HIF-2 cooperate. These include responses related to angiogenesis, lipid metabolism, redox status regulation (e.g., via regulation of mitochondrial superoxide dismutase (SOD2)) and immune functions [, ].
Taken together, hypoxia induces dose-dependent physiological responses that can result in protective adaptations (i.e., acclimatization) but also (if the hypoxic dose is too intense for the individual capacity to manage the hypoxic stress) in maladaptation and the development of high-altitude illnesses. These responses to hypoxic stress are characterized by intriguing overlaps with mental stress responses, converging for example in the activation of the sympathetic nervous system and the HPA-axis. This suggests an interdependence of the human body’s reaction to mental stress and hypoxia, potentially influencing the development of high-altitude illnesses and psychiatric diseases. Consequently, targeting the mechanisms at the crossroads between hypoxia adaptation and mental stress could be future strategies to improve mental health []. However, more knowledge on the molecular and systemic links between sensing and responses to hypoxia and mental stress are required.
Brain related altitude illnesses
When the physiological mechanisms fail to compensate for the hypoxic stress, high-altitude illnesses (HAIs) can result []. There are two main brain-related HAIs, acute mountain sickness (AMS) and high-altitude cerebral edema (HACE).
AMS is the most common HAI and is usually benign and self-limiting. It primarily affects individuals who rapidly ascend to altitudes above 2500 m without proper acclimatization [, , , ]. Symptoms include headache (cardinal symptom) along with gastrointestinal complaints, fatigue or weakness, and dizziness or lightheadedness []. Symptoms usually appear within 24 hours of arrival at altitude and develop individually during the first 6-12 hours of hypoxia exposure [], usually peaking during the first night []. The symptoms are usually assessed using the Lake Louise Scoring System (LLSS) [] or the Environmental Symptoms Questionnaire (ESQ-C) []. The LLSS scores key symptoms on a scale of 0-3. Composite scores of 3-5 indicate mild AMS, 6-9 moderate, and 10-12 severe []. The ESQ-C scores 11 items on a 0-5 scale, with AMS indicated by a weighted average score of 0.7 or higher [].
HACE can manifest 2-4 days after a rapid ascent (usually not below 4000 m) in unacclimatized individuals. Key symptoms include altered mental status and truncal ataxia, but proper diagnosis requires MRI [, ].
Epidemiology
The incidence of AMS varies based on individual susceptibility, acclimatization status, ascent rate, and altitude reached. Studies conducted at 13 different altitudes (2200 m to 4559 m) show an almost linear increase in AMS incidence from 7% to 52% with gradual ascent over 1-3 days [, ]. In contrast, rapid ascent by air travel results in a steeper increase [, ], from 39% at 3350 m [] to 84% at 3740 m []. The influence of the rate of ascent has been documented for example for Mt. Kilimanjaro (5895 m), where incidence rates of 75% and 53% were reported for 4-5 day and 6-day ascents, respectively []. In the Himalayas (Nepal), incidence rates ranged from 0% at 2500-3000 m to 34% above 5000 m [].
HACE is less common than AMS. Above 4000 m, the HACE prevalence was reported to be about 0.98% []. At altitudes between 4243 m and 5500 m in Nepal, the overall HACE prevalence was about 1%, increasing to 3.4% in trekkers with AMS []. Slow ascent rates appear to be an effective preventive measure, as evidenced by a study of Chinese railroad workers traveling to Tibet (3,500–5,000 m) over 8 days, which reported a HACE prevalence of only 0.28% [].
Pathophysiology
The mechanisms leading to AMS and HACE are not yet fully elucidated []. Insufficiently compensated high altitude-induced increases in cerebral blood flow and brain parenchyma volume [, ] may lead to brain swelling, which however is not necessarily correlate with AMS severity [, ]. Intracellular cytotoxic edema, on the other hand, resulting from hypoxia-induced energy deficiency, appear more closely linked to AMS severity [, , ]. Recent research questions the direct connection between hypoxia-induced brain edema and AMS [], highlighting the importance of other mechanisms like trigeminovascular sensitization and individual hypoxia susceptibility.
Trigeminovascular sensitization, triggered by mechanical or chemical stimuli, may develop gradually in acute hypoxia [, ], explaining AMS symptoms like nausea and vomiting through connections with related vegetative centers in the brainstem []. Various factors contribute to AMS development, including individual differences in hypoxic ventilatory responses, reduced lung diffusion capacity [], orthostatic intolerance [, ], increased sympathetic activity [, , ], and fluid retention [, , , ]. Psychological factors such as anxiety, stress levels, and nocebo/placebo effects also play a role in AMS manifestation [, , ].
The progression from AMS to HACE may involve blood-brain barrier disruption [, , ]. Some evidence suggests a potential cascade in hypoxia-induced brain edema development, from cytotoxic to vasogenic edema and hemorrhagic transformation [, ]. However, whether HACE evolves directly from AMS or develops independently remains unclear and requires further investigation [].
Prevention
Preventive measures include non-pharmacological and pharmacological approaches. Non-pharmacological prevention methods notably include gradual ascent and (pre)acclimatization. It is recommended to limit increases in sleeping altitude to 300-500 m/day, take rest days every 3-4 days, and keep the average ascent rate below 500 m/day [, ]. Pre-acclimatization strategies include staging at moderate altitudes before higher ascents and pre-exposure to high altitudes in preceding months. Hypoxia conditioning, involving simulated altitude exposure at home, is gaining popularity, with about 300 hours of adequate simulated altitude exposure (a substantial part should be close to the target altitude) being considered an optimal preparation [, ].
Pharmacological prevention primarily involves acetazolamide, which supports acclimatization [, ]. The recommended dosage is 125 mg twice daily, starting 8-24 hours before ascent and continuing for 48 hours at the destination [, ]. This can reduce AMS risk from 25% to 12% []. NSAIDs like ibuprofen have similar efficacy for headache and AMS prevention but don’t support acclimatization []. Dexamethasone is an alternative for rapid ascent but treatment should be limited to 5 days due to side effects [].
Screening for HAI susceptibility is challenging. Risk factors include previous HAI history, rapid ascent (over 400 m/day), migraine history, and certain physiological responses to hypoxia [].
Treatment
Alleviating hypoxemia through supplemental oxygen or descending to lower altitudes can improve the condition of individuals suffering from HAIs []. However, the management of AMS and HACE depends on illness severity and local conditions, including oxygen and medication availability, weather, track conditions, injuries, and transportation options [].
Those experiencing only headache or mild to moderate AMS can usually remain at altitude but should monitor their condition []. They should be strictly advised not to go higher until symptoms have completely disappeared, to perform no or only light exercise, to drink sufficiently and to take (if necessary) pain reliefers (e.g., ibuprofen) and/or antiemetics (e.g., metoclopramide) [, , , ]. While dexamethasone can effectively treat AMS, it is generally reserved for more severe cases when descent is not possible [, ]. The evidence for the efficacy of acetazolamide to treat HAIs is limited but some benefits in symptom relief and improved oxygenation have been shown [, ].
If symptoms persist or worsen over days, descent becomes necessary. Severely ill patients require passive transportation and should receive dexamethasone [, ]. When immediate transportation isn’t feasible, alternative treatments include low-flow oxygen (2-4 liters/min) or using a hyperbaric bag if available [].
If symptoms indicate a possible development of HACE, immediate treatment is of paramount importance. In this case, rapid passive descent to the lowest possible place, and administration of dexamethasone are the treatment strategies of choice []. If descent is not possible, applying low-flow oxygen or using a hyperbaric bag (if available) while continuing dexamethasone until evacuation is possible is appropriate [, ]. The recommended dosages for medication are reported in detail elsewhere [].
Differential diagnosis
Differential diagnoses of AMS include migraine, cluster headache, dehydration, carbon monoxide poisoning, psychiatric disorders, physical exhaustion, heat exhaustion and/or hangover [, , ]. The differential diagnosis of HACE includes stroke, intracranial haemorrhage, migraine, hypoglycaemia, hyponatraemia, hypothermia, drug or alcohol intoxication, and head trauma [, , ].
Mental health at high altitude
Mental health at high altitude and its association with acute mountain sickness
Higher rates of anxiety, depression, suicide and cognitive impairment have been shown in some populations of high-altitude residents who are adapted to the lower pressure of oxygen, compared to low altitude residents [, , , ]. However, these analyses are likely substantially confounded by infrastructural (such as access to healthcare) and cultural differences between lowlands and highlands. The exposure to moderate and in some cases possibly also slightly higher altitudes may even result in positive effects on the brain in the long term, i.e. after acute altitude exposures, after sufficient acclimatization or due to transgenerational adaptation [, ].
The lack of gold standard diagnostics to verify AMS has emerged as one of the major issues in dealing with HAIs and also when comparing different studies, since the diagnosis is mainly based on self-rating questionnaires []. This problem is rare in internal medicine or surgery but a common and longstanding problem in psychiatry. Psychiatric diagnoses still mostly rely on symptom clusters and objective biomarkers are rarely available. Although the LLSS is one of the most commonly used instruments to assess AMS [] false positive scorings have been observed, for example due to fatigue []. Anxiety symptom burden (often below the threshold used for diagnosing a manifest mental disorder) is associated with the prevalence and severity of AMS [, , , , ]. In addition to significant correlations [, , ], studies have shown a predictive value of anxiety prior to (OR for severe AMS 1.14; 1.006–1.28; p = 0.04 []) and during high-altitude exposure for the development of AMS (OR for AMS: 1.11; 1.07-1.15; P < .0001 [] and 1.446; 1.200–1.744; P < .0001 [], OR for severe AMS: 1.13; 1.07-1.18; P < .0001 []). Beside anxiety as the best-studied mental symptom associated with AMS, the impact of higher level of somatization at sea level, individual stress response, depression, fatigue, lack of vigor, hostility and confusion on AMS has been described [, , ]. Individuals with symptoms or a diagnosis of a psychiatric disorder prior to high-altitude exposure have a higher risk of developing AMS []. On the other hand, an analysis of health records showed that individuals who have previously received a diagnosis of AMS have a higher probability of becoming diagnosed with a mental disorder in the 10 years thereafter []. These studies demonstrate that AMS (as a subjective somatic symptom burden) and psychiatric symptoms at high altitude can co-occur but the nature of this association remains enigmatic. It could be that AMS leads to negative affective mood states and anxiety or that negative affective mood states and anxiety confound the LLS scoring of AMS due to overlapping symptoms of both conditions or that negative affective mood states and anxiety are part of the symptom cluster of AMS.
High-altitude exposure in individuals with pre-existing mental disorders
The effect of high and extreme altitude exposure on individuals with mental disorders has been relatively neglected in the scientific literature, one explanation for this gap in the literature could be the misconception that individuals with pre-existing mental disorders do not usually engage in activities at high and extreme altitude, possibly because of reports on frequently more sedentary lifestyles in such populations [, ]. From a current perspective this seems quite astonishing considering that psychiatric disorders have a high lifetime prevalence, with common mental disorders (anxiety, mood, and substance disorders) affecting ∼30% of the global population []. This lack of focus on mental health during high-altitude exposures might be linked to a lower socioeconomic status associated with some psychiatric disorders that may reduce the frequency of high-altitude sojourns for leisure purposes []. In addition, there is still a stigma associated with admitting to having “mental health problems” [], which could have reinforced the notion that individuals with pre-existing mental disorders do not travel to high and extreme altitudes. Supporting this notion, in a study at Everest Base Camp it was shown that individuals often do not disclose pre-existing mental disorders in a self-report questionnaire and only report them when directly talking to a trained healthcare professional []. In a pilot questionnaire study significantly fewer individuals with pre-existing mental disorders informed their tour partners about their conditions compared to individuals with pre-existing somatic disorders which might lead to dangerous situations when performing mountain sports at altitudes >2500 m, especially in remote areas []. In this same study it was also found that fewer individuals with mental than with somatic disorders talk to their healthcare provider about possible consequences of high-altitude exposure on their condition prior to the trip [].
In the few available studies, individuals with pre-existing mental disorders more often reported no change or an improvement of their symptoms during mountain sports at high and extreme altitude compared to individuals with somatic disorders []. This might be due to the effect of (moderate) hypoxia, the physical activity, social contact or nature exposure. On the other hand, several studies show that exposure of mentally healthy individuals to high and extreme altitude can adversely affect mood, behaviour and cognition [, , ] and that anxiety scores can increase with altitude [, ]. While the exact biochemical background underlying these effects is still unknown, it is possible that the stress response represented most prominently by the sympathetic adrenal medullary axis and the hypothalamic–pituitary–adrenal axis plays an important role in linking high and extreme altitude effects and mental health []. Larger, interventional, studies investigating the effect of high and extreme altitude on people with pre-existing mental disorders are needed to better counsel such individuals for planned high-altitude sojourns.
One important consideration for medical counselling and future studies is the level of altitude to which people are exposed. While hypoxia at moderate altitudes possibly is not associated with mental health risks [], such risks likely increase with higher altitudes and may become particularly important for insufficiently acclimatized lowlanders acutely exposed to altitudes above around 4000 m, where also cognitive symptoms occur more frequently [, ]. However, environmental conditions aside from hypoxia (e.g. weather, mountainous environments, etc.) physical activity and further stressors linked to high-altitude surroundings might influence the occurrence of mental symptoms as well [].
Psychotic symptoms during high altitude exposure
Psychotic symptoms, such as hallucinations (e.g., the “third-man phenomenon”, in which a person feels that another person is ahead or behind them and sometimes can see and hear the alleged individual without anyone being there), delusions, disorganised speech/thought, can occur at higher altitudes, especially at extreme altitude. Abnormal psychomotor behaviour, and negative symptoms, as well as impaired cognition, depression, and mania can also be part of the syndrome of psychosis []. Psychotic symptoms are transdiagnostic, meaning that they are characteristic of a single disorder but can be found in various conditions []. Psychosis at high and extreme altitude can be accompanied by further somatic or mental symptoms (Figure 2) which can aid in making the correct diagnosis.
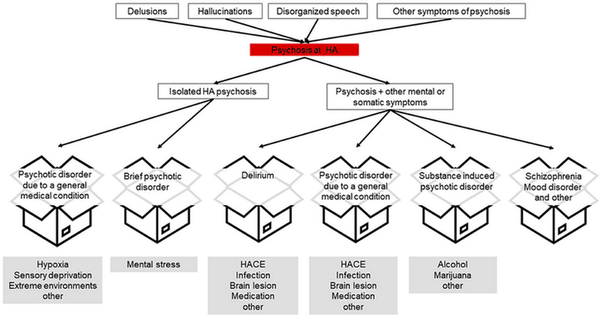
2. Psychotic symptoms are transdiagnostic, meaning that they can be a symptom of different disorders. It is essential to try and correctly assign psychotic symptoms to a diagnostic entity in order to provide the correct treatment. This can be challenging, especially at high altitude (HA). Source: []. © 2023 Hüfner, Falla, Brugger, Gatterer, Strapazzon, Iztok, Zafren, Sperner-Unterweger, Fusar-Poli. https://doi.org/10.3389/fpsyt.2023.1221047. Distributed under the terms of the Creative Commons Attribution License CC BY 4.0 (https://creativecommons.org/licenses/by/4.0).
Delirium represents an umbrella construct used to describe the mental effects of generalised brain dysfunction occurring in the context of acute illness, substance withdrawal or intoxication []. In essence, delirium describes a decompensation of cerebral function in response to pathophysiological stressors. Defining features of delirium are disturbance in attention and awareness (reduced orientation to the environment) which develop over a short period of time and usually tend to fluctuate in severity during the day. Additional disturbance in cognition (e.g., memory deficit, disorientation, language, visuospatial ability, or perception) can occur at altitude and there is always an underlying pathology that causes delirium. As mentioned, HACE is a rare disease, but must be considered as a possible cause at high and extreme altitude.
Travelling by itself, without high-altitude exposure, has been proposed to be a potential trigger for psychosis through stressors such as environmental conditions, unaccustomed physical exertion, and psychosocial factors, all of which can also occur – and can even be aggravated – in high-altitude travel []. In addition, at high and extreme altitude hypoxia has to be considered a potential trigger of psychosis as well. A diagnostic classification of psychotic symptoms at high and extreme altitude can be challenging but is essential since treatment and evacuation decisions can depend on it.
Isolated high-altitude psychosis was described as a separate medical entity [] and is characterized by psychotic symptoms which occur in the absence of other clinical features such as signs of delirium or HACE. Affected individuals can usually descend without physical assistance. Isolated high-altitude psychosis is attributed primarily to hypoxia and is usually benign and self-limiting, resolving after descent and restoration of normoxia. However, misjudgment of reality can lead to dangerous situations, so that education of mountaineers about such symptoms is advisable prior to high and extreme altitude exposure similarly to other hypoxia-associated conditions such as AMS or HACE. Persons with symptoms of psychosis must not be left alone and should not take decisions during the tour because they have an impaired reality control and a disturbed sense of judgment and are thus unable to correctly assess situations. Cognitive strategies such as reality testing can be applied by the climbers experiencing such symptoms or by a partner [].
Little is known about athletes competing at HA. However, there is one study that supports the concept that abnormal perceptual experiences can occur in runners during ultra-trailer, high-altitude competitions regardless of the context of psychiatric or neurological disorders [].
Discussion
The brain’s exquisite vulnerability to hypoxia renders it a particularly affected organ when exposed to altitude. Various cellular and systemic hypoxia responses are in place to counteract the associated risks. If they fail, HAIs, including cerebral forms like AMS and HACE, can develop. In addition, altitude/hypoxia, in particular if combined with physical exertion, can be associated with mental symptoms, such as depression, or mental disorders, such as isolated high-altitude psychosis. Therefore, it is surprising that the topic of high-altitude travelling with pre-existing mental symptoms is severely under-investigated, impeding evidence-based medical counselling. Especially the dependence of psychological effects on the magnitude of the exposure-altitude and on different psychiatric diagnoses requires future investigation. Similarly, despite considerable scientific interest in this topic, the association of mood, stress and hormonal status with the development of HAIs remains poorly understood. In addition, a major knowledge gap of cerebral and mental altitude effects concerns differences between populations and individuals. Besides different patient groups also athletes performing top-level training or competitive activities may be particularly vulnerable to cerebral/mental altitude consequences. Despite a wealth of data on physiological altitude outcomes, a detailed characterization of mental consequences of high-performance sports at altitude is lacking.
Finally, recent attempts to harness endogenous hypoxia adaptations (via the controlled induction of the innate cellular and systemic hypoxia responses) as a novel interventional treatment strategy in stress-related mental disorders have shown promise and may root in overlapping mechanisms between hypoxic and psychological stress []. Approaches like intermittent hypoxia conditioning (in which short episodes of hypoxia are repeatedly applied) appear to have potential in anxiety and depression, in particular post-traumatic stress disorder, and have been speculated to also be of value in schizophrenia and psychosis [].
Conclusion
In conclusion, high and extreme altitude environments, including their characteristic hypobaric hypoxic conditions, are powerful modulators of the human psychological state and, depending on factors like the hypoxic dose and individual vulnerabilities, can pose risks and opportunities for healthy people as well as those with mental disorders.
References
- Burtscher J, Mallet RT, Burtscher M, Millet GP. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res Rev. 2021;68:101343.
- Pun M, Falla M, Strapazzon G, Hüfner K. Neurological, neurophysiological, psychological and psychiatric effects of high altitude and hypoxia. Front Physiol. 2023;14:1329084.
- Burtscher M, Gatterer H, Burtscher J, Mairbäurl H. Extreme Terrestrial Environments: Life in Thermal Stress and Hypoxia. A Narrative Review. Front Physiol. 2018;9:572.
- Aboouf MA, Thiersch M, Soliz J, Gassmann M, Schneider Gasser EM. The Brain at High Altitude: From Molecular Signaling to Cognitive Performance. Int J Mol Sci. 2023;24.
- Burtscher J, Mallet RT, Pialoux V, Millet GP, Burtscher M. Adaptive Responses to Hypoxia and/or Hyperoxia in Humans. Antioxid Redox Signal. 2022;37:887–912.
- Treml B, Wallner B, Blank C, Fries D, Schobersberger W. The Influence of Environmental Hypoxia on Hemostasis-A Systematic Review. Front Cardiovasc Med. 2022;9:813550.
- SheikhBahaei S. Physiology: New Insights into Central Oxygen Sensing. Curr Biol. 2020;30:R1004–R1006.
- Funk GD, Gourine AV. CrossTalk proposal: a central hypoxia sensor contributes to the excitatory hypoxic ventilatory response. J Physiol. 2018;596:2935–38.
- Iturriaga R, Alcayaga J, Chapleau MW, Somers VK. Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiol Rev. 2021;101:1177–1235.
- Burtscher J, Niedermeier M, Hufner K, van den Burg E, Kopp M, Stoop R, et al The interplay of hypoxic and mental stress: Implications for anxiety and depressive disorders. Neurosci Biobehav Rev. 2022;138:104718.
- Guyenet PG, Stornetta RL, Souza G, Abbott SBG, Shi Y, Bayliss DA. The Retrotrapezoid Nucleus: Central Chemoreceptor and Regulator of Breathing Automaticity. Trends Neurosci. 2019;42:807–24.
- Kulandavelu S, Balkan W, Hare JM. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci U S A. 2015;112:6254–5.
- Dubowitz DJ, Dyer EA, Theilmann RJ, Buxton RB, Hopkins SR. Early brain swelling in acute hypoxia. J Appl Physiol (1985). 2009;107:244–52.
- Turner REF, Gatterer H, Falla M, Lawley JS. High-altitude cerebral edema: its own entity or end-stage acute mountain sickness? J Appl Physiol (1985). 2021;131:313–25.
- Moreno-Domínguez A, Ortega-Sáenz P, Gao L, Colinas O, García-Flores P, Bonilla-Henao V, et al Acute O(2) sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci Signal. 2020;13.
- Fernández-Agüera MC, Gao L, González-Rodríguez P, Pintado CO, Arias-Mayenco I, García-Flores P, et al Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015;22:825–37.
- Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–83.
- Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012;37:364–72.
- Jaśkiewicz M, Moszyńska A, Króliczewski J, Cabaj A, Bartoszewska S, Charzyńska A, et al The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol Biol Lett. 2022;27:109.
- Burtscher J, Hohenauer E, Burtscher M, Millet GP, Egg M. Environmental and behavioral regulation of HIF-mitochondria crosstalk. Free Radic Biol Med. 2023;206:63–73.
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72.
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8.
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–62.
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63.
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85.
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97.
- Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22.
- Burtscher J, Citherlet T, Camacho-Cardenosa A, Camacho-Cardenosa M, Raberin A, Krumm B, et al Mechanisms underlying the health benefits of intermittent hypoxia conditioning. J Physiol. 2024;602(21):5757–83.
- Gatterer H, Villafuerte FC, Ulrich S, Bhandari SS, Keyes LE, Burtscher M. Altitude illnesses. Nat Rev Dis Primers. 2024;10:43.
- Luks AM, Hackett PH. Medical Conditions and High-Altitude Travel. N Engl J Med. 2022;386:364–73.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Eng J Med. 2013;368:2294–302.
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–74.
- Roach RC, Hackett PH, Oelz O, Bartsch P, Luks AM, MacInnis MJ, et al The 2018 Lake Louise Acute Mountain Sickness Score. High Alt Med Biol. 2018;19:4–6.
- Burtscher M, Wille M, Menz V, Faulhaber M, Gatterer H. Symptom progression in acute mountain sickness during a 12-hour exposure to normobaric hypoxia equivalent to 4500 m. High Alt Med Biol. 2014;15:446–51.
- Burtscher J, Gatterer H, Niederseer D, Vonbank K, Burtscher M. Flying to high-altitude destinations. Minerva Med. 2024;0:0.
- Beidleman BA, Muza SR, Fulco CS, Rock PB, Cymerman A. Validation of a shortened electronic version of the environmental symptoms questionnaire. High Alt Med Biol. 2007;8:192–9.
- Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol. 2004;5:136–46.
- Mairer K, Wille M, Bucher T, Burtscher M. Prevalence of acute mountain sickness in the Eastern Alps. High Alt Med Biol. 2009;10:239–45.
- Maggiorini M, Buhler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. BMJ. 1990;301:853–5.
- Burtscher J, Swenson ER, Hackett P, Millet GP, Burtscher M. Flying to high-altitude destinations: is the risk of acute mountain sickness greater? J Travel Med. 2023;30(4):taad011.
- Caravedo MA, Mozo K, Morales ML, Smiley H, Stuart J, Tilley DH, et al Risk factors for acute mountain sickness in travellers to Cusco, Peru: coca leaves, obesity and sex. J Travel Med. 2022; 29(5):taab102.
- Murdoch DR. Altitude Illness Among Tourists Flying to 3740 Meters Elevation in the Nepal Himalayas. J Travel Med. 1995;2:255–56.
- Lawrence JS, Reid SA. Risk Determinants of Acute Mountain Sickness and Summit Success on a 6-Day Ascent of Mount Kilimanjaro (5895 m). Wilderness Environ Med. 2016;27:78–84.
- Vardy J, Vardy J, Judge K. Acute mountain sickness and ascent rates in trekkers above 2500 m in the Nepali Himalaya. Aviat Space Environ Med. 2006;77:742–4.
- Richalet JP, Larmignat P, Poitrine E, Letournel M, Canouï-Poitrine F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med. 2012;185:192–8.
- Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet. 1976;2:1149–55.
- Wu TY, Ding SQ, Liu JL, Yu MT, Jia JH, Chai ZC, et al Who should not go high: chronic disease and work at altitude during construction of the Qinghai-Tibet railroad. High Alt Med Biol. 2007;8:88–107.
- Ainslie PN, Subudhi AW. Cerebral blood flow at high altitude. High Alt Med Biol. 2014;15:133–40.
- Sagoo RS, Hutchinson CE, Wright A, Handford C, Parsons H, Sherwood V, et al Magnetic Resonance investigation into the mechanisms involved in the development of high-altitude cerebral edema. J Cereb Blood Flow Metab. 2017;37:319–31.
- Kallenberg K, Bailey DM, Christ S, Mohr A, Roukens R, Menold E, et al Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. J Cereb Blood Flow Metab. 2007;27:1064–71.
- Biller A, Badde S, Heckel A, Guericke P, Bendszus M, Nagel AM, et al Exposure to 16 h of normobaric hypoxia induces ionic edema in the healthy brain. Nat Commun. 2021;12:5987.
- Burtscher M, Likar R, Nachbauer W, Philadelphy M. Aspirin for prophylaxis against headache at high altitudes: randomised, double blind, placebo controlled trial. BMJ. 1998;316:1057–8.
- Berger MM, Sareban M, Bartsch P. Acute mountain sickness: Do different time courses point to different pathophysiological mechanisms? J Appl Physiol (1985). 2020;128:952–59.
- Luks AM, Swenson ER, Bartsch P. Acute high-altitude sickness. Eur Respir Rev. 2017;26.
- Bellovary BN, Wells AD, Fennel ZJ, Ducharme JB, Houck JM, Mayschak TJ, et al Could Orthostatic Stress Responses Predict Acute Mountain Sickness Susceptibility Prior to High Altitude Travel? A Pilot Study. High Alt Med Biol. 2023;24:19–26.
- Burtscher M, Brandstatter E, Gatterer H. Preacclimatization in simulated altitudes. Sleep Breath. 2008;12:109–14.
- Loeppky JA, Icenogle MV, Maes D, Riboni K, Scotto P, Roach RC. Body temperature, autonomic responses, and acute mountain sickness. High Alt Med Biol. 2003;4:367–73.
- Swenson ER. Pharmacology of acute mountain sickness: old drugs and newer thinking. J Appl Physiol (1985). 2016;120:204–15.
- Gatterer H, Wille M, Faulhaber M, Lukaski H, Melmer A, Ebenbichler C, et al Association between Body Water Status and Acute Mountain Sickness. Plos One. 2013; 8(8):e73185.
- Loeppky JA, Icenogle MV, Maes D, Riboni K, Hinghofer-Szalkay H, Roach RC. Early fluid retention and severe acute mountain sickness. J Appl Physiol (1985). 2005;98:591–7.
- Bärtsch P, Pfluger N, Audétat M, Shaw S, Weidmann P, Vock P, et al Effects of slow ascent to 4559 M on fluid homeostasis. Aviat Space Environ Med. 1991;62:105–10.
- Westerterp KR, Robach P, Wouters L, Richalet JP. Water balance and acute mountain sickness before and after arrival at high altitude of 4, 350 m. J Appl Physiol (1985). 1996;80:1968–72.
- Missoum G, Rosnet E, Richalet JP. Control of anxiety and acute mountain sickness in Himalayan mountaineers. Int J Sports Med. 1992;13 Suppl 1:S37–9
- Gatterer H, Bernatzky G, Burtscher J, Rainer M, Kayser B, Burtscher M. Are Pre-Ascent Low-Altitude Saliva Cortisol Levels Related to the Subsequent Acute Mountain Sickness Score? Observations from a Field Study. High Alt Med Biol. 2019;20:337–43.
- Bartsch P. The Impact of Nocebo and Placebo Effects on Reported Incidence of Acute Mountain Sickness. High Alt Med Biol. 2022;23(1):8–17.
- Luks AM, Beidleman BA, Freer L, Grissom CK, Keyes LE, McIntosh SE, et al Wilderness Medical Society Clinical Practice Guidelines for the Prevention, Diagnosis, and Treatment of Acute Altitude Illness: 2024 Update. Wilderness Environ Med. 2024; 35(1_suppl):2S–19S.
- Luks AM. Clinician’s corner: What do we know about safe ascent rates at high altitude? High Alt Med Biol. 2012;13:147–52.
- Burtscher M, Millet GP, Burtscher J. Hypoxia Conditioning for High-Altitude Pre-acclimatization. J Sci Sport Exerc. 2022;4:331–45.
- Swenson ER. New insights into carbonic anhydrase inhibition, vasodilation, and treatment of hypertensive-related diseases. Curr Hypertens Rep. 2014;16:467.
- Shlim DR. The use of acetazolamide for the prevention of high-altitude illness. J Travel Med. 2020;27.
- Basnyat B, Gertsch JH, Johnson EW, Castro-Marin F, Inoue Y, Yeh C. Efficacy of low-dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial. High Alt Med Biol. 2003;4:45–52.
- Basnyat B, Gertsch JH, Holck PS, Johnson EW, Luks AM, Donham BP, et al Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol. 2006;7:17–27.
- Gertsch JH, Lipman GS, Holck PS, Merritt A, Mulcahy A, Fisher RS, et al Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT). Wilderness Environ Med. 2010;21:236–43.
- Burtscher M, Hefti U, Hefti JP. High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med Health Sci. 2021;3:59–69.
- Irons HR, Salas RN, Bhai SF, Gregorie WD, Harris NS. Prospective Double-Blinded Randomized Field-Based Clinical Trial of Metoclopramide and Ibuprofen for the Treatment of High Altitude Headache and Acute Mountain Sickness. Wilderness Environ Med. 2020;31:38–43.
- Tang E, Chen Y, Luo Y. Dexamethasone for the prevention of acute mountain sickness: systematic review and meta-analysis. Int J Cardiol. 2014;173:133–8.
- Levine BD, Yoshimura K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Dexamethasone in the treatment of acute mountain sickness. N Engl J Med. 1989;321:1707–13.
- Grissom CK, Roach RC, Sarnquist FH, Hackett PH. Acetazolamide in the treatment of acute mountain sickness: clinical efficacy and effect on gas exchange. Ann Intern Med. 1992;116:461–5.
- Simancas-Racines D, Arevalo-Rodriguez I, Osorio D, Franco JV, Xu Y, Hidalgo R. Interventions for treating acute high altitude illness. Cochrane Database Syst Rev. 2018;6:CD009567.
- Burtscher M, Hefti U, Hefti JP. High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med Health Sci. 2021;3:59–69.
- Luks AM, Beidleman BA, Freer L, Grissom CK, Keyes LE, McIntosh SE, et al Wilderness Medical Society Clinical Practice Guidelines for the Prevention, Diagnosis, and Treatment of Acute Altitude Illness: 2024 Update. Wilderness Environ Med. 2024;35:2S–19S.
- Davis C, Hackett P. Advances in the Prevention and Treatment of High Altitude Illness. Emerg Med Clin North Am. 2017;35:241–60.
- Ortiz-Prado E, Simbana-Rivera K, Duta D, Ochoa I, Izquierdo-Condoy JS, Vasconez E, et al Optimism and Health Self-Perception-Related Differences in Indigenous Kiwchas of Ecuador at Low and High Altitude: A Cross-Sectional Analysis. High Alt Med Biol. 2022;23:26–36.
- Gao C, Ciren J, Wang D, Zhang Z, Ge R, Yan L. Assessment of Psychological and Social Fitness in Healthy Adults Permanently Living at Very High Altitude. Int J Environ Res Public Health. 2023; 20(3):2013.
- Reno E, Brown TL, Betz ME, Allen MH, Hoffecker L, Reitinger J, et alSuicide and High Altitude: An Integrative Review. High Alt Med Biol. 2018;19:99–108.
- Yan X. Cognitive impairments at high altitudes and adaptation. High Alt Med Biol. 2014;15:141–5.
- Burtscher J, Gassmann M, Ehrenreich H, Hufner K, Kopp M, Burtscher M. Cognitive effects of altitude exposure. J Travel Med. 2024; 22:taae112.
- Moore J, MacInnis MJ, Dallimore J, Wilkes M. The Lake Louise Score: A Critical Assessment of Its Specificity. High Alt Med Biol. 2020;21:237–42.
- Boos CJ, Bass M, O’Hara JP, Vincent E, Mellor A, Sevier L, et al The relationship between anxiety and acute mountain sickness. PLoS One. 2018;13:e0197147.
- Talks BJ, Campbell C, Larcombe SJ, Marlow L, Finnegan SL, Lewis CT, et al Baseline Psychological Traits Contribute to Lake Louise Acute Mountain Sickness Score at High Altitude. High Alt Med Biol. 2022;23:69–77.
- Tang X, Li X, Xin Q, Wang Q, Li S, Yang Y. Anxiety as a Risk Factor for Acute Mountain Sickness Among Young Chinese Men After Exposure at 3800 M: A cross-sectional Study. Neuropsychiatr Dis Treat. 2023;19:2573–83.
- Dong JQ, Zhang JH, Qin J, Li QN, Huang W, Gao XB, et al Anxiety correlates with somatic symptoms and sleep status at high altitudes. Physiol Behav. 2013;112–113:23–31.
- Bian SZ, Jin J, Zhang JH, Li QN, Yu J, Yu SY, et al Principal Component Analysis and Risk Factors for Acute Mountain Sickness upon Acute Exposure at 3700 m. PLoS One. 2015;10:e0142375.
- Bian SZ, Jin J, Dong JQ, Li QN, Yu J, Tang CF, et al A higher baseline somatization score at sea level as an independent predictor of acute mountain sickness. Physiol Behav. 2016;167:202–8.
- Hufner K, Caramazza F, Pircher Nockler ER, Stawinoga AE, Fusar-Poli P, Bhandari SS, et al Association of Pre-existing Mental Health Conditions with Acute Mountain Sickness at Everest Base Camp. High Alt Med Biol. 2022;23:338–44.
- Wang YH, Chien WC, Chung CH, Her YN, Yao CY, Lee BL, et al Acute Mountain Sickness and the Risk of Subsequent Psychiatric Disorders-A Nationwide Cohort Study in Taiwan. Int J Environ Res Public Health. 2023;20(4):2868.
- Peckham E, Tew G, Lorimer B, Bailey L, Beeken R, Cooper C, et al Interventions to increase physical activity and reduce sedentary behaviour in severe mental ill health: How effective are they?’- A systematic review. Ment Health Phys Act. 2023;25:100547.
- Tew GA, Peckham E, Ker S, Smith J, Hodgson P, Machaczek KK, et al Physical activity in adult users of inpatient mental health services: A scoping review. PLoS One. 2024;19:e0301857.
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43:476–93.
- Mangalore R, Knapp M, Jenkins R. Income-related inequality in mental health in Britain: the concentration index approach. Psychol Med. 2007;37:1037–45.
- Corrigan PW, Watson AC. Understanding the impact of stigma on people with mental illness. World Psychiatry. 2002;1:16–20.
- Gstir C, Schurr T, Ehlers R, Burtscher J, Sperner-Unterweger J, Hüfner K. Is it Possible for Individuals with Pre-Existing Mental Disorders to Perform Mountain Sports at High Altitude - First Evidence from a Pilot Cross-Sectional Questionnaire Study. High Alt Med Biol. 2024; in press.
- Bliemsrieder K, Weiss EM, Fischer R, Brugger H, Sperner-Unterweger B, Hufner K. Cognition and Neuropsychological Changes at Altitude-A Systematic Review of Literature. Brain Sci. 2022; 12(12):1736.
- Falla M, Hufner K, Falk M, Weiss EM, Vogele A, Jan van Veelen M, et alSimulated Acute Hypobaric Hypoxia Effects on Cognition in Helicopter Emergency Medical Service Personnel - A Randomized, Controlled, Single-Blind, Crossover Trial. Hum Factors. 2024;66:404–23.
- Shukitt-Hale B, Lieberman H. The Effect of Altitude on Cognitive Performance and Mood. In: B. Marriott and S. Carlson, Nutritional Needs In Cold And In High-Altitude Environments: Applications for Military Personnel in Field Operations. Washington (DC): National Academies Press (US); 1996.
- Roth WT, Gomolla A, Meuret AE, Alpers GW, Handke EM, Wilhelm FH. High altitudes, anxiety, and panic attacks: is there a relationship? Depress Anxiety. 2002;16:51–8.
- Flaherty GT, Chua CW. High altitude travel and mental health. J Travel Med. 2018;25(1):tay025.
- APA. Diagnostic And Statistical Manual Of Mental Disorders: Dsm-5™, 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc.; 2013.
- Fusar-Poli P, Rutigliano G, Stahl D, Davies C, Bonoldi I, Reilly T, et al Development and Validation of a Clinically Based Risk Calculator for the Transdiagnostic Prediction of Psychosis. JAMA Psychiatry. 2017;74:493–500.
- European Delirium Association, American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141.
- Felkai P, Kurimay T. Patients with mental problems - the most defenseless travellers. J Travel Med. 2017;24.
- Hufner K, Brugger H, Kuster E, Dunsser F, Stawinoga AE, Turner R, et alIsolated psychosis during exposure to very high and extreme altitude - characterisation of a new medical entity. Psychol Med. 2018; 48(11):1872–9.
- Smailes D, Alderson-Day B, Fernyhough C, McCarthy-Jones S, Dodgson G. Tailoring Cognitive Behavioral Therapy to Subtypes of Voice-Hearing. Front Psychol. 2015;6:1933.
- Carbone MG, Pagni G, Maiello M, Tagliarini C, Pratali L, Pacciardi B, et al Misperceptions and hallucinatory experiences in ultra-trailer, high-altitude runners. Riv Psichiatr. 2020;55:183–90.
- Hüfner K, Falla M, Brugger H, Gatterer H, Strapazzon G, Tomazin I, et al Isolated high altitude psychosis, delirium at high altitude, and high altitude cerebral edema: are these diagnoses valid? Front Psychiatry. 2023;14:1221047.
- Manukhina E, Downey HF, Tseilikman VE, Burtscher J. Psychiatric diseases. In: Hypoxia Conditioning in Health, Exercise and Sport. : Girard O, Burtscher J, Burtscher M, Millet G. New York: Routledge; 2024. 84–95.