Introduction
Terrestrial water storage in two-thirds of the global land area is projected to decrease under climate change scenarios by the late twenty-first century, potentially increasing the frequency of droughts (). Tropical forests account for 59% of global forest vegetation carbon and play a crucial role in climate change mitigation (; ). However, rising atmospheric vapor pressure deficit driven by global warming increased tree mortality in Australian moist tropical forests, which ultimately decreased the carbon residence duration (). Further studies are required to discover how forest dynamics and tree growth will respond to climatic factors under warming climate in different tropical regions globally (). found that carbon sink in Amazonian tropical forests has steadily declined over the past three decades due to increased tree mortality. Therefore, tree mortality and internal dynamics within forests are vital controls of the carbon sequestration potential ().
Xylem formation is a complex process underpining the dynamics of tree growth and forest productivity (), which comprises the majority of stem radial growth. Despite extensive tree-ring studies analyzing the variability of inter-annual radial growth and growth–climate relationships over a century in tropical regions (), our knowledge of intra-annual growth dynamics for individual species remains limited due to the high biodiversity () and indistinct growth ring boundaries () in the tropics. Even by applying microcore sampling, it is often difficult to directly observe the annual cycle of xylogenesis for tree species with indistinct ring boundaries. However, high-resolution dendrometer measurements can nondestructively and continuously monitor stem radius variations with high temporal resolution, which contain irreversible radial growth of xylem and phloem as well as reversible swelling or shrinking induced by stem water status (). Therefore, combining discrete xylogenesis data with continuous radial increments data may provide accurate descriptions of intra-annual xylem production and radial growth ().
In tropical monsoon regions, the intra-annual dynamics of temperature are fairly steady, but the strong seasonality of precipitation may largely impact on intra-annual wood formation and radial growth. For example, the periodicity of cambial activity and radial growth were strongly influenced by seasonal precipitation in South America (). In Indonesia, the cambium cells were consistently divided into expanding xylem cells throughout both the rainy season and the dry season with continuous precipitation (). Additionally, observations of the tropical tree species Parkia nitida and Parkia velutina in a tropical rainforest in French Guiana revealed that cambial activity primarily depended on seasonal precipitation and that leaf phenology was closely correlated with xylogenesis (). So far, investigations of intra-annual xylem formation and radial growth have been scarce in southwest China. Previous studies suggested that intra-annual stem radial growth was positively correlated with precipitation and relative humidity in a tropical karst forest and a tropical ravine rainforest (; ). Furthermore, the radial growth of pine species was mostly limited by moisture availability during the early growing season (; ), and vapor pressure deficit negatively impacted the radial growth of Toona ciliata during the dry season and the dry-to-wet transition season in southwest China (). Consequently, water stress during the pre-monsoon season might be a key determinant limiting stem radial growth in this region. Tropical montane evergreen broad-leaved forests are the dominant vegetation type in Xishuangbanna, southwest China (). Here, it is of great significance to explore the intra-annual dynamics of xylem growth and stem radial increments under extreme pre-monsoon drought, as well as to analyze how radial growth responds to climatic variables.
Betula alnoides Buch.-Ham. ex D. Don and Schima wallichii (DC.) Korth. are common tree species in the tropical montane evergreen broad-leaved forest of Xishuangbanna. Betula alnoides is a fast-growing deciduous tree species mainly inhabiting southeast Asia and southern China (). Schima wallichii is an evergreen tree species mostly distributed in the tropical forests of the north-east Himalayan region (). In this study, we monitored intra-annual wood formation and radial increments of B. alnoides and S. wallichii over two years with different hydrothermal conditions during the pre-monsoon season by combining micro-sampling and dendrometer measurements. Our objectives were (i) to compare the intra-annual patterns (onset, cessation, duration, annual growth, and maximum growth rates) of the xylogenesis and radial increments for these two species during two climatologically contrasting years, and (ii) to identify the climatic factors influencing stem radial increments of B. alnoides and S. wallichii in a tropical montane evergreen broad-leaved forest. We hypothesized that (i) growth onset of both species might be delayed during drier pre-monsoon year, especially for earlier growth onset tree species, and (ii) moisture availability may dramatically impact on the radial increments of two species.
Materials and methods
Study site
The study was conducted in a tropical montane evergreen broad-leaved forest (21.65°N, 101.47°E, 1473.6 m a.s.l.) located in Mengla County, Dai Autonomous Prefecture of Xishuangbanna in Yunnan Province, southwest China. The study site is dominated by evergreen tree species from the families Fagaceae, Euphorbiaceae, Theaceae and Lauraceae (). According to climate parameters derived from Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies in Xishuangbanna Tropical Botanical Garden (21.92°N, 101.27°E, 580 m a.s.l.), which is about 36 km away from the study site, mean annual air temperature was 21.9 °C and mean annual precipitation was 1484 mm during the period 1963 to 2019. Influenced by the tropical monsoon climate, around 84% of annual rainfall occurs during the rainy summer monsoon season (May to October), and only about 16% occurs during the dry season (November to April) (see Fig. S1 available as Supplementary data at Tree Physiology Online).
Monitoring of xylem formation and stem radius increments
In this study, we selected five healthy trees of the species B. alnoides and S. wallichii with an average diameter at breast height (DBH) of 32.97 ± 2.43 cm and 37.15 ± 6.80 cm, respectively. Microcores with a diameter of 2 mm and a length of 15 mm contained the intact cambium were collected at breast height using a Trephor corer () in biweekly intervals from January 2020 to April 2022 to investigate the dynamics of intra-annual xylem formation. These samples were extracted spirally after removing the outer bark with a distance of approximately 5 cm among each sampling position to avoid the possible influence of local wound reactions. After collection, the microcores were placed into micro-centrifuge tubes with 50% ethanol solution and stored at 4 °C to prevent cell deterioration. Microcores were then immersed in glycerol–ethanol–water solution (mixed ratio: 10:7:3) for 2 weeks to make the samples soften. Then, these samples were dehydrated in successive ethanol solutions (70%, 80%, 85%, 95%, 95% and 100%), cleared in D-limonene and finally embedded in paraffin blocks. Transverse sections with a thickness of 12 μm were cut using a rotary microtome (RM2245, Leica, Germany), dewaxed with D-limonene, stained with safranin red and astra blue, and mounted on glass slides with Eukitt mounting medium (Sigma, Germany). Finally, these sections were analyzed under polarized light using a microscope equipped with digital camera (DM2500, Leica, Germany). After staining, cambial cells and enlarging cells were stained in blue, wall-thickening cells were stained partly blue and partly red, as well as mature and lignified xylem cells were stained in red. Cambial cells were regularly arranged in close proximity, the diameter of enlarging cells nearly doubled that of cambial cells and wall thickening cells exhibited brightness under polarized light (Fig. 1). Ring boundaries of B. alnoides are characterized by two to three rows of tangential parenchyma cells (Fig. 1a), while ring boundaries in S. wallichii are indistinct. Consequently, it was unfeasible to determine the annual ring width of S. wallichii. We measured the thickness of the cambial zone, the enlarging zone and the wall-thickening zone in both study species, as well as the thickness of the mature zone and the previous year’s ring of B. alnoides in three radial rows per picture using ImageJ software (https://imagej.nih.gov/ij/).
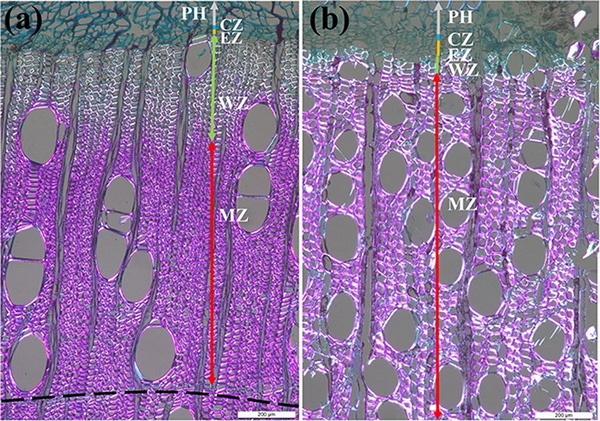
Figure 1
Wood anatomical structure of (a) B. alnoides and (b) S. wallichii under polarized light. PH, phloem; CZ, cambial zone; EZ, enlarging zone; WZ, wall-thickening zone; MZ, mature zone; dashed line in (a) indicates the ring boundary; bars = 200 μm.
Four individuals of each species among the sampling microcore trees were equipped with high-resolution band dendrometers (DRL26C, EMS Brno, Czech Republic) at breast height. Stem circumference variations were automatically recorded at 10-min intervals at a resolution of 2 μm with the in-built datalogger from January 2020 to December 2021. In order to minimize the impact of shrinking and swelling within the bark, the outermost bark layers were carefully removed before installation of the dendrometer.
Measurements of climatic factors
We installed a microclimate station to record climate variables at 10-min intervals at the study site. However, many missing data occurred because of power failure of datalogger in 2020 (see Fig. S2 available as Supplementary data at Tree Physiology Online). Meanwhile, we obtained daily air temperature, precipitation, and relative humidity (RH) from the meteorological station in Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies, Xishuangbanna Tropical Botanical Garden (XTBG). We found close correlations of climatic factors between the two sites (see Fig. S3 available as Supplementary data at Tree Physiology Online), so we used climate data from XTBG for further analyses. Vapor pressure deficit (VPD) was calculated from air temperature and RH using the RHtoVPD function in R package ‘plantecophys’ ().
Data analysis
Different xylem formation stages can be accurately represented by generalized additive models (GAMs) at an intra-annual scale (). Hence, this study describes intra-annual changes of different xylogenesis phases for two studied species as day of year (DOY) by fitting with GAMs in R package ‘mgcv’ (). Because of variable annual ring width along the stem circumference, the thickness of cambial zone, enlarging zone, wall-thickening zone and mature zone of B. alnoides were standardized by the previous year’s ring width (). In addition, we defined the onset and cessation of xylogenesis as the start of the cell enlarging and the ending of the wall thickening, respectively. The growth duration was the interval between the onset and cessation of xylogenesis. Differences in xylem growth parameters (onset day, cessation day, duration and annual xylem growth) between two species and two years were compared by a t-test.
To calculate daily radial growth, we transformed the dynamics of stem circumference monitored by high-resolution dendrometer into variations of the stem radius. The stem radial variation data were corrected with temperatures monitored by the dendrometer logger using the proc_L1 function in the R package ‘treenetproc’ (). The corrected stem radial variations data were further employed with the zero-growth model to extract irreversible radial growth caused by the formation of new xylem and phloem cells, and reversible shrinkage and expansion of the stem because of tree water deficit and resaturation with the R package ‘treenetproc’ (; ). The start and end dates of radial increments for each tree were determined as the dates when stem radius variations reached 5% and 95% of total annual radial change, respectively (). Growth duration and annual radial increments were the time periods between onset and cessation of radial variations. Differences in radial increments parameters between two years and two species were tested with a t-test.
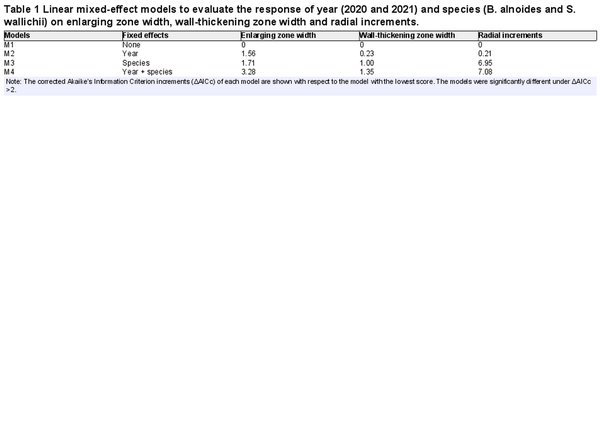
To reveal the effects of various climatic variables on wood formation and radial increments, we analyzed the relationships between weekly mean climate factors (except for weekly sum of precipitation) prior to the sampling and the thickness of enlarging zone or wall-thickening zone, as well as the correlations between weekly cumulative radial increments and concurrent climatic factors. We compared the impact of species and years on the enlarging zone width, the wall-thickening zone width and weekly radial increments (excluding zero values) by building linear mixed-effect models with individual trees as random effects and years or species as fixed effects (). Corrected Akaike’s Information Criterion (AICc) was calculated with AICc function in R package ‘MuMIn’ to evaluate the models (). The models were significantly different with AICc increments (ΔAICc) above 2. Since radial increments were significantly different in species but not years (Table 1), for the two species, we built linear mixed-effect models with the enlarging zone thickness, wall-thickening zone thickness or weekly radical increments (excluding zero values) as dependent variables, scaled climatic variables as fixed effects and individual trees nested within two years as random effects, respectively. The model is expressed as follows:
where Y represents the enlarging zone width, wall-thickening zone width or weekly radical increments of each species, X is the climate factor (precipitation, relative humidity, vapor pressure deficit, minimum air temperature, mean air temperature or maximum air temperature), α and β are the intercept and coefficient of fixed effect, and δ and ε are the variation from random effect and residual error term. The dependent variables data was log-transformed to achieve the normal distribution. All models were conducted by the lme function in the R package ‘nlme’ (). In addition, we calculated the proportion of cumulative growth on annual radial increments under different intervals of climatic factors (). Subsequent data analysis and plotting were performed in R 4.0.0 ().
Results
Climatic conditions
The mean air temperatures were 23 °C and 22.7 °C, as well as annual precipitation was 1206 mm and 1272 mm in 2020 and 2021, respectively (Fig. 2a and b). The climate conditions were warmer and drier than the long-term average (see Fig. S4 available as Supplementary data at Tree Physiology Online). The mean air temperature was higher, but precipitation was less from January to March in 2020 than in 2021 (Fig. 2a and b). The RH was lower, but VPD was higher between January and July in 2020 than 2021 (Fig. 2c and d). The year 2020 showed abnormal arid conditions during the pre-monsoon season for both RH and VPD compared with the long-term mean (see Fig. S4 available as Supplementary data at Tree Physiology Online). Therefore, the year 2020 had a significantly drier and warmer pre-monsoon season compared with 2021 (see Table S1 available as Supplementary data at Tree Physiology Online).
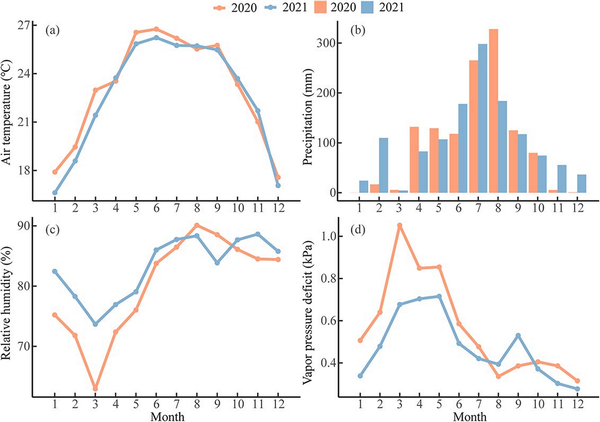
Figure 2
Monthly mean (a) air temperature, (b) precipitation, (c) relative humidity and (d) vapor pressure deficit in Menglun, Xishuangbanna during 2020 and 2021.
Intra-annual xylem formation
The dynamics of wood formation in B. alnoides and S. wallichii varied dramatically between 2020 and 2021 (Fig. 3). In 2020, cell enlargement in B. alnoides started in April (DOY: 117 ± 14) and ceased in August (DOY: 238 ± 31), whereas it started in January (DOY: 17 ± 13) and stopped in September (DOY: 246 ± 22) in 2021 (Fig. 3c). The first wall thickening cells appeared in May (DOY: 135 ± 34) and January (DOY: 27 ± 9) in B. alnoides, whereas cell wall thickening ceased in September (DOY: 259 ± 26) and October (DOY: 274 ± 34) in 2020 and 2021, respectively (Fig. 3e). The enlarging zone in S. wallichii appeared in May (DOY: 146 ± 23) and stopped in November (DOY: 325 ± 33) in 2020, while it occurred in April (DOY: 117 ± 12) and ceased in December (DOY: 364 ± 8) in 2021 (Fig. 3d). However, the wall-thickening zone in S. wallichii ceased in early February (DOY: 34 ± 20) and later February (DOY: 50 ± 18) in 2021 and 2022, respectively (Fig. 3f).
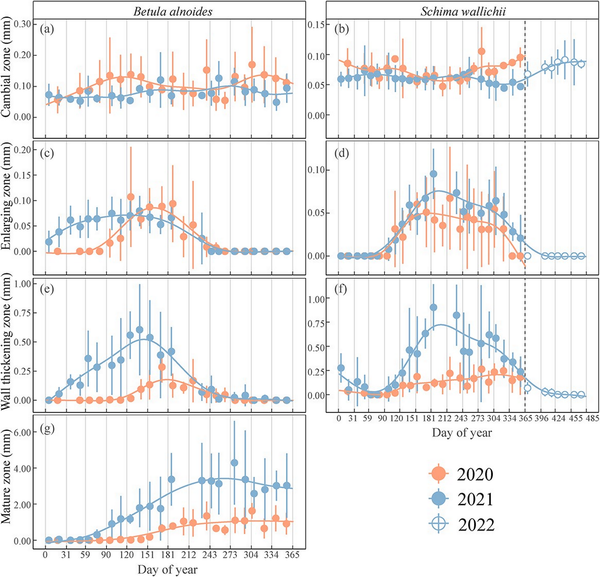
Figure 3
The thickness of (a, b) the cambial zone, (c, d) the enlarging zone, (e, f) the wall-thickening zone and (g) the mature zone in B. alnoides and S. wallichii during 2020 to 2021. Dots and bars represent means and standard deviations among five trees, respectively. Solid curves show fitting lines of generalized additive models based on mean values. The hollow dots with bars in b, d and f show the xylogenesis of S. wallichii between January and April 2022 to display an entire growing season.
The xylogenesis of both B. alnoides (P < 0.01) and S. wallichii (P = 0.04) started significantly later in 2020 than in 2021 (Fig. 4a). Furthermore, both onset (P2020 = 0.04; P2021 < 0.01) and cessation (P2020 < 0.01; P2021 < 0.01) of xylem formation were significantly earlier for B. alnoides than S. wallichii during the two years (Fig. 4a and b). The xylem growth duration of B. alnoides was significantly longer in 2021 than in 2020 (P < 0.01) (Fig. 4c). Betula alnoides grew significantly shorter than S. wallichii in 2020 (P < 0.01), while there was no significant difference in 2021 (P = 0.13) (Fig. 4c). In addition, annual xylem growth for B. alnoides was significantly lower in 2020 than in 2021 (P = 0.02) (Fig. 4d).
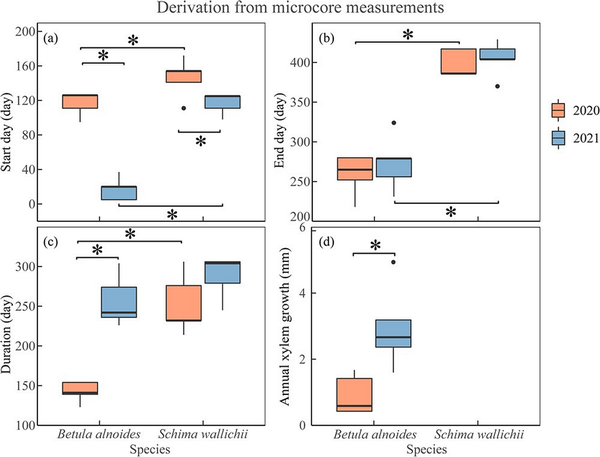
Figure 4
Boxplots of (a) start day, (b) end day, (c) duration and (d) annual xylem growth of B. alnoides and S. wallichii during 2020 to 2021. The data were derived from microcore measurements. The annual xylem growth of S. wallichii could not be measured due to its indistinct ring boundary. Asterisks indicate the significance level at P < 0.05.
Intra-annual radial increments
Radial increments of B. alnoides monitored by dendrometers mainly occurred from April (DOY: 115 ± 4) to October (DOY: 283 ± 48) in 2020 and from January (DOY: 17 ± 13) to October (DOY: 274 ± 34) in 2021 (Fig. 5a and e). A distinct tree water deficit of B. alnoides was observed between March and April in 2020, but not in 2021 (Fig. 5c). For S. wallichii, radial increments appeared during May (DOY: 138 ± 13) to late September (DOY: 268 ± 8) in 2020 and during April (DOY: 110 ± 22) to the end of October (DOY: 295 ± 3) in 2021 (Fig. 5b and f). Schima wallichii showed a strong tree water deficit from March to April in both years; however, being much more pronounced in 2020 than 2021 (Fig. 5d).
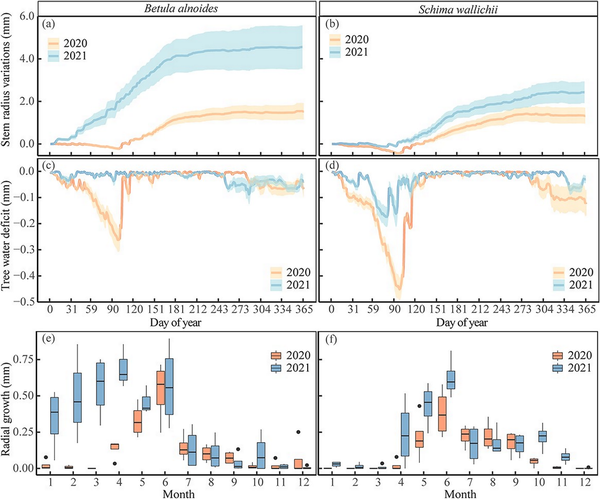
Figure 5
(a, b) Cumulative daily stem radius variations, (c, d) daily tree water deficit and (e, f) monthly radial growth of B. alnoides and S. wallichii during 2020 to 2021. Lines in a-d represent mean values and shaded areas represent standard errors among four trees.
The onset of radial increments of B. alnoides was significantly later in 2020 than in 2021 (P < 0.01), while radial increments started significantly earlier in B. alnoides than S. wallichii during 2020 (P = 0.03) and 2021 (P < 0.01) (Fig. 6a). Growth cessation was significantly later (P < 0.01) and growth duration was significantly longer (P = 0.01) for S. wallichii in 2021 than in 2020 (Fig. 6b and c). The daily maximum growth rate (P = 0.01) and annual radial increments (P = 0.049) of B. alnoides were significantly lower in 2020 than in 2021 (Fig. 6d and e). Further, B. alnoides in 2020 showed significantly higher daily maximum growth rates compared with S. wallichii in 2021 (P = 0.01) (Fig. 6d).
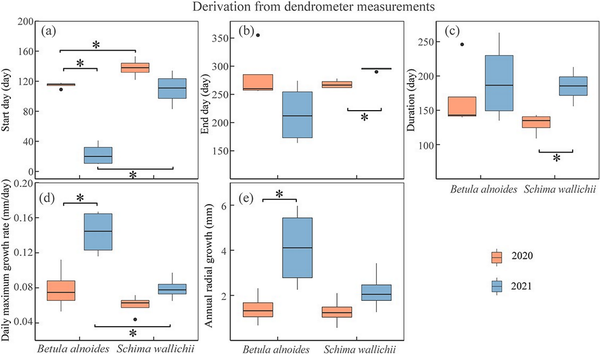
Figure 6
Boxplots of (a) start day, (b) end day, (c) duration, (d) daily maximum growth rate and (e) annual radial growth of B. alnoides and S. wallichii during 2020 to 2021. The data were derived from dendrometer measurements. Asterisks indicate the significance level at P < 0.05.
Effects of climate variables on xylogenesis and radial increments
Minimum and mean air temperature had a positive effect on the wall-thickening zone width of both species and the enlarging zone width of S. wallichii (Fig. 7). In addition, the thickness of enlarging zone and wall-thickening zone for S. wallichii was positively related to relative humidity and negatively related to VPD (Fig. 7).
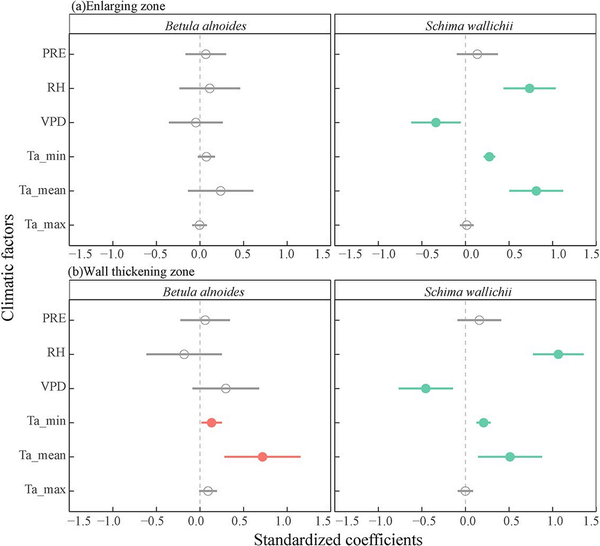
Figure 7
Effects of each weekly climatic factors prior to the sampling date on the thickness of (a) enlarging zone and (b) wall-thickening zone for B. alnoides and S. wallichii during 2020 to 2021. Significant levels (P < 0.05) were marked in closed circles. PRE, precipitation; RH, relative humidity; VPD, vapor pressure deficit; Ta_min, minimum air temperature; Ta_mean, mean air temperature; Ta_max, maximum air temperature.
Weekly radial increments of both B. alnoides and S. wallichii were significantly and positively correlated with precipitation and relative humidity, but negatively correlated with VPD and maximum air temperature (Fig. 8). The mean air temperatures showed significant negative effects on the radial increments of B. alnoides (Fig. 8a). However, the radial increments of S. wallichii were positively correlated with minimum and mean air temperatures (Fig. 8b).
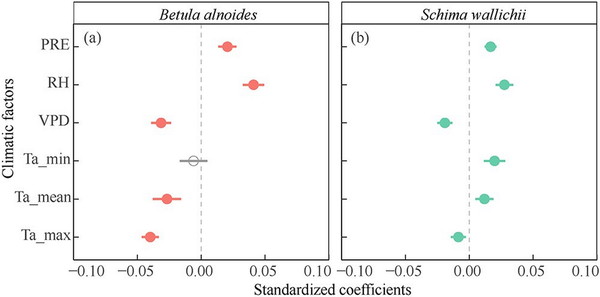
Figure 8
Effects of each climatic factor on weekly radial growth for (a) B. alnoides and (b) S. wallichii during 2020 to 2021. Significant levels (P < 0.05) were marked in closed circles. PRE, precipitation; RH, relative humidity; VPD, vapor pressure deficit; Ta_min, minimum air temperature; Ta_mean, mean air temperature; Ta_max, maximum air temperature.
Compared with S. wallichii, the radial increment of B. alnoides occurred within a wider range of temperatures (13 to 28 °C) and precipitation (0 to 120 mm) (Fig. 9a and d). However, the radial increments of both species were highest at 24 to 28 °C or VPD below 0.8 kPa (Fig. 9a and b). The two species almost ceased their radial increments when VPD reached a level higher than 1.2 kPa or RH was below 70% (Fig. 9b and c). In addition, the radial increments of S. wallichii nearly stopped during the period when there was no precipitation (Fig. 9d).
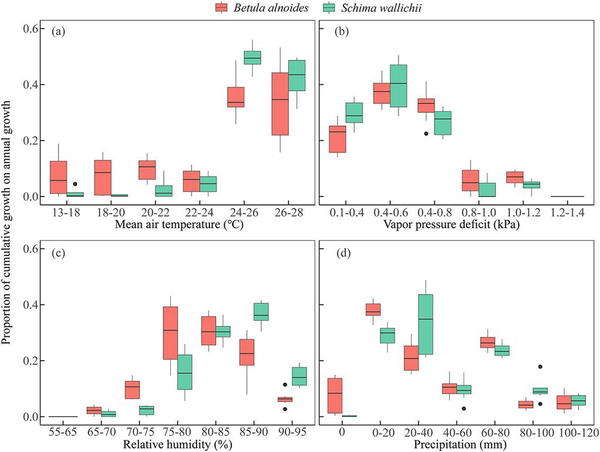
Figure 9
Boxplots of the proportion of cumulative growth on annual growth in specific climatic intervals for B. alnoides and S. wallichii during 2020 to 2021.
Comparison of microcoring and dendrometer methods
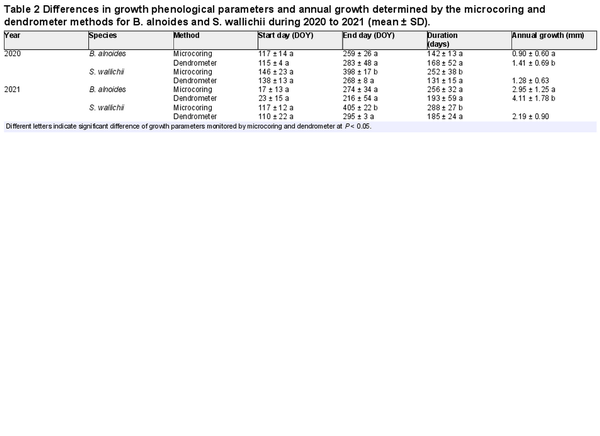
There were non-significant differences in the dates of growth onset between the microcoring and dendrometer methods for both species and both years. However, the dates of growth cessation and growth duration of S. wallichii determined by microcoring method were approximately 100 days later than those of dendrometer measurements in 2020 and 2021 (Table 2). The annual radial increments of B. alnoides determined by dendrometer method were around 0.5 mm and 1 mm higher than the xylem growth detected by microcoring method in 2020 and 2021, respectively (Table 2).
Discussion
Intra-annual xylem formation and radial increments
We found that both xylogenesis and radial increments of the two studied species in 2020 started three months (B. alnoides) and one month (S. wallichii) later compared with 2021 (Figs 3 and 5). Delayed growth onset may result from higher temperature, lower RH and higher VPD during the pre-monsoon season in 2020 (Fig. 2). Increased VPD could potentially reduce vegetation growth with global warming (). The high temperature anomaly during the pre-monsoon season in 2020 (see Fig. S4, Table S1 available as Supplementary data at Tree Physiology Online) may also intensify the effect of drought through altering plant carbon metabolism (). Similar to our results, the xylogenesis onset of Pinus ponderosa located in the Mojave Desert Mountain delayed two months in a year with hyper-arid spring compared with the year with wetter spring conditions (). Moisture conditions in the pre-monsoon season are an important driver for the start of cambial activity. observed that frequent watering during the pre-monsoon season reactivated cambial cell division of Samanea saman in subtropical Bangladesh. Delayed xylem formation in response to lower pre-monsoon moisture availability could be a strategy against hydraulic failure under dry conditions ().
Both wood formation and radial increments of B. alnoides were significantly lower in 2020 than in 2021 (Figs 4 and 6). Annual radial increments of S. wallichii were also lower in 2020 than 2021, but statistically non-significant (Fig. 6). These differences between the two species might be related to their different intra-annual growth dynamics. Betula alnoides started growing three months earlier than S. wallichii in 2021. Meanwhile, we observed that B. alnoides began budburst and flushed new leaves between December and January, but S. wallichii experienced peak leaf flushing after leaf fall in March. In 2020, B. alnoides experienced extreme drought in the early-growing season, however the growth initiation of S. wallichii occurred after the drought event. Hence, the timing of drought events may determine the extent to which radial growth is constrained. Stem radial growth sharply reduced during the early-growing season drought in Eastern North America (), while annual radial growth reduction was not observed under the European heatwave during the late-growing season in 2018 (). In addition, deciduous tree species have lower xylem cavitation resistance than evergreen tree species (), which may result in greater sensitivity to water stress for the deciduous species B. alnoides.
Effects of climatic factors on xylem formation and radial increments
We observed that the thickness of enlarging zone and wall-thickening zone for S. wallichii showed a positive response to RH, but showed a negative response to VPD (Fig. 7). Meanwhile, precipitation and RH were positively correlated with radial increments, while VPD negatively impacted the radial increments of two species (Fig. 8), indicating that stem radial increments were primarily moisture-limited in the tropical montane evergreen broad-leaved forest. Both species almost ceased their growth when no precipitation, RH below 70% or a VPD over 1.2 kPa (Fig. 9). Conversely, tree water deficits for both species occurred during the dry season (Fig. 5), especially during the warmer and drier period in 2020. Similarly, the radial growth of Pinus kesiya var. langbianensis coincided with precipitation, and tree water deficit occurred in drought in a subtropical forest in Yunnan at mid-elevations (). In a drier site, most tree species showed stronger stem shrinkage in tropical forest (). found that stem radial growth primarily depends on water conditions and secondarily on carbon allocation. That is caused by the fact that turgor-driven cell enlarging is mostly affected by water availability, whereas the process of cell-wall thickening may rely on the availability of non-structural carbohydrates (). Water availability is the most important factor explaining the production of xylem cells in black spruce saplings in a greenhouse (). Correspondingly, the width of the cambial zone was positively related to monthly precipitation in evergreen moist rain forests of Ivory Coast and Thailand (; ).
The radial increments of both species were negatively correlated to maximum air temperature, and B. alnoides was also restricted by mean air temperature (Fig. 8). The negative effect of temperature on radial increments is mainly linked with a higher VPD, which limited stem growth by reducing turgor pressure (). also revealed that high air temperature probably enhancing evapotranspiration rates and negatively affected the radial increments in two tropical dry forests. However, the wall-thickening zone width of both species was positively correlated with minimum and mean air temperature (Fig. 7). The radial increments as well as the thickness of enlarging zone for S. wallichii were positively related to minimum and mean air temperature (Figs 7 and 8). Similarly, found that stem radial growth of Cedrela montana in a humid mountain rainforest in Ecuador was positively related to temperature from January to April. Radial growth of Quercus faginea was limited by temperature in spring but limited by precipitation in summer in central Portugal ().
Though the radial increments of B. alnoides and S. wallichii responded differently to temperature, both species showed peak of growth at 24 to 28 °C (Fig. 9). Stem radial growth of three temperate broadleaved tree species peaked at 12 to 18 °C in northeastern Germany (). Maximum xylem cell production of beech occurred at around 16 °C in Slovenia (). Moreover, balsam fir started radial growth above 9 to 10 °C in a cold and humid environment (). The temperatures during radial growth occurrence in these temperate and boreal sites were lower than those of our study.
Comparison of microcoring and dendrometer methods
In our study, xylem formation and radial increments were simultaneously monitored by applying microcoring and dendrometer measurements. The annual radial increments obtained from dendrometer were higher compared with microcoring method (Table 2), which was consistent with the study by . Dendrometer measurements included phloem growth, though water-driven radius fluctuations were minimized with the zero-growth model (). Moreover, the annual radial increments recorded by dendrometer derived from stem circumference, whereas ring width from microcoring captured radial growth in only one position (). Consequently, both approaches inevitably show differences in annual radial growth.
Given that the wall thickening period failed to increase the number of xylem cells, growth cessation determined by microcoring data was later than dendrometer method. Meanwhile, stem radial variations were particularly susceptible to water fluctuations when the xylem growth rate was low (; ), so the growth cessation dates monitored by dendrometer method likely deviated from the microcoring method, especially in the late growing season. Correspondingly, we found that stem radial growth of S. wallichii derived from dendrometer method ceased significantly earlier in 2020 than 2021 (Fig. 6), as the VPD in 2020 was higher than 2021 in September (Fig. 2). Similar to our results, showed that the date of growth cessation monitored by dendrometers was one month earlier than that of the microcoring method. On the contrary, found that the growth cessation dates derived from microcoring measurements were earlier than those from dendrometer data, resulting from the fact that stem swelling increased the radial increments during the late-growing season. For coniferous trees, the application of an absolute threshold from dendrometer data, such as concrete growth rate, might be precise and unbiased for growth cessation in the southern Black Forest (). However, it is unknown whether the absolute threshold value is fit for the identification of growth ended date in tropical forests.
Compared with microcoring, dendrometer measurements are suitable for long-term monitoring but are unable to reveal the accurate growth onset and cessation dates. Hence, models combining microcoring data with high-resolution dendrometer data are required to accurately estimate stem growth parameters. Under climate change, the frequency of drought events has gradually increased in Yunnan province during the past decades (). Pre-monsoon drought events caused significant growth reductions in B. alnoides but not in S. wallichii in 2020. Therefore, we should pay more attention to the earlier growth onset tree species, which are more vulnerable to extreme pre-monsoon drought events.
Conclusion
This study simultaneously observed wood formation and stem radial variations of a deciduous tree species B. alnoides and an evergreen tree species S. wallichii during two years with differently hydrothermal pre-monsoon season in a tropical montane broad-leaved forest, southwest China. We found that the onset of xylogenesis was delayed by three months in B. alnoides and by one month in S. wallichii in 2020 (with a warmer and drier pre-monsoon season) compared with 2021. In addition, a significant growth reduction was observed in B. alnoides but not in S. wallichii during 2020. Both thickness of enlarging zone and wall-thickening zone for S. wallichii were positively correlated to relative humidity and negatively correlated to VPD. Weekly radial increments of both species showed positive correlations with precipitation and RH, and negative correlations to VPD and maximum air temperature, indicating that stem radial increments of these two species was mainly limited by water availability. Our results showed that tree species with an earlier growth onset were increasingly susceptible to pre-monsoon drought under climate warming conditions.
Acknowledgments
We thank the Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies for providing the underlying climate data used in this study. We thank the Xishuangbanna National Nature Reserve Administration Bureau for providing logistic support during field work of the present study. We also thank Ma Hong for his assistance on field sampling.
References
- Bauman D, Fortunel C, Delhaye G, Malhi Y, Cernusak LA, Bentley LP, Rifai SW, Aguirre-Gutiérrez J, Menor IO, Phillips OL, et al 2022. Tropical tree mortality has increased with rising atmospheric water stress. Nature. 608(7923):528–533. https://doi.org/10.1038/s41586-022-04737-7.
- Bi YF, Whitney C, Li JW, Yang JC, Yang XF. 2020. Spring moisture availability is the major limitation for pine forest productivity in Southwest China. Forests. 11(4):446. https://doi.org/10.3390/f11040446.
- Bonan GB. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science. 320(5882):1444–1449. https://doi.org/10.1126/science.1155121.
- Bräuning A, Volland-Voigt F, Burchardt I, Ganzhi O, Nauss T, Peters T. 2009. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde. 63(4):337–345. https://doi.org/10.3112/erdkunde.2009.04.04.
- Callado CH, Roig FA, Tomazello-Filho M, Barros CF. 2013. Cambial growth periodicity studies of south American woody species – a review. IAWA J. 34(3):213–230. https://doi.org/10.1163/22941932-00000019.
- Chen YZ, Rademacher T, Fonti P, Eckes-Shephard AH, LeMoine JM, Fonti MV, Richardson AD, Friend AD. 2022. Inter-annual and inter-species tree growth explained by phenology of xylogenesis. New Phytol. 235(3):939–952. https://doi.org/10.1111/nph.18195.
- Cruz-García R, Balzano A, Čufar K, Scharnweber T, Smiljanić M, Wilmking M. 2019. Combining dendrometer series and xylogenesis imagery—DevX, a simple visualization tool to explore plant secondary growth phenology. Front For Glob Change. 2:60. https://doi.org/10.3389/ffgc.2019.00060.
- Cuny HE, Rathgeber CBK, Kiessé TS, Hartmann FP, Barbeito I, Fournier M. 2013. Generalized additive models reveal the intrinsic complexity of wood formation dynamics. J Exp Bot. 64(7):1983–1994. https://doi.org/10.1093/jxb/ert057.
- De Micco V, Carrer M, Rathgeber CBK, Julio Camarero J, Voltas J, Cherubini P, Battipaglia G. 2019. From xylogenesis to tree rings: wood traits to investigate tree response to environmental changes. IAWA J. 40(2):155–182. https://doi.org/10.1163/22941932-40190246.
- Debel A, Foroozan Z, Häusser M, Raspe S, Bräuning A. 2024. Assessing intra-annual growth dynamics in climatically contrasting years, sites, and tree species using dendrometers and wood anatomical data. Front For Glob Change. 7:1342413. https://doi.org/10.3389/ffgc.2024.1342413.
- Deslauriers A, Rossi S, Anfodillo T. 2007. Dendrometer and intra-annual tree growth: what kind of information can be inferred? Dendrochronologia. 25(2):113–124. https://doi.org/10.1016/j.dendro.2007.05.003.
- Deslauriers A, Huang JG, Balducci L, Beaulieu M, Rossi S. 2016. The contribution of carbon and water in modulating wood formation in black spruce saplings. Tree Physiol. 170:2072–2084.
- Dié A, Kitin P, Kouamé FNG, Van den Bulcke J, Van Acker J, Beeckman H. 2012. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann Bot. 110(4):861–873. https://doi.org/10.1093/aob/mcs145.
- Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J. 1994. Carbon pools and flux of global forest ecosystems. Science. 263(5144):185–190. https://doi.org/10.1126/science.263.5144.185.
- D'Orangeville L, Maxwell J, Kneeshaw D, Pederson N, Duchesne L, Logan T, Houle D, Arseneault D, Beier CM, Bishop DA, et al 2018. Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Glob Chang Biol. 24(6):2339–2351. https://doi.org/10.1111/gcb.14096.
- Duursma RA. 2015. Plantecophys-an R package for analysing and modelling leaf gas exchange data. PloS One. 10(11):e0143346. https://doi.org/10.1371/journal.pone.0143346.
- Fan ZX, Bräuning A, Fu PL, Yang RQ, Qi JH, Grießinger J, Gebrekirstos A. 2019. Intra-annual radial growth of Pinus kesiya var. langbianensis is mainly controlled by moisture availability in the Ailao Mountains, southwestern China. Forests. 10(10):899. https://doi.org/10.3390/f10100899.
- Fu PL, Jiang YJ, Wang AY, Brodribb TJ, Zhang JL, Zhu SD, Cao KF. 2012. Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Ann Bot. 110(1):189–199. https://doi.org/10.1093/aob/mcs092.
- Hu LF, Fan ZX. 2016. Stem radial growth in response to microclimate in an Asian tropical dry karst forest. Acta Ecol Sin. 36(5):401–409. https://doi.org/10.1016/j.chnaes.2016.09.005.
- Hubau W, Lewis SL, Phillips OL, Affum-Baffoe K, Beeckman H, Cuní-Sanchez A, Daniels AK, Ewango CEN, Fauset S, Mukinzi JM, et al 2020. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature. 579(7797):80–87. https://doi.org/10.1038/s41586-020-2035-0.
- Kaewmano A, Fu PL, Fan ZX, Pumijumnong N, Zuidema PA, Bräuning A. 2022. Climatic influences on intra-annual stem radial variations and xylem formation of Toona ciliata at two Asian tropical forest sites with contrasting soil water availability. Agric For Meteorol. 318:108906. https://doi.org/10.1016/j.agrformet.2022.108906.
- Kamil B. 2022. MuMIn: multi-model inference. R package version 1.46.0. https://CRAN.R-project.org/package=MuMIn.
- Khanduri VP, Sharma CM, Kumar KS, Ghildiyal SK. 2013. Annual variation in flowering phenology, pollination, mating system, and pollen yield in two natural populations of Schima wallichii (DC.) Korth. Scientific World J. 2013(1):350157. https://doi.org/10.1155/2013/350157.
- Knüsel S, Peters RL, Haeni M, Wilhelm M, Zweifel R. 2021. Processing and extraction of seasonal tree physiological parameters from stem radius time series. Forests. 12(6):765. https://doi.org/10.3390/f12060765.
- Mendivelso HA, Camarero JJ, Gutiérrez E, Castaño-Naranjo A. 2016. Climatic influences on leaf phenology, xylogenesis and radial stem changes at hourly to monthly scales in two tropical dry forests. Agric For Meteorol. 216:20–36. https://doi.org/10.1016/j.agrformet.2015.09.014.
- Miller TW, Stangler DF, Larysch E, Honer H, Seifert T, Kahle H-P. 2022. A methodological framework to optimize models predicting critical dates of xylem phenology based on dendrometer data. Dendrochronologia. 72:125940. https://doi.org/10.1016/j.dendro.2022.125940.
- Morel H, Mangenet T, Beauchêne J, Ruelle J, Nicolini E, Heuret P, Thibaut B. 2015. Seasonal variations in phenological traits: leaf shedding and cambial activity in Parkia nitida Miq. and Parkia velutina Benoist (Fabaceae) in tropical rainforest. Trees. 29(4):973–984. https://doi.org/10.1007/s00468-015-1177-4.
- Oogathoo S, Duchesne L, Houle D, Kneeshaw D, Bélanger N. 2023. Seasonal, monthly, daily, and diel growth, and water status dynamics of balsam fir in a cold and humid boreal environment. Forests. 14(4):802. https://doi.org/10.3390/f14040802.
- Peters RL, Kaewmano A, Fu PL, Fan ZX, Sterck F, Steppe K, Zuidema PA. 2023. High vapour pressure deficit enhances turgor limitation of stem growth in an Asian tropical rainforest tree. Plant Cell Environ. 46(9):2747–2762. https://doi.org/10.1111/pce.14661.
- Pinheiro J, Bates D, DebRoy S, Sarkar D. 2020. Nlme: linear and nonlinear mixed effects models. R package version 3.1–147. https://CRAN.R-project.org/package=nlme.
- Pokhrel Y, Felfelani F, Satoh Y, Boulange J, Burek P, Gädeke A, Gerten D, Gosling SN, Grillakis M, Gudmundsson L, et al 2021. Global terrestrial water storage and drought severity under climate change. Nat Clim Change. 11(3):226–233. https://doi.org/10.1038/s41558-020-00972-w.
- Prislan P, Gričar J, de Luis M, Smith KT, Čufar K. 2013. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric For Meteorol. 180:142–151. https://doi.org/10.1016/j.agrformet.2013.06.001.
- Pumijumnong N, Songtrirat P, Buajan S, Preechamart S, Chareonwong U, Muangsong C. 2021. Climate control of cambial dynamics and tree-ring width in two tropical pines in Thailand. Agric For Meteorol. 303:108394. https://doi.org/10.1016/j.agrformet.2021.108394.
- Pumijumnong N, Muangsong C, Buajan S, Songtrirat P, Chatwatthana R, Chareonwong U. 2023. Factors affecting cambial growth periodicity and wood formation in tropical forest trees: a review. Forests. 14(5):1025. https://doi.org/10.3390/f14051025.
- R Core Team. 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rahman M, Islam M, Gebrekirstos A, Bräuning A. 2019a. Trends in tree growth and intrinsic water-use efficiency in the tropics under elevated CO2 and climate change. Trees. 33(3):623–640. https://doi.org/10.1007/s00468-019-01836-3.
- Rahman MH, Nugroho WD, Nakaba S, Kitin P, Kudo K, Yamagishi Y, Begum S, Marsoem SN, Funada R. 2019b. Changes in cambial activity are related to precipitation patterns in four tropical hardwood species grown in Indonesia. Am J Bot. 106(6):760–771. https://doi.org/10.1002/ajb2.1297.
- Rahman MH, Begum S, Nugroho WD, Nakaba S, Funada R. 2022. The effects of watering on cambial activity in the stems of evergreen hardwood (Samanea saman) during the pre-monsoon season in subtropical Bangladesh. J Wood Sci. 68(1):47. https://doi.org/10.1186/s10086-022-02053-2.
- Ren P, Rossi S, Gricar J, Liang EY, Cufar K. 2015. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan plateau? Ann Bot. 115(4):629–639. https://doi.org/10.1093/aob/mcu259.
- Rossi S, Deslauriers A, Morin H. 2003. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 21(1):33–39. https://doi.org/10.1078/1125-7865-00034.
- Rossi S, Anfodillo T, Menardi R. 2006. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 27(1):89–97. https://doi.org/10.1163/22941932-90000139.
- Rossi S, Anfodillo T, Čufar K, Cuny HE, Deslauriers A, Fonti P, Frank D, Gričar J, Gruber A, Huang JG, et al 2016. Pattern of xylem phenology in conifers of cold ecosystems at the northern hemisphere. Glob Chang Biol. 22(11):3804–3813. https://doi.org/10.1111/gcb.13317.
- Salomón RL, Peters RL, Zweifel R, Sass-Klaassen UGW, Stegehuis AI, Smiljanic M, Poyatos R, Babst F, Cienciala E, Fonti P, et al 2022. The 2018 European heatwave led to stem dehydration but not to consistent growth reductions in forests. Nat Commun. 13(1):28. https://doi.org/10.1038/s41467-021-27579-9.
- Sharma B, Fan ZX, Panthi S, Gaire NP, Fu PL, Zaw Z. 2022. Warming induced tree-growth decline of Toona ciliata in (sub-) tropical southwestern China. Dendrochronologia. 73:125954. https://doi.org/10.1016/j.dendro.2022.125954.
- Silva MDS, Funch LS, da Silva LB. 2019. The growth ring concept: seeking a broader and unambiguous approach covering tropical species. Biol Rev. 94(3):1161–1178. https://doi.org/10.1111/brv.12495.
- Stangler DF, Kahle H-P, Raden M, Larysch E, Seifert T, Spiecker H. 2021. Effects of intra-seasonal drought on kinetics of tracheid differentiation and seasonal growth dynamics of Norway spruce along an elevational gradient. Forests. 12(3):274. https://doi.org/10.3390/f12030274.
- Tumajer J, Scharnweber T, Smiljanic M, Wilmking M. 2022. Limitation by vapour pressure deficit shapes different intra-annual growth patterns of diffuse- and ring-porous temperate broadleaves. New Phytol. 233(6):2429–2441. https://doi.org/10.1111/nph.17952.
- van der Maaten E, Pape J, van der Maaten-Theunissen M, Scharnweber T, Smiljanić M, Cruz-García R, Wilmking M. 2018. Distinct growth phenology but similar daily stem dynamics in three co-occurring broadleaved tree species. Tree Physiol. 38(12):1820–1828. https://doi.org/10.1093/treephys/tpy042.
- Vieira J, Campelo F, Nabais C. 2022. Environment controls seasonal and daily cycles of stem diameter variations in Portuguese oak (Quercus faginea Lambert). Forests. 13(2):170. https://doi.org/10.3390/f13020170.
- Wang CS, Guo JJ, Hein S, Wang H, Zhao ZG, Zeng J. 2019. Foliar morphology and spatial distribution in five-year-old plantations of Betula alnoides. For Ecol Manage. 432:514–521. https://doi.org/10.1016/j.foreco.2018.09.052.
- Wang ZG, Wang CK. 2023. Interactive effects of elevated temperature and drought on plant carbon metabolism: a meta-analysis. Glob Chang Biol. 29(10):2824–2835. https://doi.org/10.1111/gcb.16639.
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 73(1):3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x.
- Worbes M. 2002. One hundred years of tree-ring research in the tropics – a brief history and an outlook to future challenges. Dendrochronologia. 20(1–2):217–231. https://doi.org/10.1078/1125-7865-00018.
- Yang RQ, Fu PL, Fan ZX, Panthi S, Gao J, Niu Y, Li ZS, Bräuning A. 2022. Growth-climate sensitivity of two pine species shows species-specific changes along temperature and moisture gradients in Southwest China. Agric For Meteorol. 318:108907. https://doi.org/10.1016/j.agrformet.2022.108907.
- Yuan WP, Zheng Y, Piao SL, Ciais P, Lombardozzi D, Wang YP, Ryu Y, Chen GX, Dong WJ, Hu ZM, et al 2019. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci Adv. 5(8):1–13. https://doi.org/10.1126/sciadv.aax1396.
- Zhao RX, Yang SQ, Sun HQ, Zhou L, Li M, Xing LS, Tian R. 2023. Extremeness comparison of regional drought events in Yunnan province, Southwest China: based on different drought characteristics and joint return periods. Atmos. 14(7):1153. https://doi.org/10.3390/atmos14071153.
- Zhu H, Cao M, Hu HB. 2006. Geological history, flora, and vegetation of Xishuangbanna, southern Yunnan, China. Biotropica. 38(3):310–317. https://doi.org/10.1111/j.1744-7429.2006.00147.x.
- Ziaco E, Truettner C, Biondi F, Bullock S. 2018. Moisture-driven xylogenesis in Pinus ponderosa from a Mojave Desert mountain reveals high phenological plasticity. Plant Cell Environ. 41(4):823–836. https://doi.org/10.1111/pce.13152.
- Zweifel R, Item H, Häsler R. 2000. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees. 15(1):50–57. https://doi.org/10.1007/s004680000072.
- Zweifel R, Haeni M, Buchmann N, Eugster W. 2016. Are trees able to grow in periods of stem shrinkage? New Phytol. 211(3):839–849. https://doi.org/10.1111/nph.13995.
- Zweifel R, Sterck F, Braun S, Buchmann N, Eugster W, Gessler A, Häni M, Peters RL, Walthert L, Wilhelm M, et al 2021. Why trees grow at night. New Phytol. 231(6):2174–2185. https://doi.org/10.1111/nph.17552.