Introduction
Acute leukemia is the most common cancer in children with an age-standardized incidence rate of 30–40 per million [, ]. The standard treatment is conventional chemotherapy (CCT), which has an overall survival (OS) rate of 70–95% and 40–60% for acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), respectively, with rates varying according to type and risk classification []. When alternative medicine (AM) is used to treat cancer, it is usually in conjunction with CCT, with different studies finding rates of 10–80% for such combined treatment [-]. There are limited studies comparing the outcomes of AM alone versus CCT, especially in pediatric leukemia.
Refusal of CCT in favor of AM upon initial diagnosis of cancer may have serious survival implications [, ]. Pediatricians, especially from developing countries such as Thailand, face the challenge of convincing families, who believe in the use of AM alone, to accept CCT. There are only a few studies evaluating the effectiveness of AM alone without CCT on survival outcomes among children, but studies on adults with breast, lung, prostate, and colon cancer have found poorer survival outcomes for patients receiving AM alone compared with CCT [, -]. The objective of this study was to compare survival outcomes between patients receiving AM alone and those receiving CCT and to assess prognostic factors for those who received AM alone among newly diagnosed child leukemia patients.
Materials and Methods
We retrospectively reviewed the hematological records of 1,149 children aged < 15 years diagnosed with acute leukemia (either ALL or AML) between January 1982 and December 2016, and treated at the Oncology Clinic, Department of Pediatrics, Faculty of Medicine, Prince of Songkla University, which is the major tertiary-referral center and the only medical institution specializing in pediatric oncology in southern Thailand. Patients are referred here for investigation, treatment, and follow-up of all types of cancers. The information recorded for each child included demographic characteristics, clinical manifestations at diagnosis, and initial laboratory investigations such as complete blood count (CBC), blood chemistry, subtype of acute leukemia (ALL or AML), and year of diagnosis. Diagnosis of AML or ALL and subtype was made according to the French-American-British (FAB) classification by morphological examination of bone marrow staining in all patients. Cytochemical stains included periodic acid-Schiff (PAS), peroxidase and α-naphthyl acetate esterase. After the year 2000, immunophenotyping of cell surface markers was additionally used to differentiate subtypes (i.e., AML from ALL, and T-cell ALL from B-cell ALL).
The treatment for each child was reviewed. Those with newly diagnosed acute leukemia who received a standard treatment protocol of chemotherapy according to their subtype of leukemia and risk stratification were classified as receiving CCT. Those who were given any form of treatment that was perceived by our medical personnel to be ineffective at the time of diagnosis, and administered by nonmedical personnel and used instead of CCT, were classified as receiving AM. Patients who had previous treatment with chemotherapy or those who relapsed and subsequently received AM treatment were excluded. Two-to-one nearest-neighbor propensity score-matching with age, sex, initial white blood cell count, phenotype of leukemia, and period of diagnosis was performed to create comparable risk groups of children receiving CCT and AM alone.
Statistical Analysis
Demographic characteristics and clinical factors were compared using the χ2 test and the t test for categorical and continuous variables, respectively. Two-to-one nearest-neighbor propensity score-matching without replacement was performed using the MatchIt package in R (R Foundation for Statistical Computing, Vienna, Austria). Univariate survival analyses were performed using the Kaplan-Meier estimator, log-rank test, and Cox proportional-hazards regression. Variables with a p value ≤0.10 on univariate analysis were entered into a multivariable Cox proportional-hazards survival model using forced entry for the 2: 1 matched sample (propensity score-matching). The assumption of proportionality was verified graphically using log-to-log survival plots. All statistical tests were two-sided and p < 0.05 was considered statistically significant.
Results
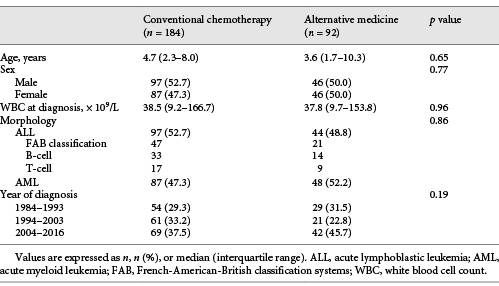
A total of 1,149 newly diagnosed children with acute leukemia were identified, 76 of whom were excluded due to incomplete data, leaving a total of 1,073 children for analysis. Of these, 92 (8.6%) received AM treatment as the first-line therapy and the remaining 981 were treated with standard CCT. All of the AM patients were treated with local herbal medicine.
The 92 AM children (44 with ALL and 48 with AML) were matched to 981 children who received CCT following two-to-one nearest-neighbor propensity score-matching on age, sex, initial white blood cell count, leukemia phenotype, and period of diagnosis, to obtain 184 CCT patients. Comparisons of demographic and hematological characteristics of the 92 AM and 184 CCT patients are shown in Table 1. There were no differences in risk variables between the 2 groups.
Survival Outcome of Acute Leukemia
OS was worse among children in the AM-alone group (p < 0.001). The 5-year OS rate for the 184 CCT patients (ALL and AML combined) was 38.2%. Among the 92 patients receiving AM, the 5-year overall survival rate for the AM group was 0% with a median survival duration of 1 month. Figure 1 shows a comparison of OS of 184 CCT and 92 AM patients.
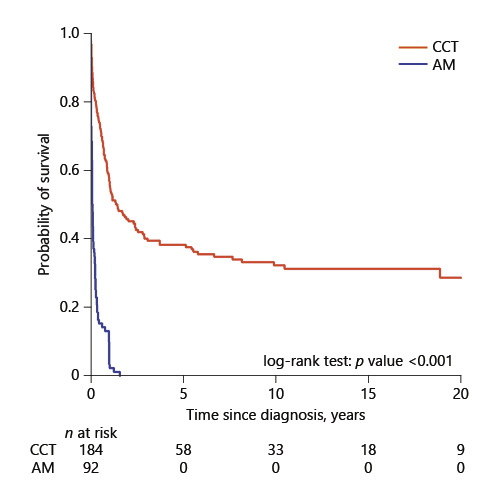
Fig. 1
Comparison of overall survival by treatment group among 276 patients. CCT, conventional chemotherapy; AM, alternative medicine.
Comparison of OS by Morphology
Among the patients with ALL, 97 received CCT and 44 received AM. Figure 2 compares the OS between these 2 groups. The 5-year and 10-year survival rate for the 97 ALL patients who received CCT was 54.6 and 50.1%, respectively. The median survival duration for the 44 AM patients was 1 month.
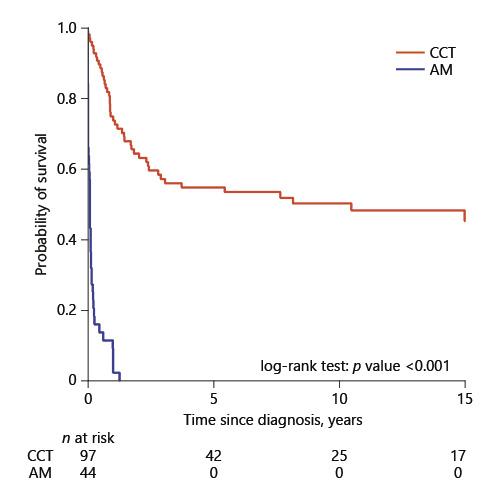
Fig. 2
Comparison of overall survival by treatment group among 141 ALL patients. CCT, conventional chemotherapy; AM, alternative medicine.
Among the patients with AML, 87 received CCT and 48 received AM. Figure 3 compares the OS between these 2 groups. Survival rates were similar for both groups during the induction phase before the curves separated in favor of CCT. The 5-year survival rate for the 87 AML patients who received CCT was 20.0%, while the 5-year survival rate for the 48 patients who received AM was 0% with a median survival duration of 1.5 months.
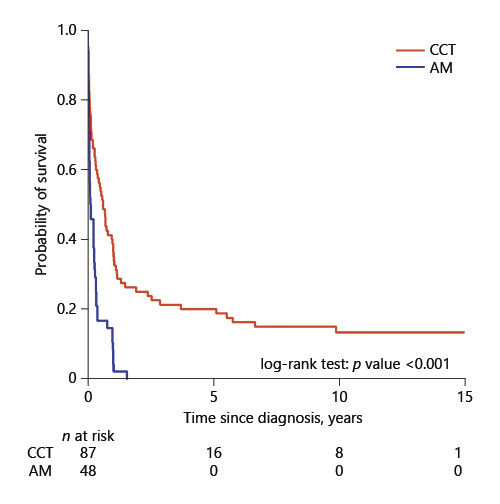
Fig. 3
Comparison of overall survival by treatment group among 135 AML patients. CCT, conventional chemotherapy; AM, alternative medicine.
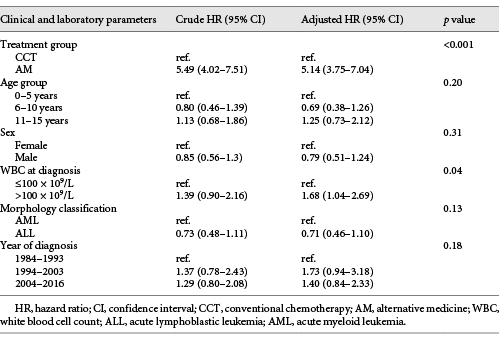
The results of the multivariate analysis for determining independent prognostic factors among all patients are shown in Table 2. Apart from treatment, white blood cell count at diagnosis of acute leukemia was the only independent factor related to OS. After adjusting for initial white blood cell count and morphology, patients receiving AM had a 5.14-times higher risk of death than patients receiving CCT.
Discussion
Acute leukemia is a common cancer in children []. The outcome of treatment is promising with standard CCT [, ]. However, some families decline CCT and prefer their children to receive AM alone as the first-line treatment. Due to the lack of studies evaluating the use and effectiveness of AM alone in childhood leukemia, it is difficult for pediatricians to give families evidence-based information on their child’s prognosis if they choose this form of treatment. Cassileth et al. [] found that 8% of adult breast cancer patients chose AM alone to treat their cancer, a figure comparable with our study among parents who cared for their children with leukemia (8.6%). Johnson et al. [] reported that overall, AM treatment alone was independently associated with a 2.5 times greater risk of death compared with CCT and among those with breast, lung, and colorectal cancer, the risk of death was higher, by factors of 5.7, 2.2, and 4.6, respectively. Refusal to receive CCT, therefore, has been documented to have a serious negative effect on the survival outcome of adult cancer patients []. The decision to rely on AM alone entails a significantly greater risk of death compared to CCT [, ]. Although there are studies on the outcomes of AM used alone to treat adult cancer patients, there are no studies to date documenting definitive evidence of clinical outcomes in childhood cancer patients. Our study found that the risk of death among childhood leukemia patients who relied on AM alone was 3.7–7.0 times higher than for those who received CCT alone.
AM covers a broad and diverse group of treatments and products such as local herbal medicine and botanicals, meditation, specialized diets, vitamins, homeopathy, massage, acupuncture, tai chi, and hypnosis, all of which do not tend to be widely use by conventional health care professionals [-]. Not only is AM not widely used by modern health care professionals, there are studies which indicate that it can even have a negative impact when used in conjunction with modern treatments []. For example, a study by Sparreboom et al. [] found that herbal remedies could cause adverse interactions with chemotherapy, and another study by Styczynski and Wysocki [] found that AM remedies might stimulate the viability of leukemia cells in vivo. On the other hand, when AM was used not as a substitute but in conjunction with CCT, studies showed an improved OS benefit for adults with AML, possibly due to the antioxidant effects of vitamin E and N-acetylcysteine, which resulted in a decrease in chemotherapy toxicity and a perceived positive effect to induce leukemic cell apoptosis in vitro [-]. These results need confirmation by more prospective studies for actual dosing and phase of treatment as well as balancing the risks and benefits of AM. It is important to note that the definition of AM differs by study in terms of treatment used with or without CCT []. Most studies conducted on pediatric cancer patients are surveys investigating the prevalence of AM used in conjunction with CCT, the type of AM, and the factors which influenced the decision to use AM such as sex, income, and family education [, , ].
There are many factors which influence the outcome of children with leukemia who receive CCT. These include the subtype of leukemia, clinical and biological risk stratification, and even the income level of the country [, ]. In high-income countries, the 5-year OS of ALL and AML patients is 95 and 60%, respectively [, ], while in low-to-middle income countries such as Thailand, this rate is 60–70% and 25–30%, respectively []. The factors which contribute to the lower survival from AML are disease severity itself, intensive treatment protocol, and supportive care. Thailand is a resource-limited country. Our setting has no isolation rooms and no air-conditioning. Induction death and septicemia within 1 month of treatment are critical problems in induction treatment of AML.
It is clear that AM alone entails a worse outcome when compared with CCT []. Although there are no studies comparing AM alone versus CCT in childhood leukemia, our multivariate analysis revealed that patients who received AM alone had a 5 times higher risk of death than patients treated with conventional CCT alone.
This study has some limitations which should be acknowledged. Results were based on a retrospective chart review over a 30-year period, thus preventing us from including potentially important variables in the analysis. Factors influencing a family’s decision to choose AM, such as household income and parent educational level, socioeconomic status, and specific types of AM such as specialized diets, homeopathy, and meditation are not routinely recorded. The use of specific types of alternative therapies by each patient/family can be a culturally sensitive issue and therefore not routinely recorded. The validity of the study is also limited by factors such as the period of diagnosis where there may have been differences in classification of ALL (from FAB to T-cell or B-cell ALL), method of diagnosis, which additionally used imunophenotyping of cell markers, the lack of cytogenetic and molecular studies, clinical risk classification, treatment protocols, and supportive care.
Nevertheless, our results indicate that the use of AM alone entails a higher risk of death than the use of CCT alone in childhood leukemia. We believe that our results can be useful in clinical practice to help pediatricians provide evidence-based advice and counseling to caregivers and to encourage patients to receive CCT.
Acknowledgement
We would like to thank Mr. Dave Patterson for his assistance with English editing. This manuscript has not been previously published and is not being submitted to any other journal at this time. All authors have made a significant contribution and have read and approved the final draft.
Disclosure Statement
There is nothing to declare. This work has no financial commercial interests.
References
- 1. Demanelis K, Sriplung H, Meza R, Wiangnon S, Rozek LS, Scheurer ME, et al Differences in childhood leukemia incidence and survival between Southern Thailand and the United States: a population-based analysis. Pediatr Blood Cancer. 2015;62(10):1790–8.
- 2. Wiangnon S, Kamsa-Ard S, Jetsrisuparb A, Sriplung H, Sontipong S, Sumitsawan Y, et al Childhood cancer in Thailand: 1995-1997. Asian Pac J Cancer Prev. 2003;4(4):337–43.
- 3. Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–65.
- 4. Gottschling S, Meyer S, Längler A, Scharifi G, Ebinger F, Gronwald B. Differences in use of complementary and alternative medicine between children and adolescents with cancer in Germany: a population based survey. Pediatr Blood Cancer. 2014;61(3):488–92.
- 5. Hamidah A, Rustam ZA, Tamil AM, Zarina LA, Zulkifli ZS, Jamal R. Prevalence and parental perceptions of complementary and alternative medicine use by children with cancer in a multi-ethnic Southeast Asian population. Pediatr Blood Cancer. 2009;52(1):70–4.
- 6. Naja F, Alameddine M, Abboud M, Bustami D, Al Halaby R. Complementary and alternative medicine use among pediatric patients with leukemia: the case of Lebanon. Integr Cancer Ther. 2011;10(1):38–46.
- 7. Bishop FL, Prescott P, Chan YK, Saville J, von Elm E, Lewith GT. Prevalence of complementary medicine use in pediatric cancer: a systematic review. Pediatrics. 2010;125(4):768–76.
- 8. Johnson SB, Park HS, Gross CP, Yu JB. Use of Alternative Medicine for Cancer and Its Impact on Survival. J Natl Cancer Inst. 2018;110(1):110.
- 9. Cassileth BR, Lusk EJ, Strouse TB, Bodenheimer BJ. Contemporary unorthodox treatments in cancer medicine. A study of patients, treatments, and practitioners. Ann Intern Med. 1984;101(1):105–12.
- 10. Han E, Johnson N, DelaMelena T, Glissmeyer M, Steinbock K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann Surg Oncol. 2011;18(4):912–6.
- 11. Chang EY, Glissmeyer M, Tonnes S, Hudson T, Johnson N. Outcomes of breast cancer in patients who use alternative therapies as primary treatment. Am J Surg. 2006;192(4):471–3.
- 12. Citrin DL, Bloom DL, Grutsch JF, Mortensen SJ, Lis CG. Beliefs and perceptions of women with newly diagnosed breast cancer who refused conventional treatment in favor of alternative therapies. Oncologist. 2012;17(5):607–12.
- 13. Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78.
- 14. Malik IA, Gopalan S. Use of CAM results in delay in seeking medical advice for breast cancer. Eur J Epidemiol. 2003;18(8):817–22.
- 15. Magi T, Kuehni CE, Torchetti L, Wengenroth L, Lüer S, Frei-Erb M. Use of Complementary and Alternative Medicine in Children with Cancer: A Study at a Swiss University Hospital. PLoS One. 2015;10(12):e0145787.
- 16. Martel D, Bussières JF, Théorêt Y, Lebel D, Kish S, Moghrabi A, et al Use of alternative and complementary therapies in children with cancer. Pediatr Blood Cancer. 2005;44(7):660–8.
- 17. Smith PJ, Clavarino A, Long J, Steadman KJ. Why do some cancer patients receiving chemotherapy choose to take complementary and alternative medicines and what are the risks?Asia Pac J Clin Oncol. 2014;10(1):1–10.
- 18.
- 19. Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;•••:
- 20. Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22(12):2489–503.
- 21. Styczynski J, Wysocki M. Alternative medicine remedies might stimulate viability of leukemic cells. Pediatr Blood Cancer. 2006;46(1):94–8.
- 22. Al-Tonbary Y, Al-Haggar M, El-Ashry R, El-Dakroory S, Azzam H, Fouda A. Vitamin e and N-acetylcysteine as antioxidant adjuvant therapy in children with acute lymphoblastic leukemia. Adv Hematol. 2009;2009:689639.
- 23. Fleischer T, Chang TT, Chiang JH, Sun MF, Yen HR. Improved survival with integration of chinese herbal medicine therapy in patients with acute myeloid leukemia: A nationwide population-based cohort study. Integr Cancer Ther. 2017;16(2):156–64.
- 24. Saedi TA, Md Noor S, Ismail P, Othman F. The effects of herbs and fruits on leukaemia. Evid Based Complement Alternat Med. 2014;2014:494136.
- 25. Cassileth BR, Deng G. Complementary and alternative therapies for cancer. Oncologist. 2004;9(1):80–9.
- 26. Clerici CA, Veneroni L, Giacon B, Mariani L, Fossati-Bellani F. Complementary and alternative medical therapies used by children with cancer treated at an Italian pediatric oncology unit. Pediatr Blood Cancer. 2009;53(4):599–604.
- 27. Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24(1):35–63.
- 28. Wiangnon S, Veerakul G, Nuchprayoon I, Seksarn P, Hongeng S, Krutvecho T, et al Childhood cancer incidence and survival 2003-2005, Thailand: study from the Thai Pediatric Oncology Group. Asian Pac J Cancer Prev. 2011;12(9):2215–20.