Introduction
Immune thrombocytopenic purpura (ITP) is characterized by a low platelet count (less than 100,000/µL = 100 × 109/L) resulting from platelet destruction and impaired platelet production []. It can lead to excessive bruising and bleeding. Autoantibodies and autoreactive CD8+ cytotoxic T cells are involved in the antiplatelet response; however, a triggering event (typically another disease or treatment) is needed to activate the autoimmune reaction []. SARS-CoV-2 infection has been recently associated with an increased occurrence of ITP [-]. A limited number of ITP cases occurring after the administration of mRNA and adenoviral vector vaccines have also been reported [-]. We report here the case of a patient with ITP who presented relapses after the second and booster doses of the BNT162b2 COVID-19 vaccine. The patient provided written informed consent for the publication of this case report.
Case Report
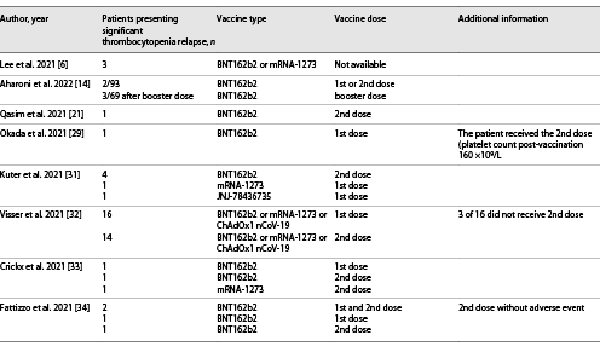
A 65-year-old male patient known for immune thrombocytopenic purpura presented with three severe episodes of thrombocytopenia (with platelet counts of 1 × 109/L at each episode) in 2007, 2015, and 2017, requiring intravenous (iv) immunoglobulin and corticosteroid treatment. His platelet count was otherwise fluctuating between 2011 and 2015 (min 118 × 109/L and max 157 × 109/L). The patient’s past medical history included Hodgkin lymphoma in 2002, colonic adenocarcinoma treated with a colectomy in 2015, and severe obstructive sleep apnoea syndrome. He received the first and second doses of the COVID-19 BNT162b2 vaccine in March and April 2021. Thirty days after the second dose, the patient visited his oncologist, complaining of asthenia. Clinical examination revealed small foot petechiae (<1 mm), while laboratory values were consistent with severe thrombocytopenia with a platelet count of 3 × 109/L (baseline platelet count 30 days before the 1st vaccine dose was 129 × 109/L). Erythrocyte and leucocyte levels were within the normal range. He was hospitalized and treated with a 4-day course of iv dexamethasone 40 mg/day, further requiring iv immunoglobulin therapy, with a progressive clinical improvement noted. The patient was not receiving any other treatment at the time of the 1st and 2nd vaccine doses.
After a multidisciplinary discussion and a shared medical decision with the patient, taking into account the risks and benefits, the booster dose was scheduled for December 2021, with close monitoring of the platelet count. The decision was taken to pursue the vaccination with the same vaccine, as an increase in systemic reactogenicity after the booster heterologous dose was observed in comparison to the homologous one []. Three weeks before the booster dose, laboratory results were as follows: negative SARS-CoV-2 antibody assay (28 BAU/mL for anti-spike IgG), absence of platelet factor 4/heparin-reactive antibodies, positive bound antiplatelet antibodies, and negative circulating antiplatelet antibodies. The baseline platelet count was 184 × 109/L. Following the multidisciplinary team’s recommendation, before the booster dose, the patient was premedicated with a 5-day course of prednisone 50 mg/day. This treatment was chosen by analogy with the treatment recommendations for ITP patients in general [-]. Eight days after the booster dose, the patient’s platelet count dropped to 29 × 109/L while remaining asymptomatic. He was treated with prednisone followed by rituximab over 4 weeks. Indeed, the patient was extremely anxious and demanding of rituximab treatment, as it allowed him to get the most durable remission from a previous ITP exacerbation. His platelet count improved progressively to 213 × 109/L 1 month after vaccination (shown in Fig. 1).
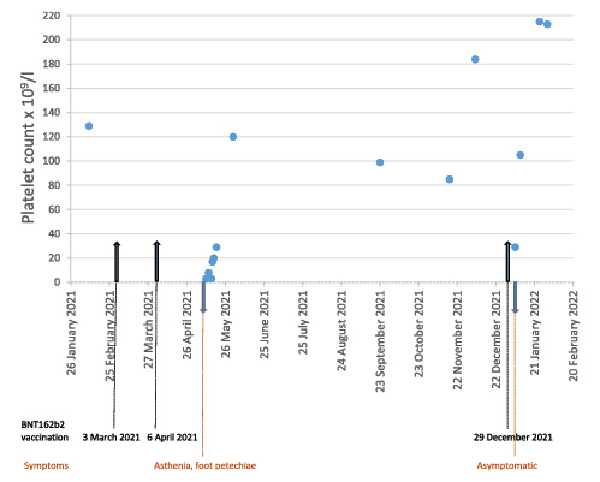
Fig. 1
Patient’s clinical course of thrombocytopenia after COVID-19 vaccination.
Due to the recurrence of thrombocytopenia in our patient after the booster dose, the causality of the COVID-19 vaccine was considered certain. The case was reported to the national pharmacovigilance centre, Swissmedic.
Conclusion
The booster dose of the COVID-19 vaccine is now recommended for all adults over the age of 12 years in Europe and Switzerland. Cases of thrombocytopenia exacerbation occurring after the first or second doses of COVID-19 vaccines have been published (Table 1). Similar data about the booster dose are scarce; however, a first retrospective study in 93 ITP patients receiving an initial and a booster dose of the BNT162b2 vaccine has been published recently []. De novo ITP was reported after a booster dose of the BNT162b2 vaccine, in a patient with a history of rheumatoid arthritis who had an uncomplicated first and second vaccine dose [].
New onset of ITP or ITP relapse has been described following several vaccines including hepatitis A and B, diphtheria-tetanus-pertussis, varicella, measles-mumps-rubella (MMR), and influenza [-]. The role of virally induced molecular mimicry in the production of autoantibodies that interact with platelet surface glycoproteins is considered the main mechanism responsible for ITP development after vaccination. Reactions to the vaccine adjuvants and preservative diluents can also be involved [, , ]. Alternatively, a mechanism associated with T-cell mediated production of pro-inflammatory cytokines and chemokines has been proposed in the anti-platelet antibody-negative cases []. The mechanisms involved in COVID-vaccines-associated new-onset or relapse of ITP are currently not clear [, ]. The presence of spike protein, a shared element between SARS-CoV-2 infection and vaccine, could be a necessary pathogenic component to enhance autoimmunity [].
Regarding the differential risks, the MMR is the only vaccine for which a causal relationship with the development of ITP is considered, with odds ratios ranging from 2.4 to 6.3 [, ]. The “probable” relation to ITP was also observed with the influenza vaccine [], and in case-control analysis, this vaccine was associated with a statistically significant 4-fold risk increase []. Moreover, in a case-control epidemiological study in HBV-vaccinated subjects, several autoimmune adverse events were observed, including thrombocytopenia with an odds ratio of 2.3 []. For other traditional vaccines, the association with ITP was mainly reported through case reports. Therefore, the differential risk is unknown.
Compared to the annual incidence of ITP which was estimated to occur in 1–6 cases per 100,000 persons per year [], the incidence of thrombocytopenia following mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS) was not higher than expected []. No association between thrombocytopenic events and the BNT162b2 vaccine was reported in the Japanese and Scottish studies either [, ]. Therefore, the benefit of vaccination outweighs the risk of developing ITP in the general population.
Our case report points out the need for specific recommendations in population subgroups, such as patients with a previous ITP diagnosis or other autoimmune diseases. Indeed, it was demonstrated that individuals with a preexisting autoimmune disease were more susceptible to developing ITP [].
Recently, the ITP exacerbation after the BNT162b2 vaccine in ITP patients was evaluated as infrequent, with ITP exacerbation rates of 2.2% after the initial dose and 4.3% after the booster dose []. These results were much lower than previously reported (around 12–14%) [, ]. This discrepancy could be potentially explained by the retrospective character of the first study, the definition of disease exacerbation, and vaccine type. The ITP exacerbation usually occurred within a week after vaccination [, ] and responded to the pharmacological treatment [, , , , ]. The risk of relapse was correlated with active ITP treatment at the start of COVID-19 vaccination [, ], a baseline platelet count of less than 50 × 109/L, and a younger age []. Regarding the booster dose, a clinically relevant platelet decrease was observed in half of the patients who experienced a platelet drop after the 1st or 2nd vaccine dose []. Interestingly, a significant decrease in the platelet count following COVID-19 vaccination was observed in 55.0% of the ITP patients and 63.0% of the healthy controls, suggesting that the risk of developing vaccine-induced thrombocytopenia might be independent of a previous diagnosis of ITP [].
In conclusion, our case report shows a strong association between the COVID-19 vaccination and the exacerbation of ITP confirmed by relapse after rechallenge, despite corticosteroid treatment. As ITP flares remain unpredictable, close monitoring should be systematically applied to individuals with a preexisting autoimmune disease to initiate early appropriate therapy.
Statement of Ethics
Ethical approval is not required for this case report in accordance with local guidelines. Written informed consent was obtained from the patient for using the details of his medical records for publication of this case report.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received to assist with the preparation of this manuscript.
Author Contributions
Laurence Favet is the physician in charge of the patient. Guy-Clébert Mutoni, Maja Ratajczak-Enselme, and Kuntheavy Ing Lorenzini evaluated safety data and provided recommendations for additional testing and choice of vaccination agent. Maja Ratajczak-Enselme wrote the manuscript. Jules Desmeules and Kuntheavy ING Lorenzini critically reviewed the manuscript. All the authors approved the final version of the manuscript.
Data Availability Statement
All data generated or analyzed during this case report are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Kuter DJ. The treatment of immune thrombocytopenia (ITP): focus on thrombopoietin receptor agonists. <X00_Journal>Ann Blood</X00_Journal>. 2021;6:7.
- 2. Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. <X00_Journal>Front Immunol</X00_Journal>. 2018;9:880.
- 3. Bomhof G, Mutsaers PGNJ, Leebeek FWG, Te Boekhorst PAW, Hofland J, Croles FN. COVID-19-associated immune thrombocytopenia. <X00_Journal>Br J Haematol</X00_Journal>. 2020;190(2):e61–4.
- 4. Zulfiqar A-A, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with covid-19. <X00_Journal>N Engl J Med</X00_Journal>. 2020;382(18):e43. https://doi:10.1056/NEJMc2010472.
- 5. Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, et al. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. <X00_Journal>Br J Haematol</X00_Journal>. 2020;190(4):e224–e229.
- 6. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. <X00_Journal>Am J Hematol</X00_Journal>. 2021;96(5):534–7.
- 7. Helms JM, Ansteatt KT, Roberts JC, Kamatam S, Foong KS, Labayog Jm S. Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine. <X00_Journal>J Blood Med</X00_Journal>. 2021;12:221–4.
- 8. Akiyama H, Kakiuchi S, Rikitake J, Matsuba H, Sekinada D, Kozuki Y, et al. Immune thrombocytopenia associated with Pfizer-BioNTech’s BNT162b2 mRNA COVID-19 vaccine. <X00_Journal>IDCases</X00_Journal>. 2021;25:e01245.
- 9. Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. <X00_Journal>Am J Hematol</X00_Journal>. 2021;96(5):E133–4.
- 10. Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. <X00_Journal>The Lancet</X00_Journal>. 2021;397(10289):2043–6.
- 11. Rodeghiero F, Besalduch J, Michel M, Provan D, Grotzinger K, Thompson G. Treatment practices in adults with chronic immune thrombocytopenia â: a European perspective. <X00_Journal>Eur J Haematol</X00_Journal>. 2010 Feb 1;84(2):160–8.
- 12. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. <X00_Journal>Blood</X00_Journal>. 2017 May 25;129(21):2829–35. https://sdoi:10.1182/blood-2017-03-754119.
- 13. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. <X00_Journal>Blood Advblood Adv</X00_Journal>. 2019 Dec 102020Jan 28;34(232):3829252–3866. https://doi:10.1182/bloodadvances.2019000966.Erratum in.
- 14. Aharoni M, Leader A, Shochat T, Raanani P, Spectre G. Exacerbation of immune thrombocytopenia following initial and booster vaccination with Pfizer-BioNTech COVID-19 vaccine. <X00_Journal>Platelets</X00_Journal>. 2022 Jul 4;33(5):781–6. https://doi:10.1080/09537104.2022.2071856.
- 15. Malayala SV, Papudesi BN, Sharma R, Vusqa UT, Raza A. A case of idiopathic thrombocytopenic purpura after booster dose of BNT162b2 (Pfizer-Biontech) COVID-19 vaccine. <X00_Journal>Cureus</X00_Journal>. 2021;13(10):e18985.
- 16. Rinaldi M, Perricone C, Ortega-Hernandez OD, Perricone R, Shoenfeld Y. Immune thrombocytopaenic purpura: an autoimmune cross-link between infections and vaccines. <X00_Journal>Lupus</X00_Journal>. 2014 May;23(6):554–67. https://doi:10.1177/0961203313499959.
- 17. Garbe E, Andersohn F, Bronder E, Salama A, Klimpel A, Thomae M, et al. Drug-induced immune thrombocytopaenia: results from the berlin case–control surveillance study. <X00_Journal>Eur J Clin Pharmacol</X00_Journal>. 2012 May;68(5):821–32.
- 18. Mantadakis E, Farmaki E, Buchanan GR. Thrombocytopenic purpura after measles-mumps-rubella vaccination: a systematic review of the literature and guidance for management. <X00_Journal>J Pediatr X</X00_Journal>. 2010 Apr;156(4):623–8. https://doi:10.1016/j.jpeds.2009.10.015.
- 19. Perricone C, Ceccarelli F, Nesher G, Borella E, Odeh Q, Conti F, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. <X00_Journal>Immunol Res</X00_Journal>. 2014 Dec;60(2–3):226–35. https://doi:10.1007/s12026-014-8597-x.
- 20. Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. <X00_Journal>J Allergy Clin Immunol</X00_Journal>. 2021 Jun;147(6):2075–82.e2. https://doi:10.1016/j.jaci.2021.04.002.
- 21. Qasim H, Ali E, Yassin MA. Immune thrombocytopenia relapse post covid-19 vaccine in young male patient. <X00_Journal>IDCases</X00_Journal>. 2021;26:e01344. https://doi:10.1016/j.idcr.2021.e01344.
- 22. Portuguese AJ, Sunga C, Kruse-Jarres R, Gernsheimer T, Abkowitz J. Autoimmune- and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. <X00_Journal>Blood Adv</X00_Journal>. 2021 Jul 13;5(13):2794–8. https://doi:10.1182/bloodadvances.2021004957.
- 23. Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. <X00_Journal>Br J Clin Pharmacol</X00_Journal>. 2003 Jan;55(1):107–11. https://doi:10.1046/j.1365-2125.2003.01790.x.
- 24. Bertuola F, Morando C, Menniti-Ippolito F, Da Cas R, Capuano A, Perilongo G, et al. Association between drug and vaccine use and acute immune thrombocytopenia in childhood. <X00_Journal>Drug Saf</X00_Journal>. 2010 Jan 1;33(1):65–72. https://doi:10.2165/11530350-000000000-00000.
- 25. Hamiel U, Kventsel I, Youngster I. Recurrent immune thrombocytopenia after influenza vaccination: a case report. <X00_Journal>Pediatrics</X00_Journal>. 2016 Dec;138(6):e20160124. https://doi:10.1542/peds.2016-0124.
- 26. Geier DA, Geier DA, Geier MR, Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. <X00_Journal>Autoimmunity</X00_Journal>. 2005 Jun;38(4):295–301. https://doi:10.1080/08916930500144484.
- 27. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. <X00_Journal>Am J Hematol</X00_Journal>. 2010;85(3):174–80.
- 28. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). <X00_Journal>Vaccin X</X00_Journal>. 2021;39(25):3329–32.
- 29. Okada Y, Sakai R, Sato-Fitoussi M, Nodera M, Yoshinaga S, Shibata A, et al. Potential triggers for thrombocytopenia and/or hemorrhage by the BNT162b2 vaccine, pfizer-BioNTech. <X00_Journal>Front Med</X00_Journal>. 2021;8:751598.
- 30. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. <X00_Journal>Nat Med</X00_Journal>. 2021;27(7):1290–7.
- 31. Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. <X00_Journal>Br J Haematol</X00_Journal>. 2021;195(3):365–70.
- 32. Visser C, Swinkels M, van Werkhoven ED, Croles FN, Noordzij H, Eefting M, et al. The effect of COVID-19 vaccine in patients with immune thrombocytopenia. <X00_Journal>Blood</X00_Journal>. 2021;138(Suppl 1):587. https://doi:10.1182/bloodadvances.2021006379.
- 33. Crickx E, Moulis G, Ebbo M, Terriou L, Briantais A, Languille L, et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. <X00_Journal>Br J Haematol</X00_Journal>. 2021;195(5):703–5.
- 34. Fattizzo B, Giannotta JA, Cecchi N, Barcellini W. SARS-CoV-2 vaccination in patients with autoimmune cytopenias: the experience of a reference center. <X00_Journal>Am J Hematol</X00_Journal>. 1 nov 2021;96(11):E413–6.