Introduction
Iron deficiency (ID) and iron deficiency anemia (IDA) are uncommon findings in healthy young males in the developed world [, ]. Although the lower limit of normal hemoglobin (Hb) for adult males suggested by the WHO almost 40 years ago is 13 g/dL, large American databases show that the lower Hb threshold for the population of white males 20–29 years of age is typically between 13.5 and 14 g/dL, while the mean Hb concentration amongst these males is usually around 15.5 g/dL []. Other definitions of anemia in males range from 13 to 14.2 g/dL [].
Serum ferritin level is the most commonly used test for the identification of ID []. Adult males have serum ferritin levels averaging 100 ng/mL []; ID is present when the level is < 30 ng/mL with a sensitivity and specificity of 92 and 98% []. The majority of studies show that, at this level, ID affects hematopoiesis []. However, the ferritin level range used in defining ID varies between studies and populations from 12 to 200 mg/L []. Transferrin saturation (TSAT) is proposed as an alternative or complementary diagnostic test for ID. Its main advantage is that the normal range is narrower than that of ferritin, which is attributable to lower physiologic variation. The TSAT threshold used for the diagnosis of ID ranges from 15 to 25% [].
The estimated prevalence of IDA in the West is 0.5–2% in adult males [, ]. The prevalence in Israeli males aged 20–40 years is reported to be 2.2% []. According to the British published guidelines, investigations for upper and lower gastrointestinal (GI) bleeding should be considered in all male patients where IDA has been confirmed [, ].
The term “athletes’ anemia” is widely used. It has been described in athletes that take part in various activities, such as running, rowing, swimming, and walking. It is also known to occur in soldiers [], especially during periods of intensive training. In some cases, the low Hb concentration can be explained by plasma volume expansion, in which case it is a pseudoanemia caused by hemodilution. Usually, this type of anemia is only temporary and occurs particularly at the beginning of training [].
Endurance athletes are particularly prone to ID as well. The etiology can be multifactorial and include occult blood loss from the GI tract caused by the reduction of intestinal perfusion during exercise, and so may contribute to progressive iron loss []. The use of nonsteroidal anti-inflammatory drugs can also cause upper GI blood loss []. Hematuria was documented in up to 90% of marathon runners [] and it may also contribute to iron depletion. Some studies also found significant hemolysis among athletes running on firm surfaces and in military recruits after a prolonged march [], sometimes known as “march hemoglobinuria.”
A similar process might occur as erythrocytes are squeezed by muscular contractions. It is also well known that iron can be lost in the urine during brisk hemolysis []. One should note that the general evidence of association between ferritin level and physical performance is partial and not unequivocal. Little is known about the iron status of male soldiers taking part in strenuous training programs. Thus, the objective of this study was to evaluate the incidences of anemia, ID, and IDA in a sample of Elite Forces trainees at the time of their recruitment to military service and during strenuous military training that lasted 15 months.
Materials and Methods
The study population consisted initially of 115 male recruits aged 18–20 years (Fig. 1). Participants from 3 elite combat units of the Israel Defense Forces took part in this study. Twelve to 18 months before recruitment, all subjects underwent a comprehensive medical examination to determine if they were healthy and eligible to start the intense training program. Recruits with a history of profound anemia (Hb < 12 g/dL), hematological diseases, or GI and absorption disorders are not eligible for service in these elite units and were not included in the study. Trainees who were prescribed any vitamin or iron supplements were excluded as well.
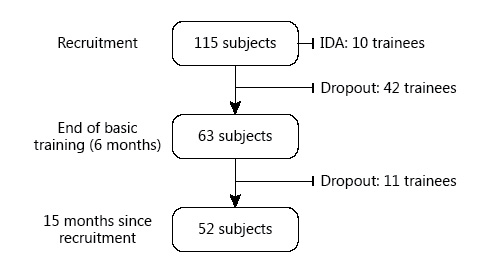
Fig. 1
Flow diagram of subjects included in the study.
Following recruitment, the participants underwent basic training for a period of 4–6 months, after which they were subjected to another extensive training program lasting an additional 14–16 months. Germane to this study, this additional training involved extensive physical training and long distance walking. All participants signed an informed consent before inclusion in the study. The study and its protocol were approved by the institutional review board of the IDF (approval No. 1467-2014).
Before commencing basic training, venous blood samples were collected from 115 soldiers. Blood was also collected at the end of basic training (6 months after recruitment). From 6 months onwards, blood samples were collected every 3 months for an additional 9 months, with all samples collected by the end of 15 months after recruitment.
All blood samples were drawn during the morning hours, after night fasting and rest. Hb, hematocrit (Hct), red blood cell count (RBC), mean corpuscular volume (MCV), white blood cell count (WBC), platelet count (Plt), and serum levels of iron, transferrin, and ferritin were assessed at all time points. Donating blood and taking iron supplements were prohibited during the whole study period.
Anemia was defined as Hb < 14 g/dL. This value is consistent with the lower limit of normal for this population as reported by the Central Laboratory of the Israeli Medical Corps and supported by other studies [, ]. ID was defined as ferritin < 30 ng/mL. Anemia in the presence of ID was defined as IDA. TSAT was calculated using the following equation: TSAT (%) = (ferrum/transferrin) × 71.24 []. A value of < 15% also indicated the presence of ID []. Relatively high cut-off values of Hb and ferritin were selected since the participants were subjected to continuous strenuous physical activity during the study period, and, as combat soldiers, they are prepared for service on the battlefield where they need to be at their peak, physically and intellectually.
The main objective of the study was to determine how often IDA developed during the training period. Trainees found to have IDA (defined as Hb < 14 g/dL and ferritin < 30 ng/mL) at recruitment or during training were excluded from the study and referred to their primary physician for complete investigation and treatment. Other end points included the development of ID with a normal Hb value, anemia without ID, and changes in hematological indices (Hb, Hct, RBC, MCV, WBC, Plt, serum iron, transferrin, and ferritin, and TSAT).
Descriptive statistics are presented as mean values and standard deviations. Before means comparisons, normal distribution of variables was determined using the Kolmogorov-Smirnov test. Normally distributed means were compared using the 2-tailed paired Student t test. Continuous variables that were not normally distributed were compared using the Wilcoxon signed-ranks test. The χ2 test was used to analyze differences between dichotomous variables. p < 0.05 was considered to be statistically significant. Data analysis was conducted using SPSS v23.0 (Chicago, IL, USA) and Microsoft Excel v14.0 (Microsoft Corp., Redmond, WA, USA).
Results
At recruitment, blood samples were collected from 115 soldiers. Due to the high rate of unsuccessful completion of this training program (unrelated to the study) and the high rate of IDA at recruitment (see below), complete data were available for only 63 subjects after 6 months, and for 52 subjects who completed both phases of training that lasted at least 9 additional months (Fig. 1). There were thus 3 independent cohorts analyzed at 3 different points of training.
Recruits
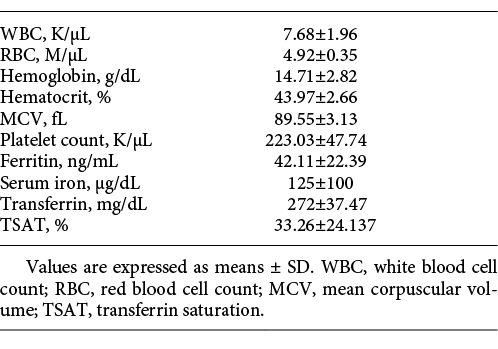
One hundred and fifteen subjects were tested at recruitment before commencing the training. Their mean hematological indices and iron profile values are presented in Table 1. The Hb level was < 14 g/dL in 32 subjects (28%), < 13.5 g/dL in 12 (10%), and < 12 g/dL in 2 (1.74%). ID, defined as ferritin < 30 ng/mL, was present in 36 subjects (31%). Ten of 115 subjects (9%) had IDA at recruitment and 8 of these had Hb < 13.7 gm/dL. TSAT of < 15% was detected in 7 subjects (6%). Forty subjects (35%) had ID according to either the ferritin or TSAT criteria.
Trainees Who Completed at Least Six Months of Training
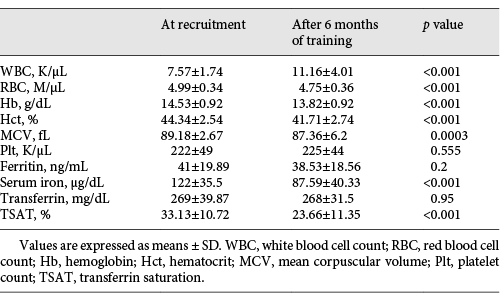
Ten subjects were excluded from the study at recruitment due to IDA and another 42 dropped out due to unrelated reasons (Fig. 1). Sixty-three trainees were followed for 6 months, and by the end of this period of training, the prevalence of anemia from all causes rose from 19 (n = 12) to 52% (n = 33) (p < 0.001). Table 2 presents the changes in mean hematological indices and iron profiles in paired samples of these 63 subjects after 6 months of training (i.e., the end of basic training) compared to the baseline values obtained at the time of recruitment. There were significant decreases in Hb, RBC, Hct, MCV, and serum iron level compared to baseline. The WBC increased significantly but ferritin and Plt values were not significantly different.
The change in Hb distribution after 6 months of training is shown in Figure 2. The Hb value decreased in 73% of the 63 subjects (mean decrease 1.3 g/dL ± 0.8). Nine (14%) developed new-onset IDA. Eight (89%) had Hb < 13.5 g/dL and 5 (56%) had Hb < 13 g/dL. The prevalence of ID as detected by a low ferritin concentration did not change significantly between the time of recruitment and the end of the first 6 months of training, being detected in 33 (n = 21) and 35% (n = 22), respectively. However, the prevalence of subjects with TSAT < 15% rose from 3% (n = 2) at the time of recruitment to 21% (n = 13) 6 months later (p < 0.001). Thus, there was a combined increase in ID from 35% at the time of recruitment to 54% after 6 months of training (p < 0.001).
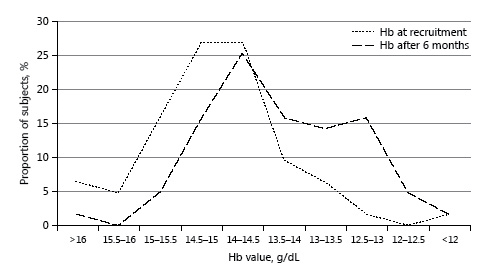
Fig. 2
Distribution of hemoglobin (Hb) values at recruitment and after 6 months of training.
Trainees Who Completed the Whole Training Program
Fifty-two trainees completed at least 15 months of training (10 were excluded from the follow-up due to IDA at recruitment and another 53 did not complete the training period due to reasons not related to the study) (Fig. 1). At the end of 15 months, 15/52 subjects (29%) developed new-onset IDA, and 33/52 (65%) had a ferritin value < 30 ng/mL at least once during this 15-month period. The prevalence of IDA after 15 months of training was significantly higher than at the time of recruitment (29 vs. 9%, respectively, p < 0.001). The difference between IDA prevalence after 6 and 15 months was also statistically significant (p = 0.01).
Of the 52 subjects followed for 15 months, 17 (33%) had ID at recruitment, 5 (29%) developed IDA at 6 months, and 2 more (12%) developed IDA during the following training period (months 9–15). In total, 41% of subjects with ID at recruitment developed IDA during the training period. Eight of 13 subjects (62%) with ferritin < 20 ng/mL at recruitment developed IDA during the 15-month period. Subjects who started their training with ID had a 2-fold-greater risk of developing IDA during the follow up period (41 vs. 23%, p < 0.05).
The Kaplan-Meier plot for the time to development of anemia, ID, and IDA among 63 subjects who completed at least 6 months of training is shown in Figure 3. The medical charts of 25 subjects who were diagnosed with IDA during the study period were reviewed. At 18 months after recruitment, none of these patients had a diagnosis of any GI or hematologic disease.
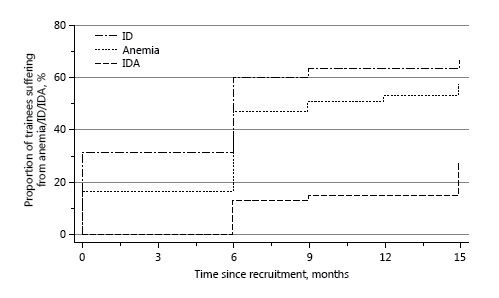
Fig. 3
A Kaplan-Meier plot of the time to the development of anemia (Hb < 14 g/dL), iron deficiency (ID; ferritin < 30 ng/mL), and iron deficiency anemia (IDA; Hb < 14 g/dL and ferritin < 30 ng/mL).
Discussion
The prevalence of ID and IDA among endurance athletes has been addressed in several studies. The prevalence of these disorders is quite variable. Wishnitzer et al. [] showed profound iron depletion in bone marrow samples in all of 12 competitive long distance runners. However, these data were published in 1983. In a more recent study, de Wijn et al. [] found that only 5–6% of the male athletes in Dutch national teams had Hb < 14 g/dL and only 7% of the anemic subjects had evidence of ID. Fallon [] found that only 5% of 303 Australian male athletes had a slightly reduced Hb concentration (13.3–13.9 g/dL) and only 19% of the athletes had a serum ferritin value of < 30 ng/mL. These differences may be attributed to the increasing popularity of the use of iron supplements by athletes [].
In Israel, the prevalence of anemia in athletes was addressed in only a few studies published in the last decade. Among 66 male members of 8 national basketball teams, anemia (Hb < 14 g/dL) was recognized in < 15%, ID (ferritin < 20 ng/mL) in < 10%, and IDA in < 5% of athletes []. Among 77 members of the Israeli national Olympic team (males and females), ID and IDA were recognized in 13 and 3.5%, respectively [].
Very few studies have addressed the iron and anemia status in strenuously training soldiers. Kehat et al. [] compared Hb values of 48 Elite Forces recruits at baseline medical fitness examination and after 2 years of training. The study did not find a significant drop in Hb but noticed a mild drop in Hct. On the other hand, Merkel et al. [] investigated the incidence of ID and anemia in 153 Israel Defense Forces recruits before and after 6 months of training and there was an anemia prevalence as high as 18% at recruitment (Hb < 14 g/dL). After 6 months of training, 50% of the subjects were anemic. The prevalence of ID (ferritin < 22 ng/mL) was 15% at recruitment and 27% 6 months later. The authors did not classify the anemia as IDA or any other type of anemia, but they noticed a strong association of ferritin depletion with anemia; this may have contributed to the anemia observed. It must be noted, however, that as many as 43% of screened trainees donated blood during the follow-up period.
Several studies clearly showed that even a small decrease in Hb level of 1–2 g/dL can cause a 20% decrease in physical and intellectual performance []. There is some evidence that ID can negatively affect emotional health and cognitive function []. Other studies claim that ID can induce muscle and hormonal dysfunction and predispose to infection []. Recently, Burden et al. [] found that iron supplementation has a positive effect on aerobic capacity (VO2 max) . In other studies, iron-supplemented subjects without anemia showed improved performance and/or VO2 max []. Most experts recommend in favor of screening ID and iron supplements for athletes with ID without anemia attributed solely to the consequences of physical activity [, , , , ]. One should note that several other studies have failed to show a positive effect of iron supplementation on performance status, and so the debate on this matter still exists [, ].
The major finding of the current prospective observational study was a high prevalence of anemia, IDA, and ID among healthy male soldiers who engaged in strenuous physical training. We reported an unexpectedly high prevalence of anemia, ID, and IDA in young healthy males who started their compulsory military service. The prevalence of IDA was significantly higher than the 2.2% in the population of young Israeli males [] (p < 0.001). The absolute values of ferritin were low, with only 2 subjects (1.74%) found to have a value of > 100 ng/mL (i.e., the average value in the male population) []. These findings could be the result of the prerecruitment training which is almost universal among those who volunteer to serve in elite units.
The changes caused by 6 months of strenuous physical training are consistent with the findings of Merkel et al. [], who found that 50% of military trainees experienced anemia and 25% had developed a depletion of iron stores after 6 months of training. These changes are explained by the physiological effect of physical activity, as explained before. The discrepancy between the unchanged ferritin values and the increase in IDA may be explained by the mean TSAT which declined from 33 to 23% (p < 0.001). Our findings suggest that ferritin may be an unreliable surrogate for total iron balance in this unique population due to inflammation-like reactions, caused by exercise and stress, that leadto persistently increased ferritin levels []. The increased WBC, unchanged Plt, and decline in MCV support this hypothesis. Using TSAT < 15% as a marker of ID led to a prevalence of ID of > 50% after 6 months of training. During military service, where repeated donations of blood donation are common among soldiers, the prevalence of ID and IDA may be even higher, making them almost universal. By extending our follow-up to 15 months, we observed that the main changes in hematological indices and the depletion of iron stores took place during the first 6 months of training.
Conclusion
In conclusion, we found a high prevalence of anemia, ID, and IDA in healthy military recruits who engaged in continuous strenuous physical training. We believe that this phenomenon is caused, at least partly, by the physiological changes caused by strenuous physical activity. Further research and the development of specific guidelines addressed to this unique population are necessary.
References
- 1. Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL: Prevalence of iron deficiency in the United States. J Am Med Assoc 1997; 277: 973–976.
- 2. Killip S, Bennett JM, Chambers MD: Iron deficiency anemia. Am Fam Physician 2007; 75: 671–678.
- 3. Beutler E, Waalen J: The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006; 107: 1747–1750.
- 4. Camaschella C: Iron-deficiency anemia. N Engl J Med 2015; 372: 1832–1843.
- 5. Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J (eds): Harrison’s Principles of Internal Medicine, ed 19. New York, McGraw Hill, 2015.
- 6. Mast AE, Blinder MA, Gronowski AM, Chumley SM: Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 1998; 44: 45–51.
- 7. Clénin GE, Cordes M, Huber A, Schumacher Y, Noack P, Scales J, Kriemler S: Iron deficiency in sports – definition, influence on performance and therapy. Swiss Med Wkly 2016; 64: 6–18.
- 8. Peyrin-biroulet L, Williet N, Cacoub P: Guidelines on the diagnosis and treatment of iron deficiency across indications : a systematic review 1. Am Soc Nutr 2015; 102: 1585–1594.
- 9. Novack V, Finestone AS, Constantini N, Shpilberg O, Weitzman S, Merkel D: The prevalence of low hemoglobin values among new infantry recruits and nonlinear relationship between hemoglobin concentration and physical fitness. Am J Hematol 2007; 82: 128–133.
- 10. Goddard AF, James MW, McIntyre AS, Scott BB: Guidelines for the management of iron deficiency anaemia. Gut 2011; 60: 1309–1316.
- 11. Short M, Domagalski J: Iron deficiency anemia: evaluation and management. Am Fam Physician 2013; 87: 98–104.
- 12. Watts E: Athletes’ anaemia. A review of possible causes and guidelines on investigation. Br J Sports Med 1989; 23: 81–83.
- 13. Zoller H, Vogel W: Iron supplementation in athletes – first do no harm. Nutrition 2004; 20: 615–619.
- 14. Riddoch C, Trinick T: Gastrointestinal disturbances in marathon runners. Br J Sports Med 1988; 22: 71–74.
- 15. Blacklock NJ: Bladder trauma in the long-distance runner: “10,000 m haematuria.” Br J Urol 1977; 49: 129–132.
- 16. Davidson RJ: Exertional haemoglobinuria: a report on three cases with studies on the haemolytic mechanism. J Clin Pathol 1964; 17: 536–540.
- 17. Rowland T: Iron Deficiency in athletes: an update. Am J Lifestyle Med 2012; 6: 319–327.
- 18. Hussien A-MA, Abdalla Hussein M, Abd El Mageed AD, Abdel-Baky AM: Cranberry extract as a functional food in treatment of oxidative stress in iron-induced hepatic toxicity in rats. J Drug Metab Toxicol 2015; 6.
- 19. Wishnitzer R, Vorst E, Berrebi A: Bone marrow iron depression in competitive distance runners. Int J Sports Med 1983; 4: 27–30.
- 20. de Wijn JF, de Jongste JL, Mosterd W, Willebrand D: Hemoglobin, packed cell volume, serum iron and iron binding capacity of selected athletes during training. Nutr Metab 1971; 13: 129–139.
- 21. Fallon KE: Screening for haematological and iron-related abnormalities in elite athletes – analysis of 576 cases. J Sci Med Sport 2008; 11: 329–336.
- 22. Rodenberg RE, Gustafson S: Iron as an ergogenic aid: ironclad evidence? Curr Sports Med Rep 2007; 6: 258–264.
- 23. Dubnov G, Constantini NW: Prevalence of iron depletion and anemia in top-level basketball players. Int J Sport Nutr Exerc Metab 2004; 14: 30–37.
- 24. Eliakim A, Nemet D, Constantini N: Screening blood tests in members of the Israeli national Olympic team. J Sports Med Phys Fitness 2002; 42: 250–255.
- 25. Kehat I, Shupak A, Goldenberg I, Shoshani O: Long-term hematological effects in Special Forces trainees. Mil Med 2003; 168: 116–119.
- 26. Merkel D, Huerta M, Grotto I, Blum D, Rachmilewitz E, Fibach E, Epstein Y, Shpilberg O: Incidence of anemia and iron deficiency in strenuously trained adolescents: results of a longitudinal follow-up study. J Adolesc Heal 2009; 45: 286–291.
- 27. Chatard JC, Mujika I, Guy C, Lacour JR: Anaemia and iron deficiency in athletes. Practical recommendations for treatment. Sports Med 1999; 27: 229–240.
- 28. Burden RJ, Morton K, Richards T, Whyte GP, Pedlar CR: Is iron treatment beneficial in, iron-deficient but non-anaemic (IDNA) endurance athletes? A meta-analysis. Br J Sports Med 2014; 49: 1–10.
- 29. Schumacher YO, Schmid A, König D, Berg A: Effects of exercise on soluble transferrin receptor and other variables of the iron status. Br J Sports Med 2002; 36: 195–199.
- 30. Pedlar CR, Whyte GP, Burden R, Moore B, Horgan G, Pollock N: A case study of an iron-deficient female Olympic 1,500-m runner. Int J Sports Physiol Perform 2013; 8: 695–698.
- 31. Burden RJ, Pollock N, Whyte GP, Richards T, Moore B, Busbridge M, Srai SK, Otto J, Pedlar CR: Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Med Sci Sports Exerc 2015; 47: 1399–1407.
- 32. Leonard BJ: Hypochromic anaemia in R.A.F. recruits. Lancet 1954; 263: 899–902.
Danny Epstein and Ariel Borohovitz contributed equally to this work.