Introduction
Obesity rates have been increasing since the 1980s, and between 2000 and 2018 the prevalence of obesity increased from 30.5% of the US population to 42.4% (). Weight loss and maintenance have become critical aspects of health care owing to the increased risk of disease associated with obesity as well as economic costs and poor quality of life. Weight gain or weight loss occurs when energy intake and energy expenditure become unbalanced. On the energy intake side, the behavior surrounding eating is complex and numerous factors affect how appetite is perceived and acted on. Rooted in biology, circulating hormones (), neural components (), and cellular/molecular pathways () may play important roles in determining satiation or satiety. On the environmental side, demographics, the food environment, social/economic, and psychological circumstances also are factors. Although the current article focuses on the biological aspects of appetite regulation in the context of energy intake regulation, it is important to understand social/demographic/economic/cultural determinants as well and all are active areas of investigation.
Individuals may choose a variety of dietary approaches involving calorie restriction (CR) to achieve the goal of inducing weight loss. A constant daily level of CR has traditionally been used to elicit an energy deficit, causing related changes to hunger and satiety signaling as the body responds to a change in energy balance. However, recent insights into the importance of circadian signaling have demonstrated that the daily window in which one consumes calories may have an impact on the metabolic factors related to food regulation (). As a result, time-restricted feeding (TRF), when one limits the hours of the day during which one consumes one's meals and extends the time spent fasting, has become a popular dietary pattern used to achieve weight loss. These alterations to biochemical signaling and neural networks may elicit slight changes in appetite and satiety, but to our knowledge, the differences between TRF and constant daily CR in this area have not been investigated. Further, the change in appetite and satiety within the circadian cycle in TRF or CR compared with ad libitum eating has not been examined.
Several hormones are linked to orexigenic and anorexigenic aspects of eating behavior () such as ghrelin, glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK), peptide YY (PYY3-36), glucose-dependent insulinotropic polypeptide (GIP), pancreatic polypeptide (PP), insulin, amylin, and leptin (). Numerous other peptides have been hypothesized to affect appetite (). The current article focuses on those that have been studied within the context of CR and TRF in humans. Appetite describes the overall drive to eat food that manifests in searching/foraging behaviors, as well as choosing what foods to eat (), whereas satiety reduces appetite and affects the frequency of meals throughout the day. The magnitude of food consumed in each meal influences satiation—the feeling of “fullness" that increases during food consumption and which may bring about the end of a single meal, although factors other than fullness can result in the termination of a meal (, ).
Hunger and satiety mechanisms that are associated with food intake behavior also play a critical role in both weight loss and weight maintenance. The focus of this review is to present evidence about how hormonal hunger and satiety signals are affected by TRF and CR paradigms, to glean insight into what that might mean for adherence to these regimes. A companion review will address the evidence with regard to the central neuroendocrine systems affected by TRF and CR.
CR
CR involves a consistent reduction in daily caloric intake, but without malnourishment or underconsumption of essential vitamins, minerals, and trace elements (), and is often touted as the best antiaging intervention (). By design, CR places an individual in a negative energy balance when the energy consumed is less than what is expended, making it integral to losing weight in overweight/obese individuals (). CR can be achieved by several approaches including diet, exercise, and appetite-altering pharmacological and surgical interventions. Of particular note, leptin, ghrelin (), insulin (), PYY3-36, and CCK-8 () have been evaluated under fasting and CR paradigms. The current report will focus on CR of 15%–75% restriction whether it was intended for weight loss or not, and its effect on pathways of peripheral and central regulation of satiety.
TRF
Fasting is defined by brief periods of ceasing caloric intake and differs from starvation, which is a degenerative condition of prolonged malnutrition. Intermittent fasting (IF) is a broader term used to describe a range of extended fasting periods. This may include alternate-day fasting (ADF), where one fasts every other day or undertakes a 1- to 2-d fast each week with the remaining days following an ad libitum feeding pattern. Another subset of temporal dietary patterns under the umbrella of IF is referred to as TRF. A TRF dietary pattern calls for daily periods of fasting, traditionally defined by avoiding energy intake while allowing for water intake during a certain time frame (). There are variations in TRF allowing individuals to follow a 12- to 21-h fasting period, with an ad libitum feeding cycle lasting from 3 to 12 h (). A common TRF pattern calls for fasting 16 h of the day and eating freely for the remaining 8 h (16:8 diet), and it may have gained popularity because it is a reasonable approach in comparison with some of the more restrictive feeding windows ().
Traditional CR diets typically do not restrict feeding times but focus on decreasing caloric intake by 15%–40% (). TRF does not necessarily result in CR because it can be adhered to with normative caloric intake (). However, decreased feeding windows may cause an individual to consume less energy (). Also eating meals closer together in time, as happens during a 16:8 diet, can reduce the time available to empty gastric contents between meals, potentially reducing the capacity for caloric intake (). Fasting catalyzes a shift to using endogenous substrate stores, causing a spectrum of metabolic reactions dependent on the time spent in an energy deficit. This can affect the orchestration of hormonal hunger and satiety signals by the hypothalamus, a key regulator of neuroendocrine signaling in response to energy status (). Few studies have directly assessed the effects of TRF on appetite, typically including a few select hormones as part of a study with aims centered on metabolic effects (, ).
Current Status of Knowledge: Hormonal Control of Food Intake
Studies were identified by searching the PubMed and Google Scholar electronic databases for peer-reviewed, English-language publications. The search terms included “leptin," “insulin," “ghrelin," “GLP-1/glucagon-like peptide-1/GLP," “PYY/PYY3-36/peptide YY," “amylin/IAPP/islet/insulinoma amyloid polypeptide/DAP/diabetes-associated peptide," “GIP/gastric inhibitory peptide/glucose‐dependent insulinotropic polypeptide," “PP/pancreatic polypeptide," “CCK/cholecystokinin," and “orexin" with “calorie/caloric restriction" and “time-restricted feeding/TRF" and “weight loss." Only reports with human participants were included, with the exception of orexin and while addressing the background of each hormone. Acute feeding challenges that involved single-meal or single-day protocols were excluded. Tables 1 and 2 are summaries of outcomes from studies that have looked at either CR or TRF, respectively, and their effect on satiety hormones. Figure 1 presents a graphical summary of satiety hormones, and their interplay at the level of the gastrointestinal tract, along with highlighting the overarching changes seen in CR and TRF conditions in the fasted state.
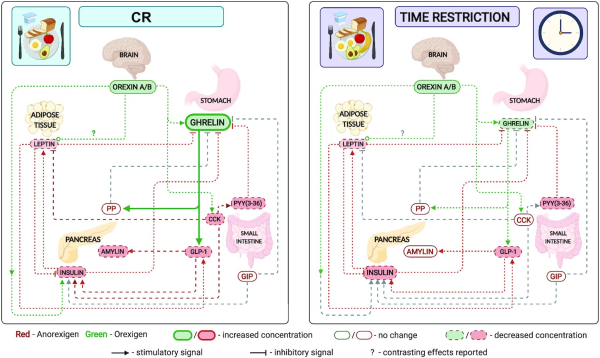
FIGURE 1
Effect of CR and TRF on circulating satiety hormones. Left: CR; right: time restriction. An overview of the relation of circulating satiety hormones and a comparison of the change in fasting satiety hormones in response to CR or TRF. Several satiety hormones decrease in both CR and TRF (leptin, insulin, and PYY); however, the orexigenic hormones ghrelin and orexin differ. Although orexin decreases in CR, there is also an increase in circulating ghrelin, suggesting either an increase or no change in hunger and a decrease in satiety during the fasting state. Ghrelin decreases and orexin remains unchanged in TRF, suggesting a decrease in both hunger and satiety signals in the fasting state. Created with Biorender.com. CCK, cholecystokinin; CR, calorie restriction; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; PP, pancreatic polypeptide; PYY, peptide YY; TRF, time-restricted feeding.
Long-term regulators
Leptin
Leptin, a 146-amino-acid peptide hormone, is released from white adipose tissue, mammary epithelial cells, and bone marrow (), and readily crosses the blood–brain barrier (). Secretion and production of leptin are dependent on triglyceride stores (), making it a unique hormone that can influence food intake through both acute and long-term signals of energy status (). Originally, leptin was thought to induce satiety, decrease food intake, and increase energy expenditure and weight loss (), and recent evidence suggests that sensitization/desensitization and resistance to leptin affect how leptin functions (). Fasting leads to decreases in both stored and circulating triglyceride levels, thus decreasing the magnitude of leptin secretion.
CR and weight loss are known influencers of leptin. CR results in a reduction in leptin (), independent of weight loss (, ), and disrupts its chrono-rhythmicity. This reduction in leptin has been associated with reduction in both subjective appetite and compensatory food intake; however, there is a lack of clarity about whether decreases in leptin are proportional to the level of caloric compensation (). CR reduces dopamine receptor availability and increases motivation and sensitivity to reward (), which have been linked to the reduction in leptin in circulation ().
Several studies have shown that both chronic (continuous) and intermittent CR, as a set reduction of dietary energy (∼500 kcal/d), a percentage of the total required intake (15%–75% of total required energy intake), or very-low-calorie diets (VLCDs) (430–800 kcal/d), for 3–24 wk, reduce serum leptin (, ) when compared with no CR. However, no significant change in fasting leptin concentrations was reported after 8 wk of a 500-kcal/d deficit CR diet (), and after 20 wk with an intake of 1000–1200 kcal/d (). The decrease in leptin was seen both with and without significant weight loss, suggesting that the change in leptin is likely due to the acute negative energy balance. In the CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) study, a 6-mo CR with intake 25% below required energy needs, not just fasting but 24-h diurnal leptin was dampened (). An ∼11-kg drop in body weight among individuals who were overweight was associated with a 44% reduction in 24-h mean circulating leptin. With weight loss and CR, there is a resultant metabolic adaptation, in the form of reduced 24-h and sleeping energy expenditure (). In the CALERIE study, the metabolic adaptation resulted in a ∼125-kcal/d reduction on average, and the decrease in leptin was an independent determinant of this metabolic adaptation (). This association has been shown in other CR intervention trials as well (, ) and attributed largely to movement economy (). However, the change in leptin does not explain the entirety of this metabolic adaptation (). In summary, fasting leptin is reduced after CR; however, whether this is sustained through diurnal and meal-induced changes remains unknown.
Similarly to CR, in some studies, TRF (6 h, 8 h, or 12 h or Ramadan feeding regimens), without intentional CR or weight loss, has been shown to reduce fasting leptin concentrations (, ). In contrast, in studies where TRF was practiced without CR, with or without weight loss, leptin concentrations were not affected (, ). After CR, when energy intake returns to prerestricted conditions, leptin concentrations also rise to match prerestriction values (). In free-living conditions, the rise in caloric intake is proposed to be due to the decrease in satiety, partly due to the lesser anorexigenic effect resulting from the reduced leptin in circulation. The evidence suggests that CR, with or without TRF, lowers circulating leptin. Although this would increase caloric intake to compensate acutely (weeks to months), the effect this has on appetite and food intake in the long run (years) remains contested, and a unifying theory of its effects is yet to be established.
Insulin
Insulin is a peptide hormone, comprised of a 21-amino-acid A chain and a 30-amino-acid B chain. It is released from pancreatic β-cells in the islets of Langerhans () primarily in response to the absorption of glucose, although other signals () and nutrients () can influence its secretion, and elicits a corresponding increase in intracellular calcium concentrations (). Insulin has a central effect on the brain, orchestrating alterations to energy balance, metabolism, appetite, and neural activity (). Insulin that is released into the systemic circulation, in the postprandial state, crosses the blood–brain barrier (), binds to insulin receptors located across the brain, elicits an anorexigenic effect, and diminishes orexigenic signals. Owing to insulin's sensitivity to long-term energy balance as well as acute energy fluctuations, it may function as a contributor to both long- and short-term regulation of hunger and satiety signals.
CR to 20%–75% of total required energy intake; ketogenic, very-low-energy diets (430–660 kcal/d); and 500- to 750-kcal restriction of the total daily energy intake required for maintenance for durations of 3 wk–6 mo have been shown to reduce fasting insulin (, , , , , , ), postprandial/all-day insulin (, , ), insulin-like signaling, and insulin sensitivity (). In contrast, very few studies reported no significant change in fasting insulin after 8- to 18-wk interventions in normal-weight or obese adults on caloric deficits ranging from VLCDs (430 kcal/d) to 500- to 600-kcal/d deficits (, , ). However, other metabolic benefits, such as reduction in circulating cholesterol (LDL, VLDL), triglycerides, and insulin resistance, are concurrent (), and CR is often recommended in managing and preventing type 2 diabetes (). Based on the studies reviewed here, a majority of the evidence suggests a reduction in insulin after CR.
TRF, along with CR, has been deemed safe and effective in managing body weight in individuals with type 2 diabetes (). TREAT (Time-Restricted Eating on Weight Loss Trial) identified that CR, both with and without TRF (16:8), resulted in no change in insulin, suggesting no unique impact from TRF (). Several TRF regimens lasting from 5 d to 12 wk, studying varying feeding-fasting temporal patterns (14–20 h fasting, 4- to 10-h eating windows), with or without concurrent CR or weight loss, showed no effect of TRF on fasting or postprandial insulin (, , , , ). In contrast, TRF (5 wk–12 wk) reduced fasting and postprandial insulin in men with prediabetes, without weight loss (); men and women with type 2 diabetes, accompanied by weight loss (); women with polycystic ovarian syndrome, accompanied by weight loss (); and normal healthy individuals, accompanied by weight loss (). It is important to note here that other IF regimens like ADF or 2-d/wk fasting protocols have shown consistent success in reducing fasting insulin (), but data from within-day TRF appear less consistent. In summary, there is weak evidence to suggest insulin is reduced after TRF, but often studies that fall under the general umbrella of IF protocols fail to differentiate between ADF, TRF, or other fasting regimens ().
Acute-phase regulators
Ghrelin
Ghrelin is a 28-amino-acid peptide hormone that is primarily produced and secreted from the X/A cells in the stomach, specifically the gastric fundus region (). Ghrelin is secreted preprandially and circulating concentrations peak immediately before meal initiation, then fall within 1 h of meal consumption (). This gives rise to ghrelin's classification as the “hunger hormone," even though it is unclear if it causes or reflects hunger (). Ghrelin has been shown to stimulate growth hormone release from the anterior pituitary, as well as influence homeostatic controls of food intake and appetite, taste sensations, and reward behaviors (). Ghrelin stimulates gastric motility and gastric acid secretion in anticipation of food intake (). A review by Briggs and Andrews () suggests the prevailing theory is that ghrelin influences appetite and hunger by stimulating neuropeptide Y/Agouti-related peptide (NPY/AgRP) neurons in the arcuate nucleus of the hypothalamus and the paraventricular nucleus (PVN).
Circulating ghrelin concentrations follow a diurnal pattern in humans and rodents that occurs owing to the suppression of ghrelin by sleep, and which is independent of meals (). Ghrelin also increases during fasting, whereas refeeding leads to a reduction of plasma concentrations (, ). There is inconsistency in the form of ghrelin reported across studies, with some reporting the active (acylated) form and others reporting total ghrelin. Despite this, CR led to significant increases in fasting (, , , , ) and postprandial concentrations (, , , ) of plasma ghrelin, with some exceptions (, , ). In addition, following a VLCD (800 kcal/d) over 8 wk led to higher ghrelin concentrations, which remained higher beyond 1 y of weight maintenance, after the initial loss (). This sustained elevation has been associated with the continuous “grazing" pattern of food intake (), which could consequently contribute to increased energy intake and a higher probability of regaining weight in the long term. Overall, it is likely that fasting and postprandial ghrelin concentrations increase following a CR regimen.
The ability of a TRF dietary pattern to modulate ghrelin concentrations remains unclear. Studies have shown no significant differences in fasting ghrelin concentration following an isocaloric TRF regimen (4–8 wk) when compared with controls (, ), regardless of whether it was accompanied by weight loss. In contrast, some studies have shown significant decreases in fasting ghrelin concentrations with TRF regimens lasting between 4 d and 30 d when compared with baseline measures (, ), also regardless of whether the TRF was accompanied by weight loss. One study that reported on soccer players that observed Ramadan fasting noted significantly higher concentrations of ghrelin post-Ramadan than preintervention values, which was accompanied by significant weight loss (). However, the fact that they are athletes and physically active suggests the involvement of exercise-induced appetitive changes, setting it apart from the other studies discussed in this section. Major concerns while interpreting from the collection of TRF studies are the lack of standardized TRF regimens, duration of the interventions, and lack of postprandial measurements. Case in point, Hutchison et al. () tested the differences between early morning TRF (eating window between 08:00 and 17:00) and afternoon TRF (eating window between 12:00 and 21:00) in free-living adults with overweight or obesity. After 7 d on the assigned TRF regimen, fasting ghrelin concentrations were significantly lower in the early morning TRF group, but postprandial ghrelin incremental area under the curve (iAUC) was lower in the afternoon TRF group, suggesting that timing of the eating window can play a role in the diurnal modulation of ghrelin concentrations by TRF (). In summary, TRF, with or without CR or weight loss, does not increase fasting ghrelin (unlike CR) and may even reduce it. Future research, factoring in the time of day for restriction and conducted for longer durations (>5 wk), is necessary for clarifying the effect of TRF on ghrelin.
GLP-1
GLP-1, an incretin, is a 30-amino-acid peptide hormone () secreted from the L cells in the small intestine in response to nutrients in the luminal intestinal space. GLP-1 is known to influence glucose homeostasis, by inducing insulin secretion and reducing glucagon secretion and gastric motility (). The significant effects of GLP-1 in modulating circulating blood glucose have led to the development and use of pharmaceutical agonists to lower blood glucose and manage body weight ().
Iepsen et al. () reported no change in fasting but an increase in postprandial GLP-1 after following a VLCD (800 kcal/d) for 8 wk, which was sustained at 52 wk. Another VLCD (550–660 kcal/d), conducted by Nymo et al. (), resulted in an increase in postprandial GLP-1 AUC at <1–3 wk. In contrast, Adam et al. () reported a reduction in postprandial GLP-1 after a 6-wk VLCD (∼600 kcal/d), which returned to baseline after 3 mo of sustained weight maintenance. A VLCD (10 wk, 500–550 kcal/d) also resulted in a small but significant reduction in GLP-1, not immediately after CR, but at the 62-wk follow-up test (). A less severe restriction of calories (intake reduced by 500 kcal/d or 20%) did not affect fasting or postprandial GLP-1 concentrations (, ). In summary, CR has reduced fasting GLP-1 concentrations, although not consistently. The lack of similarity in these interventions is important to note, with large variability in the sample type, extent of CR, duration of the study, and follow-up testing times. The inconsistencies between studies make it difficult to reach conclusions about the physiological effect of CR on GLP-1.
In the context of TRF, no change in fasting, postprandial, or all-day GLP-1 was reported following a 16:8 TRF regimen after 5 d () and after 5 wk (). On the other hand, a Ramadan-type feeding regimen was evaluated by Zouhal et al. () and reported a reduction in fasting GLP-1. This reduction in fasting GLP-1 was also seen after a 18:6 TRF intervention for 4 d () and a 15:9 TRF intervention for 7 d (); however, no differences in postprandial GLP-1 were reported. In summary, TRF appears to reduce fasting GLP-1; however, the evidence for this stems from very short-term studies (spanning days), and longer-term studies are needed to corroborate this possibility.
Amylin
Amylin, or islet/insulinoma amyloid polypeptide (IAPP)/diabetes-associated peptide (DAP), is a 37-amino-acid peptide hormone (, ). Amylin is colocalized, copacked, and cosecreted with insulin (molar ratio 1:15, respectively) from the pancreatic β cells within the islets of Langerhans, in response to a stimulus in the form of nutrient intake. Amylin acts by reducing food intake and promotes negative energy balance (). We have learned from rat model studies that exogenous amylin injected intraperitoneally has been shown to reduce meal size, in a dose-dependent manner (), and irrespective of whether the amylin is administered peripherally or centrally, its effects appear to be mediated via central mechanisms ().
Sumithran et al. () reported that after 10 wk of CR (500–550 kcal/d) in 50 obese men and women, there was a decrease in fasting and 30-min postprandial amylin. A 500-kcal/d reduction in energy intake for 12 wk did not affect fasting or postprandial amylin, in a group of obese men and women (). Further, in 74 adolescent participants (10–17 y old), compared with a control group with no dietary restriction, a 20% CR as part of either a low-carbohydrate (35% carbohydrate; 30% protein; 35% fat) or low-fat diet (55% carbohydrate; 30% protein; 25% fat) for 12 wk () resulted in no significant differences in fasting amylin. The contrasting findings may relate to the extent and/or duration of CR, and future research is needed to definitively determine the effects of CR on amylin. One study assessed the effects of 15:9 TRF on amylin; no effect on fasting or postprandial amylin was reported (). Overall, amylin may be reduced after CR in response to a VLCD, but it is unclear if this would be the case if the CR was more moderate. The evidence evaluating the effect of TRF on amylin is insufficient to draw conclusions.
PP
PP is a 36-amino-acid peptide, primarily produced in the F cells of the pancreatic islets of Langerhans, but is also secreted in smaller quantities from the large intestine (). PP diminishes gastric emptying, motility, and contraction of the gallbladder as well as preventing exocrine secretions from the pancreas through vagal signaling, contributing to satiation from the current meal episode. PP binds to Y4 receptors from the family of G-coupled protein receptors, which are found in the hypothalamus, stomach, pancreas, duodenum, ileum, and colon (, ). PP rises with caloric intake with peaks typically being observed 15 min into the postprandial state, yet values have remained high 6 h after an 830-calorie meal (, ).
After a VLCD (10 wk, 500–550 kcal/d, adults), mean (fasting and postprandial combined) PP concentration was higher than at baseline after initial weight loss and remained elevated at the 62-wk weight maintenance follow-up period (). The study did not report significant changes in fasting PP. However, postprandial PP AUC was higher at week 10 and week 62 than at baseline. Contradictory to this, in a study comparing a 20% CR with no CR in adolescents (for 12 wk), there were no changes in PP at fasting, and postprandial time points were not measured (). So, it appears that severe CR or significant weight loss can induce changes in postprandial PP, whereas fasting values do not change with moderate or severe CR. Future studies need to confirm this observation. To our knowledge, PP has not been evaluated in any TRF study to date. Hence, although CR could increase PP over the long term, further studies need to measure PP in both CR and TRF regimens to better understand their effects.
GIP
GIP is a 42-amino-acid polypeptide functioning as an incretin much like GLP-1. GIP is secreted from/by the K cells of the duodenum and jejunum in response to carbohydrates and lipids in the small intestine (). GIP activity increases insulin secretion from pancreatic β-cells in hyperglycemic conditions and increases glucagon secretion from α-cells in euglycemic conditions. Additional downstream actions of GIP include upregulation of lipoprotein lipase and increasing lipogenesis (). GIP and GLP-1 work additively to increase insulin secretion but do not appear to have the additive effect on reducing food intake seen in animal models (). As insulin concentrations rise after a meal, GIP secretion falls, creating a negative feedback loop ().
Whereas fasting concentrations of GIP remained unaffected by CR (, , , ), postprandial GIP iAUC was elevated after weight loss (, ), but this was not observed in all the studies (, ). After a severe CR (800 kcal/d), 1 study found postprandial GIP concentrations increased, only to return to baseline concentrations after 1 y of weight maintenance (), whereas another study showed GIP increased with CR and remained higher than baseline concentrations after 62 wk of weight maintenance (). Two studies have looked at the effect of TRF on GIP. One study in obese adults following a 16:8 TRF regimen compared with a 9:15 regimen reported that total GIP AUC was not significantly different between the groups (). Early morning or afternoon feeding times in TRF did not affect fasting or postprandial concentrations of GIP in a different study (). In summary, although CR may result in higher postprandial GIP, there was no effect observed at fasting, and in the very short term (5–7 d) TRF does not appear to affect GIP. Longer-duration interventions, with replicated study designs, are needed to draw more accurate conclusions about the effect of both CR and TRF on GIP.
PYY
PYY is a peptide hormone secreted from L cells primarily in the ileum and large intestine in response to food intake, especially protein ingestion, and postprandial concentrations are thought to be proportional to the number of calories ingested (). PYY circulates as PYY1-36 and PYY3-36 and the latter is thought to be primarily involved in regulating food intake (). In the current report, owing to a lack of studies that only focused on PYY3-36, we have summarized studies that reported on both PYY and PYY3-36.
Peripheral infusion of PYY3-36 in humans leads to decreased food intake and increased feelings of fullness during a standard meal, although higher infusions lead to increased feelings of nausea, and these higher infusions were considered to be pharmacological (as opposed to physiological) doses (). A 90-min infusion of PYY3-36, reported to be in the physiological concentration range by Batterham et al. (), reduced total calorie intake by 33% in humans. Moreover, 12 h after the 90-min infusion, no reported differences in fullness were reported. Taken together this indicates that PYY3-36 plays a bigger role in acute satiety and satiation. In addition, PYY3-36 (along with GLP-1) inhibits gastric acid secretion, gastric emptying, and gastrointestinal motility, known as the “ileal brake" ().
Weight loss trials utilizing VLCDs (∼500–810 kcal/d) demonstrated decreases in fasting PYY in most (, , ), but not all, studies (). However, the effects of VLCDs on postprandial PYY are inconsistent, possibly due to the differing study designs and PYY forms that were measured in each study. Essah et al. () found a decrease in fasting and postprandial total PYY after 8 wk of a moderate CR (500-kcal deficit/d). In contrast, another study also using a 500-kcal deficit/d CR regimen reported no significant changes in fasting or postprandial PYY3-36 concentrations after a 12-wk controlled-feeding weight-loss trial in obese men and women (). Similarly, Jensen et al. () reported no effect of a 20% CR on fasting PYY3-36 in 74 adolescents. McNeil et al. () reported no significant changes in total PYY after 6 mo of a 600- to 800-kcal/d restriction; however, they did report negative associations between fasting PYY concentrations and weight change and fat-free mass change. In summary, extreme CR may likely reduce PYY, but mild to moderate CR has inconsistent effects on PYY.
The effects of TRF on PYY or PYY3-36 are varied. Ravussin et al. () observed an increase after a 6-h evening fast in PYY3-36 after a 4-d trial of early morning TRF in obese adults. In contrast, in a 5-d feeding study, a 16:8 TRF regimen (no CR) did not lead to a difference in PYY 21-h AUC in overweight and obese men (n = 11) (). In agreement with these findings, Hutchison et al. () found no change in fasting or 3-h postprandial PYY in men at risk of type 2 diabetes (n = 15) after 7 d following a 15:9 TRF regimen (no forced CR). On the contrary, another study in overweight men with prediabetes (n = 8) following an 18:6 TRF regimen for 5 wk (weight stable and no CR) reported a decrease in fasting PYY (). Ramadan fasting reduced fasting PYY concentrations in obese men after 30 d (). These studies suggest that the reduction of fasting PYY in response to TRF may require a >7-d intervention duration. More research is needed to verify this and to explore the long-term effects of TRF on postprandial PYY or PYY3-36 concentrations.
CCK
CCK was one of the first gut-related hormones discovered to have effects on food intake and satiety (, ). CCK is synthesized in I cells found throughout the gastrointestinal tract, but primarily concentrated in the duodenum and jejunum (). The presence of dietary lipids and proteins in the gut triggers CCK secretion from these cells and stimulates the release of bile acids and pancreatic juices into the duodenum, increasing intestinal motility, and decreases gastric emptying rate (). The preprohormone is a peptide residue containing 115 amino acids and can be cleaved into varying lengths (CCK-5, CCK-33, CCK58, and CCK-83) by different enzymes (, ), although CCK-8 is frequently investigated and therefore better understood (). Using a meal challenge protocol, infusion of exogenous CCK in humans led to a significant decrease in food intake and feelings of satiety immediately after the infusion (). Hyperphagia and morbid obesity have resulted from loss of function mutations of CCK receptor A in humans (). Several rodent studies reported that total food intake did not decrease long term, thus showing that CCK infusion failed to have longer-lasting effects on overall food intake and satiety (); however, recent evidence using a CCK analog targeting the CCK-1 receptor in domestic pigs has reported decreased food intake after 13 wk (). Thus, whether CCK has long-term effects on food consumption is still under active investigation.
Although most studies report total CCK, some studies report CCK-8. Hence, in the current article we present data on both, which adds to inconsistencies making it challenging to understand the effect of CR or TRF on this hormone. Moderate reduction of daily total caloric intake by 500 kcal showed no significant changes in CCK after 12 wk at the fasting or postprandial state (). On the other hand, a VLCD (430–660 kcal/d) along with weight loss led to decreases in postprandial CCK/CCK-8 AUC after 8 wk, but there were no changes in fasting CCK/CCK-8 concentrations (, , , ). In summary, VLCDs may reduce postprandial CCK/CCK-8, but moderate or less extreme forms of CR do not seem to affect fasting CCK/CCK-8 significantly.
Regarding TRF, Zouhal et al. () determined that fasting CCK concentrations in obese men dropped after 30 d of Ramadan fasting; however, concentrations increased back to baseline concentrations 21 d after the end of Ramadan. Aside from this, no other studies have looked at CCK or CCK-8 under TRF conditions, warranting further study.
Orexin
Orexins or hypocretins are hypothalamic neuropeptides (). Orexin A (hypocretin 1) is a 33-amino-acid neuropeptide; orexin B (hypocretin 2) is a 28-amino-acid neuropeptide. These are produced by ∼70,000 orexin-producing neurons in the dorsolateral hypothalamus (with projections to dorsal raphe nuclei, amygdala, basal forebrain, suprachiasmatic nucleus, locus coeruleus, and the spinal cord) and the perifornical area of the brain (). Orexins act via the activation of G-protein coupled receptors—orexin-1 and -2 receptors (OX1R/OX2R), and both are present in different regions of the brain (). OX1R has also been detected in testes, kidneys, adrenal glands, and throughout the gastrointestinal tract (stomach, ileum, colon, and colorectal epithelial cells) in humans (, ), suggesting they have roles to play in peripheral tissues. Orexin A is more potent than orexin B, and OX1R has a 100-fold higher affinity for orexin A than for orexin B (). Although orexins are abundant in circulating cerebrospinal fluid, they are also present in the plasma () and are capable of rapidly crossing the blood–brain barrier by simple diffusion (). They are implicated in sleep/arousal, spontaneous physical activity, reward-seeking, drug addiction, and food intake regulation (). Orexins increase food intake and gastric motility: both intraperitoneal and intranasal administration result in increased food intake for ≤4 h after exposure (, ). Orexin precursors become more abundant (elevated mRNA expressions) during the fasted state because they are responsible for the fasting-induced increase in wakefulness that helps in food foraging behaviors (). A rise in blood glucose inhibits orexin-secreting neurons, and excitatory activity ensues with reductions in glucose ().
The effects of CR (50% for 6 d, rat model), without a change in feeding time, did not lead to changes in hypothalamic preproorexin mRNA levels () but the authors failed to measure protein concentrations in the brain or circulation. A ketogenic VLCD in adults (10 men and 10 women, 700–900 kcal/d) for 8 wk led to an increase in fasting plasma orexin-A concentrations (). Because orexin stimulates food anticipatory activity (FAA) and leads to food intake, this likely would result in increased feelings of hunger and energy intake.
In regard to TRF, orexin is suspected to play an important role in changes of FAA (increased movement and wakefulness time) when feeding time is restricted in mice (); however, evidence to support or negate this suspicion is both sparse and weak. In healthy men (n = 8) following a Ramadan fasting regimen, plasma orexin-A increased at the fasting time point, and the diurnal rhythm was flipped (inverted, based on 5 measurements in 24 h) in comparison with the nonfasting group (). In rats, when food intake was restricted to 2 h/d for 3 wk, the activity of orexin-containing neurons increased in the lateral hypothalamus, which led to a downregulation of the orexin receptor gene in the PVN (), effectively balancing the secretion of orexin with its activity via the OX2R receptor. More studies looking at the effect of orexin on TRF, for longer durations (>4 wk) as well as maintaining consistent hours of restriction, are necessary. At present the evidence suggesting an effect of TRF on orexin is inconclusive.
Conclusions
CR, either from VLCDs or from modest caloric restriction, with or without weight loss, increases fasting ghrelin and decreases fasting leptin and insulin. Reductions in orexin, amylin, and CCK after CR have been reported; however, the evidence for these is weak owing to very few studies evaluating these outcomes, nonuniform study designs, lack of consistency in the form reported (CCK compared with CCK8, total PYY compared with PYY3-36), and varying measurement assays. Potentially, these changes suggest a homeostatic response to counter the reduction in caloric intake, prompting an increase in energy consumption by reduced satiety.
TRF also reduces fasting concentrations of satiety signals: leptin, GLP-1 (with stronger evidence than CR), and PYY. The expected reduction in fasting insulin which is seen after CR is inconsistently observed after TRF. Also, based on the limited evidence available, after TRF, fasting ghrelin decreases or remains unchanged. Evidence with regard to changes in amylin, CCK, GIP, and PP after TRF is ambiguous or inadequate, warranting further study. Overall, in consideration of the circulating peripheral hormones, the hunger–satiety balance after CR likely tips toward hunger, which appears less likely following a TRF regimen. However, with the evidence available at this time, drawing firm conclusions about differences between CR and TRF is not possible owing to the variable study design aspects such as participants’ size and type, duration of intervention, lack of control group, and inconsistent TRF regimens. In addition, differing methodological parameters such as type of hormone measured (active compared with total forms), types of assays (ELISA, RIA, MS), and prepreparation of biological samples (addition of protease inhibitors, acidification) add to the challenge of comparing results across studies. Gut peptides/hormones are one of several factors affecting eating behavior, including cultural and socioeconomic determinants that are not included in this review, but play significant roles. Longer-term studies are needed to elucidate the response of these hormones to TRF (both with and without CR).
It is important to note the hormonal processes discussed here are integrated by the central mechanisms with other signals in the brain. These then manifest as satiation or feelings of satiety and ultimately influence energy intake regulation. In a companion review, the central mechanisms involved in the regulation of satiety after CR and TRF are presented.
Limitations and directions for future research
There are both overlapping as well as potentially distinct peripheral hormonal features affecting satiety in TRF and CR. Among several determinants, the balance of the hunger and satiety systems influences eating behavior and energy balance, and restricted eating times may potentially sway this balance. Whereas satiety systems are suppressed as a function of CR and TRF (with or without CR), the effect of TRF on hunger systems may set the 2 apart. However, more human studies are needed that compare CR alone, CR with TRF, and TRF alone, so that the actual mechanisms underlying hunger and satiety can be better understood. The number of studies that have looked at TRF in humans is small, and the variety of types of TRF tested are inadequate for empirical conclusions. This information is critical to our understanding of how these different approaches to eating are sustained and whether body weight changes are maintained in the long term.
Acknowledgments
We are grateful to Fanny Lee for her review of the manuscript. The authors’ responsibilities were as follows—SK and GPK: conceived the idea; SK, NLK, DKMT, and APT: were involved in critically assessing identified literature/articles; DKMT, APT, CER, and SK: were involved in developing the early manuscript versions; NLK: independently expanded and improved the manuscript; WFH and GPK: helped to critically assess the manuscript; and all authors: were involved in literature collection and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
NLK and SK contributed equally to this work.
Abbreviations used: ADF, alternate-day fasting; CALERIE, Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy; CCK, cholecystokinin; CR, calorie restriction; FAA, food anticipatory activity; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; iAUC, incremental area under the curve; IF, intermittent fasting; PP, pancreatic polypeptide; PVN, paraventricular nucleus; PYY, peptide YY; TRF, time-restricted feeding; VLCD, very-low-calorie diet.
References
- 1.
- 2. Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–62.
- 3. Posovszky C, Wabitsch M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: characteristics of enteroendocrine cells and their capability of weight regulation. Horm Res Paediatr. 2015;83(1):1–10.
- 4. Murphy KG, Bloom SR. Gut hormones in the control of appetite. Exp Physiol. 2004;89(5):507–16.
- 5. Amin T, Mercer JG. Hunger and satiety mechanisms and their potential exploitation in the regulation of food intake. Curr Obes Rep. 2016;5(1):106–12.
- 6. Moran TH, Ladenheim EE. Physiologic and neural controls of eating. Gastroenterol Clin North Am. 2016;45(4):581–99.
- 7. Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring). 2008;16(S3):S11–22.
- 8. Sternson SM, Eiselt AK. Three pillars for the neural control of appetite. Annu Rev Physiol. 2017;79(1):401–23.
- 9. Cornier MA. Is your brain to blame for weight regain?. Physiol Behav. 2011;104(4):608–12.
- 10. Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11_supplement_1):s37–50.
- 11. Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, O'Connor EC. Homeostasis meets motivation in the battle to control food intake. J Neurosci. 2016;36(45):11469–81.
- 12. Woods SC. Metabolic signals and food intake. Forty years of progress. Appetite. 2013;71:440–4.
- 13. Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9(6):489–98.
- 14. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53.
- 15. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60.
- 16. Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–15.
- 17. Yeung AY, Tadi P, editors. Physiology, obesity neurohormonal appetite and satiety control. Treasure Island, FL: StatPearls Publishing; 2020.
- 18. Austin J, Marks D. Hormonal regulators of appetite. Int J Pediatr Endocrinol. 2009:141753.
- 19. Posovszky C, Wabitsch M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 2: therapeutic potential of enteroendocrine cells in the treatment of obesity. Horm Res Paediatr. 2015;83(1):11–18.
- 20. Hill CM, Qualls-Creekmore E, Berthoud H-R, Soto P, Yu S, McDougal DH, Münzberg H, Morrison CD. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161(3):bqaa019.
- 21. Freire RH, Alvarez-Leite JI. Appetite control: hormones or diet strategies?. Curr Opin Clin Nutr Metab Care. 2020;23(5):328–35.
- 22. Covasa M, Stephens RW, Toderean R, Cobuz C. Intestinal sensing by gut microbiota: targeting gut peptides. Front Endocrinol (Lausanne). 2019;10:82.
- 23. de Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004;79(6):946–61.
- 24. Tremblay A, Bellisle F. Nutrients, satiety, and control of energy intake. Appl Physiol Nutr Metab. 2015;40(10):971–9.
- 25. Van Kleef E, Van Trijp JC, Van Den Borne JJ, Zondervan C. Successful development of satiety enhancing food products: towards a multidisciplinary agenda of research challenges. Crit Rev Food Sci Nutr. 2012;52(7):611–28.
- 26. Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14(2):275–87.
- 27. Liang Y, Liu C, Lu M, Dong Q, Wang Z, Xiong W, Zhang N, Zhou J, Liu Q, Wang X. Calorie restriction is the most reasonable anti-ageing intervention: a meta-analysis of survival curves. Sci Rep. 2018;8(1):5779.
- 28. Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119(7_Part_2):688–93.
- 29. Beasley JM, Ange BA, Anderson CA, Miller ER III, Holbrook JT, Appel LJ. Characteristics associated with fasting appetite hormones (obestatin, ghrelin, and leptin). Obesity. 2009;17(2):349–54.
- 30. Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Effect of extended morning fasting upon ad libitum lunch intake and associated metabolic and hormonal responses in obese adults. Int J Obes. 2016;40(2):305–11.
- 31. Shahrbanoo H, Reza AHS, Majid RN. Investigating the effect of fasting on appetite regulatory hormones in thin and obese females. Jundishapur J Chronic Dis Care. 2018;7(2):e65282.
- 32. Paoli A, Tinsley G, Bianco A, Moro T. The influence of meal frequency and timing on health in humans: the role of fasting. Nutrients. 2019;11(4):719.
- 33. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, Leeuwenburgh C, Mattson MP. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26(2):254–68.
- 34. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11(10):2442.
- 35. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–5.
- 36. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290.
- 37. Noakes TD, Rehrer NJ, Maughan RJ. The importance of volume in regulating gastric emptying. Med Sci Sports Exerc. 1991;23(3):307–13.
- 38. Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359–72.
- 39. Myers MG, Greenwald-Yarnell M. Leptin. In: Kastin AJ, editor. Handbook of biologically active peptides. 2nd ed. Boston, MA: Academic Press; 2013. p. 1129–34.
- 40. Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7(2):125–33.
- 41. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70.
- 42. Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21(3):263–307.
- 43. Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and leptin resistance: where are we 25 years later?. Nutrients. 2019;11(11):2704.
- 44. Mars M, de Graaf C, de Groot LC, Kok FJ. Decreases in fasting leptin and insulin concentrations after acute energy restriction and subsequent compensation in food intake. Am J Clin Nutr. 2005;81(3):570–7.
- 45. Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf). 2004;61(3):332–8.
- 46. Näätänen M, Kolehmainen M, Laaksonen DE, Herzig K-H, Poutanen K, Karhunen L. Post-weight loss changes in fasting appetite- and energy balance-related hormone concentrations and the effect of the macronutrient content of a weight maintenance diet: a randomised controlled trial. Eur J Nutr. 2021;60(5):2603–16.
- 47. Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS. Short- and long-term changes in serum leptin in dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab. 1998;83(1):214–18.
- 48. Fogteloo J, Meinders E, Frölich M, McCamish M, Pijl H. The decline in plasma leptin in response to calorie restriction predicts the effects of adjunctive leptin treatment on body weight in humans. Eur J Intern Med. 2003;14(7):415–18.
- 49. Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91(5):459–72.
- 50. Dunn JP, Abumrad NN, Kessler RM, Patterson BW, Li R, Marks-Shulman P, Tamboli RA. Caloric restriction-induced decreases in dopamine receptor availability are associated with leptin concentration. Obesity (Silver Spring). 2017;25(11):1910–15.
- 51. Cho Y, Hong N, Kim K-W, Cho SJ, Lee M, Lee Y-H, Lee Y-H, Kang ES, Cha B-S, Lee B-W. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. 2019;8(10):1645.
- 52. Strohacker K, McCaffery JM, MacLean PS, Wing RR. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes. 2014;38(3):388–96.
- 53. Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, Varady KA. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. 2018;37(6):1871–8.
- 54. Krishnan S, Adams SH, Witbracht MG, Woodhouse LR, Piccolo BD, Thomas AP, Souza EC, Horn WF, Gertz ER, Van Loan MD, et al Weight loss, but not dairy composition of diet, moderately affects satiety and postprandial gut hormone patterns in adults. J Nutr. 2021;151(1):245–54.
- 55. Jensen DE, Nguo K, Baxter KA, Cardinal JW, King NA, Ware RS, Truby H, Batch JA. Fasting gut hormone levels change with modest weight loss in obese adolescents. Pediatr Obes. 2015;10(5):380–7.
- 56. Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr. 2008;87(5):1238–46.
- 57. Most J, Redman LM. Impact of calorie restriction on energy metabolism in humans. Exp Gerontol. 2020;133:110875.
- 58. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604.
- 59. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30.
- 60. Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of weight loss by a low-fat diet and a low-carbohydrate diet on peptide YY levels. Int J Obes. 2010;34(8):1239–42.
- 61. Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011;96(9):E1512–16.
- 62. Hall KD. Metabolic adaptations to weight loss. Obesity (Silver Spring). 2018;26(5):790–1.
- 63. Camps SG, Verhoef SP, Westerterp KR. Leptin and energy restriction induced adaptation in energy expenditure. Metabolism. 2015;64(10):1284–90.
- 64. McNeil J, Schwartz A, Rabasa-Lhoret R, Lavoie JM, Brochu M, Doucet É. Changes in leptin and peptide YY do not explain the greater-than-predicted decreases in resting energy expenditure after weight loss. J Clin Endocrinol Metab. 2015;100(3):E443–52.
- 65. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring). 2019;27(8):1244–54.
- 66. Zouhal H, Bagheri R, Triki R, Saeidi A, Wong A, Hackney AC, Laher I, Suzuki K, Ben Abderrahman A. Effects of Ramadan intermittent fasting on gut hormones and body composition in males with obesity. Int J Environ Res Public Health. 2020;17(15):5600.
- 67. Al-Rawi N, Madkour M, Jahrami H, Salahat D, Alhasan F, Bahammam A, Al-Islam Faris ME. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: a prospective observational study. PLoS One. 2020;15(8):e0237922.
- 68. Alzoghaibi MA, Pandi-Perumal SR, Sharif MM, Bahammam AS. Diurnal intermittent fasting during Ramadan: the effects on leptin and ghrelin levels. PLoS One. 2014;9(3):e92214.
- 69. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, et al Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–34.
- 70. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–21.e3.
- 71. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12(2):505.
- 72. Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19(1):31–44.
- 73. Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53.
- 74. Newsholme P, Krause M. Nutritional regulation of insulin secretion: implications for diabetes. Clin Biochem Rev. 2012;33(2):35–47.
- 75. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, Mckee LJ, Bauer TL, et al Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N Engl J Med. 1996;334:292–5.
- 76. Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232–43.
- 77. Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82–93.
- 78. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–48.
- 79. Lee C, Longo V. Dietary restriction with and without caloric restriction for healthy aging. F1000Res. 2016;5:117.
- 80. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164(4):302–11.
- 81. Iepsen EW, Lundgren J, Holst JJ, Madsbad S, Torekov SS. Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3–36. Eur J Endocrinol. 2016;174(6):775–84.
- 82. Nymo S, Coutinho SR, Jørgensen J, Rehfeld JF, Truby H, Kulseng B, Martins C. Timeline of changes in appetite during weight loss with a ketogenic diet. Int J Obes. 2017;41(8):1224–31.
- 83. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–44.
- 84. Adam TC, Lejeune MP, Westerterp-Plantenga MS. Nutrient-stimulated glucagon-like peptide 1 release after body-weight loss and weight maintenance in human subjects. Br J Nutr. 2006;95(1):160–7.
- 85. Taylor R. Calorie restriction for long-term remission of type 2 diabetes. Clin Med. 2019;19(1):37–42.
- 86. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, et al Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491.
- 87. Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724–32.
- 88. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, et al Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104.e5.
- 89. Alsubheen SAA, Ismail M, Baker A, Blair J, Adebayo A, Kelly L, Chandurkar V, Cheema S, Joanisse DR, Basset FA. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br J Nutr. 2017;118(12):1023–30.
- 90. Aksungar FB, Sarıkaya M, Coskun A, Serteser M, Unsal I. Comparison of intermittent fasting versus caloric restriction in obese subjects: a two year follow-up. J Nutr Health Aging. 2017;21(6):681–5.
- 91. Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab. 2021;18(1):88.
- 92. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19(1):148.
- 93. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366–78.e3.
- 94. Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E. Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity (Silver Spring). 2020;28(S1):S29–37.
- 95. Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72(5):308–18.
- 96. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–61.
- 97. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–19.
- 98. Lippl F, Erdmann J, Steiger A, Lichter N, Czogalla-Peter C, Bidlingmaier M, Tholl S, Schusdziarra V. Low-dose ghrelin infusion—evidence against a hormonal role in food intake. Regul Pept. 2012;174(1–3):26–31.
- 99. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, et al Ghrelin. Mol Metab. 2015;4(6):437–60.
- 100. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats.Biochem Biophys Res Commun. 2000;276(3):905–08.
- 101. Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2019;93(1):48–57.
- 102. Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–93.
- 103. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–8.
- 104. Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1071–9.
- 105. Coutinho SR, Halset EH, Gåsbakk S, Rehfeld JF, Kulseng B, Truby H, Martins C. Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clin Nutr. 2018;37(3):815–23.
- 106. Lean MEJ, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence?. Int J Obes. 2016;40(4):622–32.
- 107. Abedelmalek S, Denguezli M, Chtourou H, Souissi N, Tabka Z. Does Ramadan fasting affect acylated ghrelin and growth hormone concentrations during short-term maximal exercise in the afternoon?. Biol Rhythm Res. 2015;46(5):691–701.
- 108. Chia CW, Egan JM. Incretins in obesity and diabetes. Ann N Y Acad Sci. 2020;1461(1):104–26.
- 109. Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87(3):1239–46.
- 110. Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14(7):390–403.
- 111. Scherbaum WA. The role of amylin in the physiology of glycemic control. Exp Clin Endocrinol Diabetes. 1998;106(2):97–102.
- 112. Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev. 2015;67(3):564–600.
- 113. Lutz TA. Pancreatic amylin as a centrally acting satiating hormone. Curr Drug Targets. 2005;6(2):181–9.
- 114. Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995;58(6):1197–202.
- 115. Lutz TA, Del Prete E, Scharrer E. Subdiaphragmatic vagotomy does not influence the anorectic effect of amylin. Peptides. 1995;16(3):457–62.
- 116. Meneguetti BT, Cardoso MH, Ribeiro CFA, Felício MR, Pinto IB, Santos NC, Carvalho CME, Franco OL. Neuropeptide receptors as potential pharmacological targets for obesity. Pharmacol Ther. 2019;196:59–78.
- 117. Kim W, Fiori JL, Shin Y-K, Okun E, Kim JS, Rapp PR, Egan JM. Pancreatic polypeptide inhibits somatostatin secretion. FEBS Lett. 2014;588(17):3233–9.
- 118. Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–4.
- 119. Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, Polex-Wolf J, Lam BY, Zvetkova I, Pan W, et al Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019;30(5):987–96.e6.
- 120. Sirinek KR, Pace WG, Crockett SE, O'Dorisio TM, Mazzaferri EL, Cataland S. Insulin-induced attenuation of glucose-stimulated gastric inhibitory polypeptide secretion. Am J Surg. 1978;135(2):151–5.
- 121. Orr J, Davy B. Dietary influences on peripheral hormones regulating energy intake: potential applications for weight management. J Am Diet Assoc. 2005;105(7):1115–24.
- 122. Reidelberger R, Haver A, Chelikani PK. Role of peptide YY(3–36) in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab. 2013;304(9):E944–50.
- 123. Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3–36 on food intake in humans. Gastroenterology. 2005;129(5):1430–6.
- 124. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650–4.
- 125. Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145(1–3):12–16.
- 126. Ivy AC, Oldberg E. A hormone mechanism for gall-bladder contraction and evacuation. Am J Physiol Legacy Content. 1928;86(3):599–613.
- 127. Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97(1):411–63.
- 128. Sekiguchi T. Cholecystokinin. In: Takei Y, Ando H, Tsutsui K, editors. Handbook of hormones. San Diego, CA: Academic Press; 2016. p. 177–e20B-3.
- 129. Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16(10):858–65.
- 130. Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005;6(4):297–306.
- 131. Solomon TE, Yamada T, Elashoff J, Wood J, Beglinger C. Bioactivity of cholecystokinin analogues: CCK-8 is not more potent than CCK-33. Am J Physiol Gastrointest Liver Physiol. 1984;247(1):G105–G11.
- 132. Goebel-Stengel M, Stengel A, Wang L, Ohning G, Taché Y, Reeve JR Jr. CCK-8 and CCK-58 differ in their effects on nocturnal solid meal pattern in undisturbed rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(8):R850–60.
- 133. Lieverse RJ, Jansen J, Masclee AAM, Lamers C. Satiety effects of cholecystokinin in humans. Gastroenterology. 1994;106(6):1451–4.
- 134. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–9.
- 135. Christoffersen BØ, Skyggebjerg RB, Bugge A, Kirk RK, Vestergaard B, Uldam HK, Fels JJ, Pyke C, Sensfuss U, Sanfridson A, et al Long-acting CCK analogue NN9056 lowers food intake and body weight in obese Göttingen minipigs. Int J Obes. 2020;44(2):447–56.
- 136. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85.
- 137. Chieffi S, Carotenuto M, Monda V, Valenzano A, Villano I, Precenzano F, Tafuri D, Salerno M, Filippi N, Nuccio F, et al Orexin system: the key for a healthy life. Front Physiol. 2017;8:357.
- 138. Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, Williams AJ. The hypocretin/orexin system. J R Soc Med. 2002;95(5):227–30.
- 139. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015.
- 140. Zhang S, Blache D, Vercoe PE, Adam CL, Blackberry MA, Findlay PA, Eidne KA, Martin GB. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 2005;124(1–3):81–7.
- 141. Heinonen MV, Purhonen AK, Mäkelä KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiologica. 2008;192(4):471–85.
- 142. Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Commun. 2000;267(2):527–31.
- 143. Mäkelä KA, Karhu T, Jurado Acosta A, Vakkuri O, Leppäluoto J, Herzig K-H. Plasma orexin-A levels do not undergo circadian rhythm in young healthy male subjects. Front Endocrinol. 2018;9:710.
- 144. Blais A, Drouin G, Chaumontet C, Voisin T, Couvelard A, Even PC, Couvineau A. Impact of orexin-A treatment on food intake, energy metabolism and body weight in mice. PLoS One. 2017;12(1):e0169908.
- 145. Bulbul M, Babygirija R, Ludwig K, Takahashi T. Central orexin-A increases gastric motility in rats. Peptides. 2010;31(11):2118–22.
- 146. Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, et al Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13.
- 147. Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105(33):11975–80.
- 148. Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48(11):2132–7.
- 149. Valenzano A, Polito R, Trimigno V, Di Palma A, Moscatelli F, Corso G, Sessa F, Salerno M, Montana A, Di Nunno N, et al Effects of very low calorie ketogenic diet on the orexinergic system, visceral adipose tissue, and ROS production. Antioxidants. 2019;8(12):643.
- 150. Mieda M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24(46):10493–501.
- 151. Almeneessier AS, Alzoghaibi M, BaHammam AA, Ibrahim MG, Olaish AH, Nashwan SZ, BaHammam AS. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann Thorac Med. 2018;13(1):48–54.
- 152. Kurose T, Ueta Y, Yamamoto Y, Serino R, Ozaki Y, Saito J, Nagata S, Yamashita H. Effects of restricted feeding on the activity of hypothalamic orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats. Regul Pept. 2002;104(1–3):145–51.