The first report using free fat autografts stems back to 1893 when Neuber described the use of fat grafting as a means to correct for unilateral facial atrophy. Various methods of fat grafting were then applied to treat malar and chin deficits in 1909 and later other facial deficits in the 1920s upon the invention of injectable fat grafting. Despite the historic use of autologous fat grafting, its incorporation into the aesthetic field largely took effect in the 1980s, when Illouz first introduced its reuse as an injectable graft following liposuction and it was standardized in Coleman’s reports., Although fat grafting has become almost synonymous with body and facial contouring, one of its most significant contributions to the aesthetic field has been its incorporation into standard facelift techniques to address volume losses in soft tissue and bone. As such, facial rejuvenation surgery has evolved from a 2-dimensional process that targeted the dermal and the superficial musculoaponeurotic system (SMAS) fascial layer to a 3-dimensional (3D) process that replaces volume loss in both superficial and deep structural compartments of the face.
As described by Coleman and Grover, decreased skin elasticity, tissue atrophy, and ptosis, along with bone resorption and remodeling, collectively manifest facial aging. By addressing these individual deep and superficial compartments associated with facial aging, restoration of a youthful face can be achieved through correction of tissue ptosis and replacement of volume loss. As such, many facelift techniques now incorporate fat grafting with tissue repositioning and removal. Furthermore, fat grafting can be used to restore contour to the mandible and gonial angle as well as volume restoration of deep and superficial compartments of the temporal region and lower lateral brow.
When analyzing volume losses in any given face, regardless of ethnic background, a variety of patterns with different degrees of severity present in both deep and superficial fat compartments—along with specific regions of the craniofacial skeleton—become apparent. Through facial analysis, one can correlate these particular losses in specific anatomic areas of the face with topographical changes on the surface. This permits more accurate treatment planning. Furthermore, the degree of skin thinning and epithelial/dermal injury can be evaluated with more advanced imaging techniques, profilometry, and histology.
At present there is generally little biologic benefit of fillers. Fillers enhance features, but the tissues around them continue to age. Despite their aesthetic enhancement, they contribute little to the patient’s individual rate of tissue decay while remaining expensive, impermanent, foreign, and immunogenic with possible, albeit unlikely, disease transmission. In contrast, autologous fat grafting remains inexpensive, natural, readily available, and biocompatible. Furthermore, with the introduction of microfat, millifat, and nanofat, fat grafting has allowed the surgeon to modify or retrieve individually sized adipose parcels to treat the same range of facial aging concerns that are presently addressed with cosmetic fillers: from fine lines and rhytids, to larger atrophic facial defects, such as gaunt facial contours due to deep compartment facial fat loss (ie, buccal and temporal regions).
Rigotti and his team demonstrated neo-angiogenisis and histologic signs of reversal of architectural changes of aging in elastin and collagen employing mechanically obtained stromal vascular fraction and expanded mesenchymal stem cells., Neo-angiogenesis has been shown with most types of fat injections, leading one to speculate that these regenerative approaches may act as replacement techniques that delay tissue aging by replacing losses in tissue and blood supply as they occur. Despite these findings, the question remains: “What effect does autologous fat grafting have on the longevity of facial volume?” One of the important questions this study raises is the following: If an increase in facial volume does exist, how does it progress and how long does it last when fat is used in a protocol of specific sizes for different fat compartments and layers of the face? As a means to follow the short-and long-term effects of simultaneous facelift and fat grafting, we progressively tracked facial volume changes of the midfacial zones during an 18- to 24-month period. Herein, we provide novel findings of a regenerative, long-lasting effect that provides an in-vivo extension of a molecular theory of fat graft survival.
METHODS
We retrospectively evaluated midfacial volume in a subset of patients during an 18- to 24-month period using 3D Photometric Imaging. This study was part of a larger prospective study tracking patient satisfaction and outcome that originally used 3D photometry as a secondary measure. A consent form, subject’s bill of rights, and media authorization form were given. These patients underwent trans-oral buccal fat pad augmentation along with premaxillary and prezygomatic deep fat compartment augmentation. 3D analysis of the midfacial region was chosen because this area of fat grafting was least affected by superior and posterior elevation of the skin and SMAS flaps. The fat was harvested with a 2-mm smooth-holed cannula obtained through a 14-gauge needle puncture and was cleaned with normal saline. The fat was emulsified into 3 product sizes: millifat (2- to 2.4-mm parcel size), microfat (1- to 1.2-mm parcel size), and nanofat (400- to 600-micron parcel size). Injection was performed into the deep fat compartments, preperiosteal in the pyriform aperture and prezygomatic and premaxillary areas, the medial and lateral sub-orbicularis oculi fat (SOOF), and into the deep medial cheek utilizing an 18-gauge side port cannula through an 18-gauge needle incision. Millifat was also used in the buccal space and injected transorally through a needle incision either 1 cm below or above Stensen’s duct. Millifat is also used in the deep temporal region and in the preperiosteal brow as well as into the upper and lower lip and chin when indicated based on clinical diagnosis of areas of fat and bone loss. In our procedures, the midfacial technique was relatively consistent and involved some degree of SMAS elevation. In most cases, fat was placed after performing the desired facelift. However, we place fat prior to doing eyelids and brow surgery if indicated.
Of 24 patients entered into a prospective BioMED institutional review board-approved study investigating fat grafting in conjunction with facelift surgery, a subset of 6 patients (12 midfacial regions) were evaluated in this retrospective study from May 2012 to May 2015. Remaining patients were excluded due to: (1) not receiving fat grafting to the trans-oral buccal fat pad and/or the deep fat pad compartments of the midface; (2) incomplete photometric imaging at the preoperative, 1- to 2-month postoperative, and/or 18- to 24-month postoperative visit(s); and (3) extensive rhytids before receiving a facelift. The Vectra 3D Imaging System is only able to detect changes in volume and cannot differentiate between skin and adipose tissue. Excessive rhytides cause wrinkling of the skin that adds “volume” to Vectra baseline measurements and causes a discrepancy between preoperative and postoperative measurements.
As an estimate of volume changes, the midfacial region was measured preoperatively and postoperatively at 1 to 2 months and 18 to 24 months with the Vectra XT 3D Imaging System (Canfield Scientific Inc., Parsippany-Troy Hills, NJ). Preoperative and postoperative photos were overlaid and aligned according to consistent anatomical points and rigid structures of the face that remained invariable over time. Once the photos were overlaid, volume changes in the midfacial zone were measured. The lateral portion of the nasolabial fold, the inferior border of the zygomatic arch, and the superior border of the mandible anatomically defined the perimeter of the buccal space. The amount of volume measured as percentage of fat injected was calculated as follows: the difference in volume between the preoperative and postoperative photo was compared with the amount of injected fat recorded at the time of the procedure. Subsequent edema following surgery produces a false baseline effect; therefore, the recorded amount of fat injected at the time of the procedure was utilized in place of taking an immediate postoperative photo. If available, 3D photometry between the 1- to 2-month and 18- to 24-month postoperative marks of these same patients were measured and assessed in the same manner.
Given the small study size, both parametric and nonparametric tests were performed to determine statistical significance between the 1- to 2-month and 18- to 24-month postoperative measurements of the midfacial zone. Average follow-up times were 1 month (range 1-2 months) and 20 months (range 18-24 months). A 2-tailed, repeated-measures t test and 2-tailed Wilcoxon Signed-Rank test were conducted on patients exhibiting facial volume progression. Statistical significance was defined as P < 0.05 prior to the study.
RESULTS
The average age at the time of the procedure was 62.5 years (range, 52-85 years). All patients were female and underwent either a mini-facelift with lateral smasectomy or a high SMAS facelift in addition to fat transfer to the face. Three patients received necklifts, 2 patients received upper blepharoplasties, 4 underwent lower blepharoplasties, 2 had rhinoplasty, and 1 received an endoscopic browlift. In addition, patient weight and BMI were recorded preoperatively and postoperatively and were virtually unchanged from the start to the completion of the study. The average preoperative and postoperative weight were both 119 lbs. The average preoperative BMI was 20.7 kg/m2 and the average postoperative BMI was 20.6 kg/m2. 3D imaging demonstrated an average improvement in facial volume in the midfacial region.
At the 1- to 2-month follow-up period, average facial volume was 49.60% of the initial fat injected. At the 18- to 24-month follow-up period, average facial volume was 73.64% of the initial fat injected, indicating an increase in midfacial volume (Figure 1). Both parametric and nonparametric tests proved statistically significant when comparing volume changes in patients that showed progressive improvement (P < 0.05).
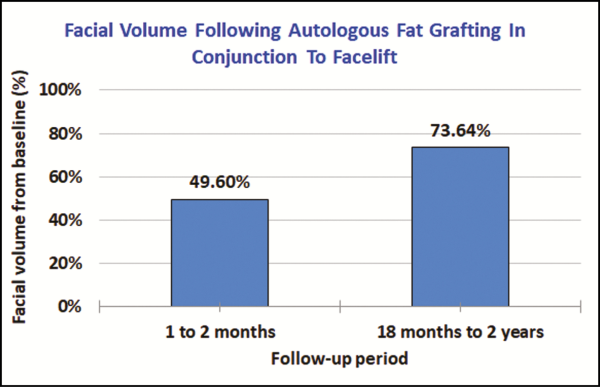
Figure 1
Average percent of increased facial volume at 1- to 2-month and 18- to 24-month periods following autologous fat grafting and facelift. Values were obtained via the Vectra 3D imaging system. Of the midfacial zones assessed at 1 to 2 months, 3-dimensional imaging showed an average of 49.60% of grafted fat retained. At 18 to 24 months, 3-dimensional imaging showed an average of 73.64%.
Further analysis of available 3-, 6-, and 12-month postoperative 3D photos demonstrated that all patients experienced an increase of midfacial volume at some point during the study period; 67% of patients showed a increase in facial volume by the end of the study period (Figures 2 and 3). Upon graphing available photometric data, dynamic changes in facial volume were observed. In 5 midfacial zones, facial volume appeared to initially decline (average decline, 49.0% of original fat injection), troughing at 10 months (range, 2-15 months), but later inclined (average increase in volume, 95.9% of original fat injection), peaking at around 16 months (range, 4-24 months) (Figure 4A,B). Remaining midfacial zones either showed a sigmoidal trend similar to the above or did not exhibit continuous facial volume restoration, tapering off between 4 to 7 months (average, 5.5 months) after treatment (Figure 4). Human error in 3D photometry was calculated to be 0.2187 mL. No complications occurred in any of these patients.
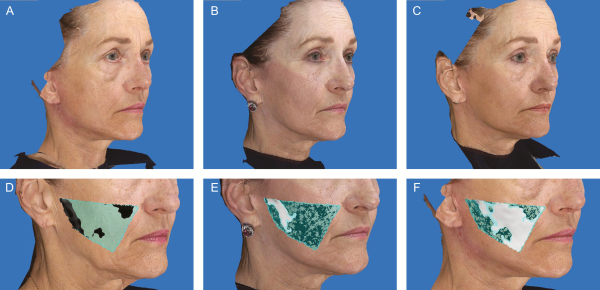
Figure 2
This 63-year-old woman underwent a facelift with high SMAS approach, septoplasty, bilateral upper and lower blepharoplasties, and facial fat grafting. Of the 34 mL of fat injected into the face, 2 mL of fat was injected into each midfacial zone, totaling 4 mL. (A) Preoperative, (B) 1-month postoperative, and (C) 18-month postoperative photographs. (D) 3-Dimensional imaging at baseline. (E) 3-Dimensional imaging at the 1-month postoperative period, showing 0.4867 mL (24.34%) of fat retained upon overlay of preoperative photo. (F) 3-Dimensional imaging at 18-month postoperative period showing 1.7528 mL (87.64%) of fat retained upon overlay of preoperative photo.
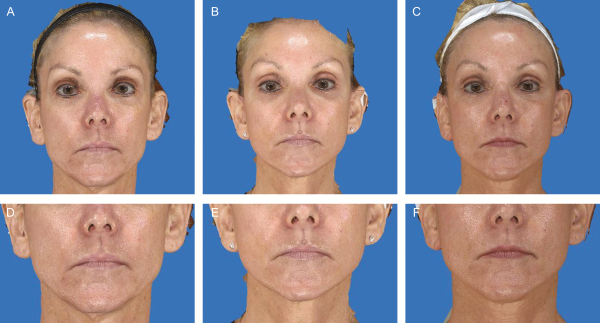
Figure 3
This 52-year-old woman presented with facial aging and depression. Of the 42 mL of fat injected into the face, 5 mL of fat was injected into each midfacial zone, totaling 10 mL. (A,D) Preoperative photographs. (B,E) Nine-month postoperative photographs. 3-Dimensional photometry measured an averaged volumetric increase of 1.70 cc in the midfacial zones when overlaying postoperative and preoperative images in the Vectra XT system. (C,F) Eighteen-month postoperative photographs. 3-Dimensional photometry measured an averaged volumetric increase of 3.16 cc in the midfacial zones when overlaying postoperative and preoperative images.
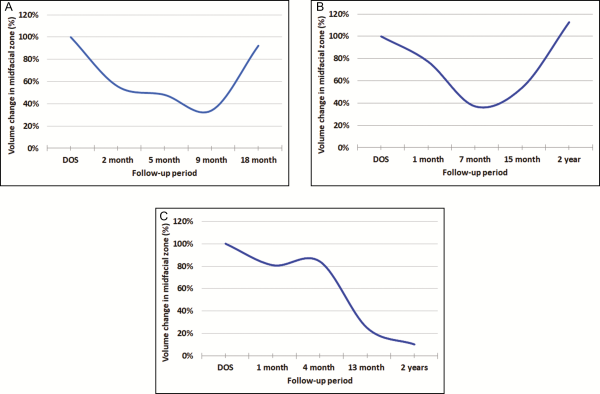
Figure 4
Facial volume changes following an 18- to 24-month period. (A,B) These graphs represent findings of an apparent trend in facial volume following simultaneous fat grafting and facelift. In 5 midfacial zones, facial volume appeared to initially decline (average decline, 49.0% of original fat injection), troughing at 10 months (range, 2-15 months), but later inclined (average increase in volume, 95.9% of original fat injection), peaking around 16 months (range, 4-24 months). (A) This graph represents facial volume changes in the left midfacial zone of a 52-year-old woman after she received 5 mL of autologous fat injection. At 2 months, photometric data measured 2.78 mL of increased facial volume compared with the patient’s preoperative baseline. At 5 months, volume measured 2.40 mL. At 9 months, photometric data measured 1.70 mL of volume, and at 18 months, volume was 4.61 mL. (B) This graph represents facial volume changes in the right midfacial zone of a 57-year-old woman after she received 5 mL of autologous fat injection. At 1 month, photometric data measured 3.86 mL of increased facial volume compared with the patient’s preoperative baseline. At 7 months, volume measured 1.85 mL. At 15 months, volume measured 2.70 mL, and at the 2-year follow-up, volume measured 5.64 mL. (C) This graph represents a different 57-year-old woman who did not exhibit continuous facial volume restoration.
DISCUSSION
Despite the abundant amount of clinical research behind fat grafting, a limited amount of literature discusses the molecular theory behind fat graft survival, with even less research devoted to the long-term effects following facial fat grafting in conjunction with facelifting. To date, 2 well-known theories, the cell survival theory and the host replacement theory, have remained pertinent throughout the field as contrasting theories behind fat graft survival.
In 1923, Neuhof and Hirschfield proposed the host replacement theory wherein grafted adipose tissue immediately dies following transplantation and acts as scaffolding for recruited host adipocytes and connective tissue. However, in 1950, Peer proposed the cell survival theory wherein grafted adipocytes compete for favorable transplantation sites and survive by simple diffusion of nutrients prior to undergoing anastomosis., Although the host replacement theory is now considered outdated, the cell survival theory has been supported and extended.
Mouse model studies conducted by Zhao et al have shown that grafted fat survives through neovascularization. Through the utilization of green fluorescent proteins, Yoshimura showed that host bone marrow and fat graft equally contribute to the development and expansion of capillary networks. Although Yoshimura’s study shows some support of the cell survival theory, only a small subset of adipocytes undergoes angiogenesis and neovascularization. Instead, Yoshimura and colleagues have shown that extensive adipocyte death occurs due to the severe ischemic/hypoxic environment created upon transplantation; therefore his group proposed a new theory revolving around fat graft survival termed the graft replacement theory.
Biochemical assays have shown that most aspirate adipose tissue is viable following harvesting yet ignore the stimulated inflammatory agents in host tissue that follow implantation. The graft replacement theory suggests that graft adipose-derived stem cells survive the initial hypoxic environment of host tissue and eventually replace the dead grafted adipose tissue upon differentiation. Kato et al have provided studies showing differential adipocyte fates dependent on 3 fat graft zones (surviving, regenerating, and necrotizing zones) that lead to 4 different outcomes: survival, successful regeneration, failed regeneration/cicatrization, and oil cyst formation.,
The surviving zone, which is superficial to the fat graft, adjacent to host tissues, and approximately 100 to 300 µm thick, contains both adipocytes and adipose-derived stem cells that survive transplantation. Given their proximity to host tissues, grafted adipocytes can survive initially through plasmatic diffusion prior to neovascularization, as originally suggested by the cell survival theory. However, directly below the survival zone is the regenerating zone, in which all grafted adipocytes die upon transplantation and are replaced by adipose-derived stem cells. Lastly, at the core of the transplanted graft is the necrotizing zone, in which both adipocytes and adipose-derived stem cells die and either undergo cicatrization or oil cyst formation dependent on adipocyte size. Pu has provided an extensive review on the current literature behind theories of fat graft survival.
Emile Durkheim referred to modern society as an organic solidarity in which different specializations work interdependently to complement the functioning unit as a whole. The aging face represents a collapsing network between skin, tissue, and bone as the decay of one compartment leads to a systematic collapse of others. As such, rejuvenation surgery requires targeting of both superficial and deep structural compartments. To date, the graft replacement theory has appeared to be the most significantly relevant to the field, and by 3D photometry, we provide clinical observations that appear to support and possibly extend the graft replacement theory.
At the 1- to 2-month follow-up period, average facial volume was 49.60% of the initial fat injected. At the 18- to 24-month follow-up period, average facial volume was 73.64% of the initial fat injected. Eight of the 12 midfacial zones (67%) showed an increase in facial volume at the end of the study period; 5 of those 8 midfacial zones underwent an initial decline in facial volume (49.0%) followed by a restoration phase (95.9%). Yoshimura’s mouse models showed that adipose-derived stem cells replace dead grafted adipocytes up to 3 months following fat graft injection with slow lipid droplet absorption of adipose tissue that can persist up to 12 months following injection. Through clinical observations and 3D photometry, our results appear to support the graft replacement theory in that facial volume decreased during the first several months (average, 10 months; range, 3-15 months) following transplantation and reached its nadir in similar time as Yoshimura’s animal studies (10 months). Necrotic adipose tissue and latent swelling likely contribute to the initial volume seen within the 1- to 2-month postoperative period. It is also likely that the apparent decline in adipose tissue is due to lipid droplet adsorption for up to 15 months following injection. The second wave of progressive volume restoration in midfacial volume (at approximately 16 months) provides a finding we have not seen in any other data thus far and may give plausible insight into the long-term underlying dynamic changes occuring in facial volume after simultaneous fat transfer and facelift. These dynamic changes may be due to differentiating stem cells from the original fat graft that act as a continual source of facial volume restoration. It is well known that cells will continue to divide until they are impeded by contact inhibition. This is most evident in how cells in a petri dish will continue to divide until forming a monolayer. The host replacement theory claims that grafted adipose tissue immediately dies following transplantation and acts as scaffolding for recruited host adipocytes and connective tissue. Does this scaffolding of dead adipose tissue that undergoes lipid droplet formation create new parameters of facial volume that stimulate the grafted stem cells to continuously differentiate? Further studies should be conducted to provide further insight. These findings, together with Rigotti’s work,, show a histologic reversal of aging architecture that points to a new paradigm in the management of facial atrophy: injectable tissue replacement. Injectable tissue replacement keeps up with biological losses as they become phenotypically apparent to the patient. Given that volume reflects facial mass, by improving volume over a prolonged period we can attenuate the curve of decay through fat graft injection.
Although most patients commented that their skin texture and thickness improved, it was difficult to quantitate. In part, this may be related to improvement in blood supply and some degree of reversal of architectural changes in elastin and collagen. How to quantitate these findings without the aid of histological information is a bit more challenging, especially when changes may be more subtle.
Limitations
Although 3D imaging has been shown to be fairly accurate, we reported a 0.2187-cc human error. Every face shape produces a different midfacial zone; therefore, we did not use a set region to record midfacial volume between individuals. Instead, a specific region was outlined for each individual and used continuously throughout the study period. To meet the aesthetic means of our patients, different volumes of fat were transplanted into the midfacial zones (range, 2-5 cc per midfacial zone) and although consistent anatomical points of both 3D preoperative and postoperative photos were aligned, clenching of the teeth, tightening of the jaw, and other subtle movements can slightly affect photometric overlays. As suggested, human error was calculated by taking the standard deviation from 10 analyses of the same 3D photometric comparison of the midfacial zone. Given this study was clinically based, we were unable to standardize the growth stage of the injected adipocytes. Moreover, every patient is subjected to their own intrinsic rates of tissue decay, which we believe was responsible for the additional trends observed. Furthermore, considering this institutional review board-approved study was volunteered based, patient enrollment and commitment were relatively difficult, limiting our study size and leading to inconsistent follow-ups. Patient BMI remained consistent throughout the course of the study period, and no severe rhytids were observed. In addition, results excluded patients who received fillers or secondary facial fat grafting to the midfacial zone. As such, it is unlikely that an external variable is contributing to the progressive improvement in facial volume shown. The midface area seemed the easiest from to retrieve a reliable measurement in volume and determine changes over time; however, we recognize results may be affected if patients receive a variety of additional facial surgeries. A comparison group of facelift patients without autologous fat grafting would have further validated the results of this study. The effect of brow-lifting and eyelid surgery would need to be measured to determine the effects of simultaneous surgery with this type of fat grafting technique. Furthermore, this study was part of a larger prospective study tracking patient satisfaction and outcome. Originally, 3D photometry was used as a secondary measure to solely track volume changes over time. We took a retrospective approach in looking further into the secondary measures and therefore did not use a standardized control that would have definitively validated our results. In future studies, we will assess volume changes over time in patients who do not receive facial rejuvenation surgery. In addition, several authors have reported poor maintenance in grafted adipose tissue in older patients. Given the amount of fat injected into our subset of patients ranged from 2 to 5 mL, our reported values were too small to provide further insight.
CONCLUSIONS
To our knowledge, this is the first report to document changes in midfacial volume following combined facelift and fat grafting 18 to 24 months after treatment. Earlier applications of injectable tissue replacement and regenerative approaches may delay tissue decay and affect cellular aging. Studies are underway to further verify these intriguing observations. Furthermore, we encourage others to investigate additional strategies in regenerative medicine such as platelet rich plasma (PRP) treatments and growth factors and in the future, possibly intravenous supplementation of cells in pharmaceuticals used to slow the aging process.
Disclosures
Dr Cohen consults for Tulip Medical, Inc., Millenium Medical Technologies, Inc., and Galderma; is a paid investigator for Ampersand, Allergan, and Thermigen; is an MID distractor with royalties on invention for Stryker; and is a consultant for and shareholder in Cytori, Inc. The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Presented at: The Aesthetic Meeting 2017 in San Diego, CA, in April 2017 and was the recipient of the Tiffany Award for Best Scientific Presentation.
REFERENCES
- 1. Neuber GA. Fett transplantation. Verh Dtsch Ges Chir. 1893;22:66.
- 2. Van de Graaf RC, Korteweg SFS. Gustav Adolf Neuber (1850-1932) and the first report on fat auto-grafting in humans in 1893. Hist Plast Surg. 2010;1(1):7–11.
- 3. Miller CG. Cannula Implants and Review of Implantation Techniques in Esthetic Surgery. Chicago, IL: Oak Press; 1926.
- 4. Illouz YG. L’avenir de la réutilisation de la graisse après liposuccion. Rev Chir Esthétique Lang Fran. 1984;36: 13–14.
- 5. Agris J, Illouz YG, Pitanguy I. Liposuction: The Franco-American Experience. Medical Aesthetics, Incorporated. Am J Cosmet Surg. 1987;(4)2:89-94.
- 6. Illouz YG. The fat cell “graft”: a new technique to fill depressions. Plast Reconstr Surg. 1986;78(1):122–123.
- 7. Illouz YG. Present results of fat injection. Aesthetic Plast Surg. 1988;12(3):175–181.
- 8. Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19(5):421–425.
- 9. Coleman SR. The technique of periorbital lipoinfiltration. Oper Tech Plast Reconstr Surg. 1994;1(3):120–126.
- 10. Guerrerosantos J. Simultaneous rhytidoplasty and lipoinjection: a comprehensive aesthetic surgical strategy. Plast Reconstr Surg. 1998;102(1):191–199.
- 11. Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9.
- 12. Winters R, Moulthrop T. Is autologous fat grafting superior to other fillers for facial rejuvenation?Laryngoscope. 2013;123(5):1068–1069.
- 13. Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, et al Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36(3):261–270.
- 14. Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C, et al Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. 2015;135(4):999–1009.
- 15. Cohen SR, Fireman E, Hewett S, Saad A. Buccal fat pad augmentation for facial rejuvenation. Plast Reconstr Surg. 2017;139(6):1273e–1276e.
- 16. Gause TM 2nd, Kling RE, Sivak WN, Marra KG, Rubin JP, Kokai LE. Particle size in fat graft retention: a review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 2014;3(4):273–279.
- 17. Patel AJ, Benson JR, Malata CM. Chapter 29: The science of autologous fat grafting. In: Querci della Rovere G, Benson JR, Nava M, eds. Oncoplastic and Reconstructive Surgery of the Breast. Boca Raton, FL: CRC Press; 223–233.
- 18. Neuhof H, Hirshfeld S. The transplantation of tissues. New York, NY: Appleton, 1923.
- 19. Peer LA. loss of weight and volume in human fat grafts: with postulation of a cell survival study. Plast Reconstr Surg. 1950;5(3):217–230.
- 20. Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946). 1955;16(3):161–168.
- 21. Zhao J, Yi C, Li L, et al Observations on the survival and neovascularization of fat grafts interchanged between C57BL/6-gfp and C57BL/6 mice. Plast Reconstr Surg. 2012;130(3):398e–406e.
- 22. Doi K, Ogata F, Eto H, et al Differential contributions of graft-derived and host-derived cells in tissue regeneration/remodeling after fat grafting. Plast Reconstr Surg. 2015;135(6):1607–1617.
- 23. Suga H, Eto H, Aoi N, et al Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126(6):1911–1923.
- 24. Eto H, Kato H, Suga H, et al The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129(5):1081–1092.
- 25. Yoshimura K, Eto H, Kato H, Doi K, Aoi N. In vivo manipulation of stem cells for adipose tissue repair/reconstruction. Regen Med. 2011;6(6 Suppl):33–41.
- 26. Lalikos JF, Li YQ, Roth TP, Doyle JW, Matory WE, Lawrence WT. Biochemical assessment of cellular damage after adipocyte harvest. J Surg Res. 1997;70(1):95–100.
- 27. von Heimburg D, Hemmrich K, Haydarlioglu S, Staiger H, Pallua N. Comparison of viable cell yield from excised versus aspirated adipose tissue. Cells Tissues Organs. 2004;178(2):87–92.
- 28. Wolter TP, von Heimburg D, Stoffels I, Groeger A, Pallua N. Cryopreservation of mature human adipocytes: in vitro measurement of viability. Ann Plast Surg. 2005;55(4):408–413.
- 29. Jauffret JL, Champsaur P, Robaglia-Schlupp A, Andrac-Meyer L, Magalon G. Arguments in favor of adipocyte grafts with the S.R. Coleman technique. Ann Chir Plast Esthet. 2001;46(1):31–38.
- 30. Butterwick KJ. Lipoaugmentation for aging hands: a comparison of the longevity and aesthetic results of centrifuged versus noncentrifuged fat. Dermatol Surg. 2002;28(11):987–991.
- 31. Kato H, Mineda K, Eto H, et al Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133(3):303e–313e.
- 32. Doi K, Ogata F, Eto H, et al Differential contributions of graft-derived and host-derived cells in tissue regeneration/remodeling after fat grafting. Plast Reconstr Surg. 2015;135(6):1607–1617.
- 33. Pu LL. Mechanisms of Fat Graft Survival. Ann Plast Surg. 2016;77(Suppl 1):S84–S86.
