More than 20 years ago, we wrote a review evaluating whether or not angiotensin-converting enzyme inhibitor (ACE)-associated elevations in serum creatinine were of concern after the initiation of these drugs []. The beneficial effects of renin-angiotensin system (RAS) blockers, ACE inhibitors, and angiotensin receptor blockers (ARBs) have been known for almost 30 years. These drugs cause an approximate 10–30% initial reduction in estimated glomerular filtration (eGFR) when therapy is initiated [, ]. This initial drop in eGFR will vary depending on hydration status and poorly controlled blood pressure duration. It is also well known that these drugs reduce blood pressure and albuminuria and slow the progression of kidney disease in people with and without diabetes [].
This initial drop in eGFR had been a significant concern for many clinicians and remains so today. In the current issue of the journal, Cherney et al. [] report the effects of a sodium-glucose cotransporter (SGLT 2) inhibitor on initial changes in estimated glomerular filtration rate (eGFR) and describe that with the initial dip in eGFR, there is an associated improvement in kidney function decay over time (DOI: 10.1159/000524889). These observations are entirely consistent with prior observations with other SGLT 2 inhibitors, ACE inhibitors, and angiotensin receptor blockers (ARBs) as a class []. These data add to our understanding that the therapeutic advantage of specific drug classes that ultimately slow diabetic kidney disease progression and reduce cardiovascular (CV) events lowers blood pressure and albuminuria. Still, all have an initially limited decline in eGFR.
In the past 5 years, in addition to the SGLT2 inhibitors [, ], an agent from a new class, the nonsteroidal mineralocorticoid receptor antagonists, finerenone, has also been demonstrated to slow the decline in kidney function among those with diabetes, when added to maximally tolerated doses of either ACE inhibitors or ARB []. These drug classes also lower blood pressure, reduce albuminuria, and slow the progression of kidney disease, but they are associated with an initial dip in GFR that is somewhat less than that described with ACE inhibitors or ARB [, ] Fig. 1. The consistency of the observation that dipping is associated with cardiorenal protection is comforting for clinicians who observe an upward trend in the serum creatinine early in drug initiation.
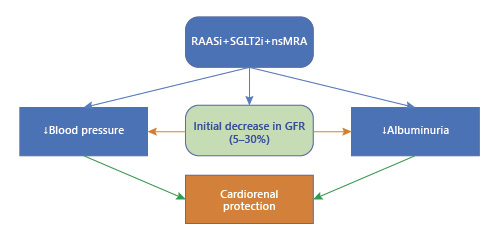
Fig. 1
Interaction between cardiorenal protective drugs and eGFR drops.
The actual mechanism of the eGFR dip is not entirely known. Some suspect it may be simply a reduction in glomerular capillary pressure due to a decrease in systemic blood pressure and selective effects on either efferent glomerular arteriolar dilation or some degree of pre-glomerular vasoconstriction, or a combination of the two. There may also be effects on the glomerular ultrafiltration coefficient as well. The magnitude of reduction in eGFR is a function of initial eGFR values; the lower the eGFR, the less the dip [, ]. Nonetheless, the consistency of these observations is an important consideration when utilizing these drugs in clinical practice, either alone or in combination with other medications that affect blood pressure or blood volume. Clinicians should be aware that changes in GFR will occur upon the initiation of these drugs.
In clinical practice, clinicians often utilize renal protective drugs with other commonly used antihypertensive drugs such as thiazide and thiazide-like diuretics. These agents facilitate not only better blood pressure reduction but also a further reduction in albuminuria. They can also potentiate the dip observed with renoprotective classes of medications []. Many clinicians may stop both diuretics and renal protective drugs because of this initial increase in serum creatinine. However, this is not a wise consideration given that these drugs do have a long-term benefit on kidney function and CV events, and the continuation of these therapies reduces the long-term risk of significant adverse clinical outcomes irrespective of initial changes in serum creatinine. Moreover, a review of older studies, some with as long as a 10-year follow-up, demonstrates the benefits of slowed progression of kidney disease with an initial rise in serum creatinine of up to 30% upon initiation of therapy [, ].
Despite the consistency of cardiorenal benefits, many clinicians do not utilize these renal protective drugs or use them in doses not proven to slow kidney disease progression. This is a significant public health concern, and more effort should be provided to educate clinicians that these acute and self-limited changes in kidney function need to be monitored but should not be an indication of a cessation of these cardiorenal protective therapies. With a persistent increase in serum creatinine of 30%, or more, clinicians should be sure that their patients are not taking nonsteroidal anti-inflammatory drugs, are not volume depleted, and rule out clinically significant renal artery stenosis, if necessary, with imaging. The abundance of data supports the continued use of these drugs. It expands the use of these renal protective therapies in clinical practice to take full advantage of their potential for arresting the rate of progression of kidney and cardiovascular disease. This is evident from data on many recent outcome studies [, ].
Conflict of Interest Statement
George Bakris: supported by T32 NIH grant DK07011, consultant to Bayer, KBP Biosciences, Ionis, Alnylam, Astra Zeneca, Quantum Genomics, Horizon, Novo Nordisk, Dia Medica Therapeutics, InREGEN. Matthew Weir: supported by R01 DK116095, U01 DK1061022, R01 DK120886, U01 DK129884, and Scientific advisor: AstraZeneca, Bayer, Merck, Boehringer-Ingelheim, Janssen, NovoNordisk, Akebia, Vifor.
Funding Sources
There is no funding involved in this editorial.
Author Contributions
Equal contributions were made by both Dr. G.L. Bakris and Dr. M.R. Weir.
References
- 1. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern?Arch Intern Med. 2000 Mar 13;160(5):685–93. http://dx.doi.org/10.1001/archinte.160.5.685.
- 2. Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011 Aug;80(3):282–7.
- 3. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
- 4. Cherney DZI, Cosentino F, Dagogo-Jack S, McGuire D, Pratley RE, Federich R, et al. Initial eGFR changes with ertugliflozin and associations with clinical parameters: analyses from he VERTIS CV trial. Am J Nephrol. 2022. (In Press). DOI: 10.1159/000524889.
- 5. Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, et al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021 Mar;99(3):750–62.
- 6. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295–306.
- 7. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
- 8. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
- 9. Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021 Apr;99(4):999–1009.
- 10. Bakris G, Oshima M, Mahaffey KW, Agarwal R, Cannon CP, Capuano G, et al. Effects of Canagliflozin in patients with baseline eGFR <30 mL/min per 1.73 m2: subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol. 2020 Dec 7;15(12):1705–14.
- 11. Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020 May;31(5):1128–39.
- 12. Appel LJ, Wright JT, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–29.
- 13. Collard D, Brouwer TF, Olde Engberink RHG, Zwinderman AH, Vogt L, van den Born BH. Initial estimated glomerular filtration rate decline and long-term renal function during intensive antihypertensive therapy: a post hoc analysis of the SPRINT and ACCORD-BP randomized controlled trials. Hypertension. 2020 May;75(5):1205–12. http://dx.doi.org/10.1161/HYPERTENSIONAHA.119.14659.
- 14. Beddhu S, Shen J, Cheung AK, Kimmel PL, Chertow GM, Wei G, et al. Implications of early decline in eGFR due to intensive BP control for cardiovascular outcomes in SPRINT. J Am Soc Nephrol. 2019 Aug;30(8):1523–33.
