Introduction
Peripheral arterial disease (PAD) is a chronic atherosclerotic process that causes narrowing or occlusion of peripheral arteries, particularly in the lower limbs. Although the majority of patients remain asymptomatic, symptomatic progression often manifests as intermittent claudication (IC) or, in severe cases, chronic limb-threatening ischemia (CLTI). Chronic limb-threatening ischemia is associated with a particularly poor prognosis, with an estimated annual amputation and mortality rate of 12% and 25%, respectively. Much of the associated mortality risk associated with PAD is attributable to the increased risk of myocardial infarction and stroke, for which PAD is an independent risk factor. ,
The management for PAD is multimodal. Conservative treatment centers around the optimization of cardiovascular (CV) risk factors. Patients with short distance claudication or CLTI are offered open or endovascular revascularization, with the aim of improving blood flow and tissue perfusion, in order to prevent limb loss and improve symptomatology. Observational studies have demonstrated higher healing rates in patients with CLTI and tissue loss undergoing revascularization as compared with those undergoing conservative management. Additionally, PAD is associated with systemic endothelial dysfunction and inflammation, which can accelerate existing CV disease. – This is thought to be the result of reduced vessel wall shear stress and repeated ischemia–reperfusion injury. Therefore, it is postulated that achieving improvements in flow and perfusion with revascularization may improve systemic endothelial function and also lead to a wider protective effect to the CV system.
Flow, perfusion, and systemic endothelial function may well be closely related. However, this is a relatively recent concept with little evidence supporting it. In order to effectively investigate this association in future studies, we must first better understand the effect of revascularization on each of these parameters. Therefore, the aim of this systematic review is to explore the impact of revascularization on (1) lower limb blood flow, (2) tissue perfusion, and (3) systemic endothelial function.
Methods
Eligibility Criteria
Observational studies of patients undergoing revascularization for CLTI or claudication in which lower limb flow, tissue perfusion, or systemic endothelial function were measured. All eligible studies were included. Conference abstracts, protocol papers, and studies involving animals were excluded.
Search
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations, an electronic database search was conducted using MEDLINE, Embase, and Web of Science to include articles from January 1, 1956, to November 30, 2019, written in English. Reference lists were examined from the retrieved full-text articles. In our search strategy, we used the following key terms: “lower limb,” “bypass,” “angioplasty,” “revascularization,” “haemodynamics,” “perfusion,” “pressure,” “flow,” “velocity,” “microcirculation,” and “endothelial function.” Search results were then imported into Covidence (Covidence.org) for duplicate removal and study selection. Titles and abstracts were independently reviewed by 2 investigators (S.K. and P.N.). Full-text articles were then reviewed, and data collected on the methods, participants, intervention, outcomes, and findings. Disagreements were resolved through discussion between all the authors.
Outcomes
We collected data relating to changes in lower limb blood flow, perfusion, or systemic endothelial function following revascularization. No specific limitation was set on the surrogate measures for these outcomes of interest. For the purpose of this review, surrogates of flow were defined as any measure (direct or indirect) of lower limb macrovascular blood flow. Surrogates of perfusion were defined as any measure of lower limb tissue perfusion or microvascular function. Accepted measures of systemic endothelial function included biochemical markers of inflammation and endothelial function as well as brachial flow-mediated dilatation (FMD).
Results
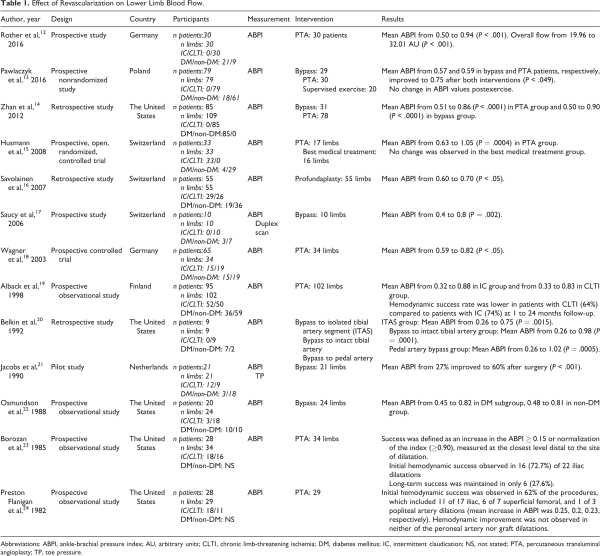
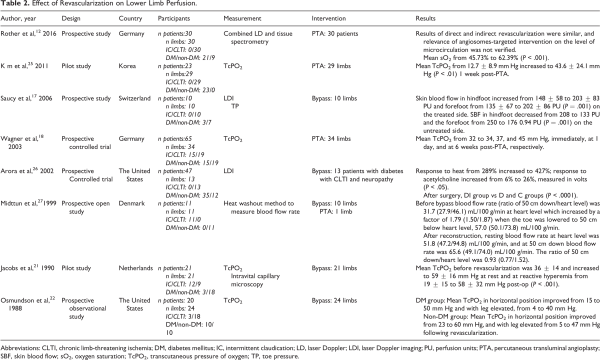
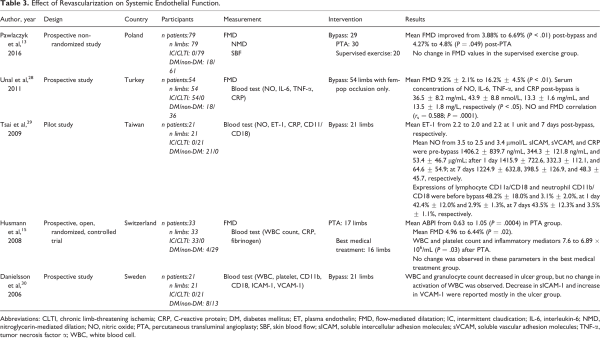
Our initial search identified 4642 papers. Of these, 63 publications were selected for full-text review based on their title and abstract (Figure 1). A full-text screening resulted in the final selection of 19 studies; 13 of these studies reported the effect of revascularization on flow (Table 1), 9 articles reported tissue perfusion measurements (Table 2), and 5 articles reported on systemic endothelial function (Table 3). Key findings are presented in Figure 2.
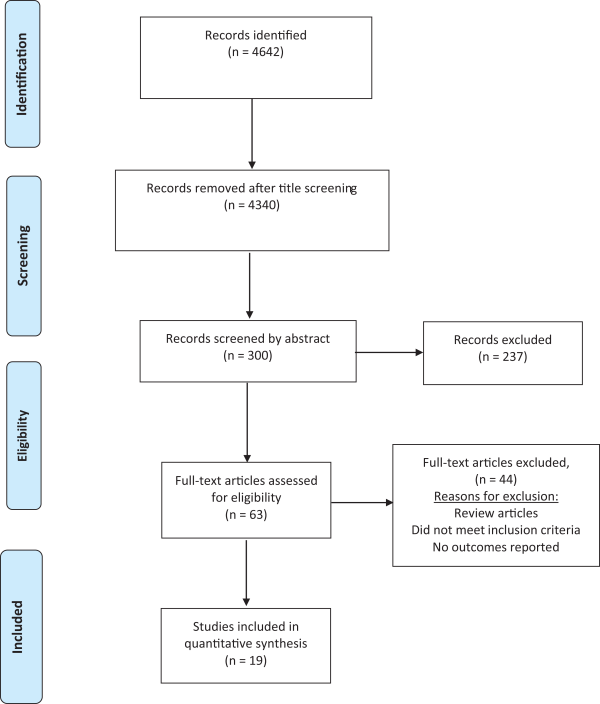
Figure 1
PRISMA systematic review flow diagram.
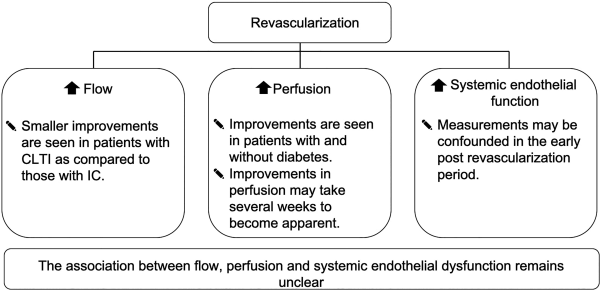
Figure 2
Summary of key findings. CLTI indicates chronic limb-threatening ischemia; IC, intermittent claudication.
Description of Studies
Only 1 study was a randomized controlled trial. Of the remaining studies, 15 were prospective and 3 were retrospective. Sample sizes ranged from 9 to 85, with a total number of 707 participants across all studies. A total of 697 limbs underwent endovascular or open bypass revascularization, and 86 participants participated as controls in 5 studies (angiography [n = 31], exercise treatment [n = 16], best medical therapy [n = 20], and healthy volunteers [n = 12]). A total of 261 patients with IC and 375 patients with CLTI were included across the studies. A total of 316 (43.8%) patients had diabetes mellitus (DM) and 4 studies recruited only patients with DM. Studies that included patients with DM and non-DM reported separate results for each group. Mean age of patients in the included studies ranged between 47 and 94 years, with 65% men and 36% women. Study duration ranged from 14 days to 48 months.
Studies Assessing Flow
Thirteen studies reported ankle brachial pressure indices (ABPIs) after revascularization. ,,,,,,,,, Nine of these studies reported significant improvement in initial ABPI. ,,,,,,,, Overall, success in improving flow was lower in patients with CLTI compared to patients with IC at 1 to 12 months follow-up; Savolainen et al performed revascularization on 55 cases (26 CLTI and 21 IC) and reported a better outcome in patients with IC compared to patients with CLTI. Alback et al reported 83% and 66% rate of hemodynamic success at 1-month follow-up in patients with IC and CLTI, respectively.
A randomized controlled trial compared percutaneous transluminal angioplasty (PTA) with best medical treatment in 17 patients with IC and reported significant increase in ABPI in the PTA group only (0.63-1.05; P = .0004).
Borozan et al observed a 72% initial ABPI improvement in 34% of patients with IC treated with PTA, followed by a significant deterioration of flow during the follow-up period. Continued hemodynamic success was maintained in 37%, and overall long-term success was 27% during a mean follow-up of 16 months. The average time to deterioration in ABPI following PTA was 14 months.
Flanigan et al observed an immediate improvement in ABPI in only 62% of patients with IC treated with PTA, despite achieving 93% immediate anatomical success. Only 48% of patients maintained hemodynamic success during a mean follow-up duration of 9 months.
Pawlaczyk and colleagues measured flow in 3 groups of patients: bypass (group 1, n = 30), PTA (group 2, n = 29), exercise only (group 3, n = 20). They reported a significant improvement in mean ABPI in groups 1 and 2 at 90 days postprocedure (mean ABPI: 0.57 and 0.59 in groups 1 and 2, respectively, improved to 0.75 in both groups; P < .049). No change in ABPI values was observed in the exercise group (group 3).
Studies Assessing Tissue Perfusion
We identified 8 studies that measured changes in perfusion after PTA and bypass in 162 patients (CLTI, n = 122; IC, n = 40; DM, n = 85) using 3 different methods (laser Doppler method, transcutaneous pressure of oxygen [TcPO2], and heat washout technique). ,,,,,,, All 8 studies observed an improvement in perfusion when compared with preintervention measurements, regardless of the method of perfusion assessment. Kim et al reported an increase in mean TcPO2 from 12.7 ± 8.9 to 43.6 ± 24.1 mm Hg (P < .01) at 1 week post-PTA. Jacobs et al reported an increase in mean TcPO2 from 36 ± 14 to 59 ±16 mm Hg at rest. They also demonstrated an increase in TcPO2 in reactive hyperemia from 19 ± 15 to 58 ± 32 mm Hg post-op (P < .001). Osmundson et al provided separate results following open revascularization for patients with DM and non-DM with CLTI. In the DM group, mean TcPO2 in the horizontal position improved from 15 to 50 mm Hg and mean TcPO2 with the leg elevated improved from 4 to 40 mm Hg. In the non-DM group, the corresponding values were 23 to 60 and 5 to 47 mm Hg.
Wagner et al demonstrated that improvements in perfusion may take as long as 6 weeks to manifest following endovascular revascularization (mean TcPO2: prior to revascularization 32 mm Hg, at completion 34 mm Hg, 37 mm Hg 1 day following revascularization, and 45 mm Hg 6 weeks following revascularization). Saucy et al (n = 10) reported a significant increase in skin blood flow (SBF) on the treated side 10 days post-bypass (hindfoot from 148 ± 58 to 203 ± 83 perfusion units [PU] and forefoot from 135 ± 67 to 202 ± 86 PU, P = .001). Interestingly, they observed a statistically significant decrease in SBF on the untreated side at 10 days postoperation (hindfoot from 208 to 133 PU and the forefoot from 250 to 176 0.94 PU, P = .001). No decrease in flow was observed on the untreated side. The mechanism behind the decrease in SBF was not clear. However, the authors hypothesized that it may be due to a neuromediated vasoconstriction of microvascular networks. In this study, the authors reported a significant increase in flow as measured by toe pressures (P = .001) on the treated side and demonstrated a good correlation between SBF and toe pressure (ie, perfusion and flow).
Rother et al measured microcirculatory changes (using laser Doppler tissue spectrometry) continuously during and immediately following tibial PTA in 30 patients with CLTI (20 diagnosed with DM) at different angiosome regions of the index foot and reported a statistically significant increase in microcirculation in all patients with overall mean oxygen saturation (sO2) improvement of 62.4%. They also compared directly and indirectly revascularized angiosomes and found no difference between the 2 groups in either flow or sO2 parameters postintervention. This suggests that changes in tissue perfusion following revascularization are global and not restricted to angiosome-defined borders.
Arora et al compared the post-bypass vasodilatory response (using laser Doppler imaging to measure vasodilation of the foot skin in response to heat and to iontophoresis of 1% acetylcholine and 1% sodium nitroprusside) in 13 patients with diabetic neuropathy and CLTI (group DI) with 3 different groups of patients who did not undergo revascularization (diabetic neuropathy without CLTI [group DN, n = 15], no neuropathy or ischemia [group D, n = 7], and healthy individuals [group C, n = 12]). The response post-bypass was considerably improved when compared with baseline measurements (response to heat: 289% increased to 427%; response to acetylcholine: 6% increased to 26%, measured in volts P < .05). However, the post-bypass response was comparable with the CLTI without neuropathy group and it was still significantly lower (P < .0001) when compared with no neuropathy and healthy groups despite a successful revascularization.
Studies Assessing Systemic Endothelial Function
Overall, 5 (165 limbs) studies reported the impact of revascularization on systemic endothelial function. ,,, – Tsai et al investigated the effect of bypass surgery on plasma endothelin (ET)-1 and nitric oxide (NO) concentrations, plasma soluble intercellular adhesion molecules (sICAMs), soluble vascular adhesion molecules (sVCAMs), C-reactive protein (CRP), lymphocyte CD11a/CD18, and neutrophil CD11b/CD18 before and at 1 and 7 days postsurgery in 21 diabetic patients. They found no significant change in any of these parameters.
Danielsson et al investigated the effect of revascularization on white blood cell (WBC) and platelet count, expression of CD11b/CD18 on granulocytes and monocytes, and CD41 on platelets and soluble endothelial markers (sICAM-1 and VCAM-1, soluble E-selectin and soluble P-selectin) 4 weeks following bypass. They found a decrease in WBCs and granulocyte counts 4 weeks postrevascularization in the subgroup of patients with ulcers and gangrene. They also noted a decrease in the endothelial marker ICAM-1 and increase in VCAM-1. No significant changes were seen in patients with rest pain, but no tissue loss (n = 8).
Three studies measured FMD to evaluate the effect of revascularization on systemic endothelial function. Flow-mediated dilatation is the expression of the change in diameter of brachial artery after a period of arm ischemia, which indicates the endothelial response to shear stress. Pawlaczyk and colleagues measured FMD in 3 groups of patients (n = 79) with severe IC: bypass (group 1), PTA (group 2), and exercise only (group 3). They reported a significant improvement in FMD in groups 1 and 2, 90 days postprocedure (mean FMD: 3.88%-6.69%, P < .01; and 4.27%-4.8%, P = .049, respectively). No change in FMD values was observed in the exercise group (group 3).
Unal et al reported significant increase in FMD values in 54 patients with IC 4 weeks post-bypass (9.2% ± 2.1% to 16.2% ± 4.5%; P < .01) as well as considerable decrease in serum concentrations of interleukin-6, tumor necrosis factor α, and CRP post-bypass (36.5 ± 8.2 mg/mL, 43.9 ± 8.8 nmol/L, 13.3 ± 1.6 mg/mL, and 13.5 ± 1.8 mg/L, respectively, P < .05). They also reported significant positive correlation between FMD and serum NO (r = 0.51; P = .0093).
A randomized controlled trial compared PTA with best medical treatment in 17 patients with IC and reported a significant increase in FMD (4.96%-6.44%; P = .02) and a statistically significant decrease in WBC (7.6-6.9 × 106/mL; P = .03) at 4 weeks post-PTA revascularization. No change was observed for CRP or fibrinogen. No change in these parameters was observed in the best medical treatment group.
Discussion
Currently, lower limb revascularization is performed to increase limb perfusion by improving flow. Peripheral arterial disease has also been strongly associated with systemic endothelial dysfunction and inflammation, , but the effect of revascularization on these parameters remains unclear. This is the first systematic review to explore the effect of revascularization on flow, perfusion, and systemic endothelial dysfunction.
The results from the limited number of studies in this review suggest that improvement in perfusion following revascularization is comparable when considering patients with and without DM. Patients with DM and neuropathy are at highest risk of microcirculatory dysfunction. However, Arora et al suggest that foot perfusion and response to heat and acetylcholine can be significantly improved with revascularization in patients with diabetic neuropathy, although the response appears to be inferior to that for patients without DM and those with DM without neuropathy.
Included studies suggest that the likelihood of improving flow is lower in patients with CLTI compared to those with IC. , Reasons for this observation are likely multifactorial and may relate to the often multilevel and complex anatomy of disease in patients with CLTI and also the limitations in methods used to assess flow, such as the confounding effect of calcification on ABPI measurements.
When considering PTA, anatomical success may not always translate into hemodynamic success. Flanigan et al reported a 93% anatomical success but only 62% hemodynamic success immediately post-PTA. Clearly defined flow or perfusion end points may better translate to clinical benefit, although this remains to be investigated. Importantly, as observed in a number of studies in this review, improvements in perfusion may take weeks to become apparent. Studies measuring systemic endothelial dysfunction using FMD were typically performed at least 4 weeks following revascularization. When considering systemic endothelial dysfunction following bypass surgery, measurements in the first few weeks may also be confounded by inflammation related to the operation. Additionally, patients may require hemodynamic support following the procedure, which may further confound measurements of flow, perfusion, and systemic endothelial dysfunction. This may offer an explanation for the conflicting results regarding the effect of revascularization on biochemical markers of systemic endothelial dysfunction. Although a number of studies found significant reductions in biochemical markers, ,, Tsai et al only performed measurements 7 days following bypass surgery and did not observe such changes.
The magnitude of improvements in ABPI and FMD following revascularization seems to be equivocal for bypass and PTA. However, there is paucity of long-term follow-up data regarding the effect of revascularization on flow, perfusion, and systemic endothelial dysfunction. The limited available evidence suggests that deterioration in flow parameters such as ABPI is common even in claudicants, with an average time to deterioration of approximately 14 months after revascularization. ,
Although one study assessed the correlation between flow and perfusion, none of the studies directly assessed the association between flow or perfusion with systemic endothelial dysfunction. Pawlaczyk et al and Husmann and colleagues did demonstrate improvements in flow and FMD but did not assess the correlation between them. ,
One study suggested that improvements in systemic endothelial dysfunction measured with FMD are only observed in those with tissue loss. However, other studies, assessing systemic endothelial dysfunction in patients with IC and CLTI, with and without tissue loss, noted improvements when measurements were taken a few weeks following revascularization. , There are a number of mechanisms that may be contributing to improvements in systemic endothelial function, including healing ulcers, increase in shear stress, and a reduction in inflammation secondary to a decrease in ischemia–reperfusion injury. However, studies included in this review do not adequately investigate this hypothesis. Importantly, they lack direct measures of flow (eg, Duplex ultrasound and strain gauge plethysmography) and local endothelial function proximal and distal to the site of revascularization. Future studies should address these limitations in order to further our understanding of the association between flow, perfusion, and systemic endothelial function.
Limitations
There is a paucity of recent evidence on this topic as most studies were conducted over a decade ago. Studies were also limited by small sample size, short follow-up period, and significant heterogeneity in methodology including inclusion criteria, revascularization approach, outcome metrics, and measurement technique. The latter is particularly relevant to the assessment of FMD, which can only be reliably measured if standard protocols are followed. The included studies did not report FMD methodology in sufficient detail or reference up-to-date FMD assessment guidelines. Additionally, none of the studies controlled for optimization of medical therapy such as initiation of statin therapy or level of physical activity, which may change postrevascularization due to improved IC and influence flow, perfusion, and systemic endothelial function measurements. Furthermore, none of the included studies provided a meaningful assessment of the association between flow, perfusion, and systemic endothelial function following revascularization.
Conclusion
Current evidence suggests that revascularization has a positive effect on flow, perfusion, and systemic endothelial dysfunction. There is a need for well-designed studies to explore the association between flow, perfusion, and systemic endothelial dysfunction.
Authors’ Note All authors contributed to (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Pasha Normahani and Sodabeh Khosravi are the first authors.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Pasha Normahani
https://orcid.org/0000-0002-6362-7535
Mohamed Aslam
https://orcid.org/0000-0002-5927-3201
References
- 1. Rhee SY, Guan H, Liu ZM, et al. Multi-country study on the prevalence and clinical features of peripheral arterial disease in Asian type 2 diabetes patients at high risk of atherosclerosis. Diabetes Res Clin Pract. 2007;76:82–92.
- 2. Novo S. Classification, epidemiology, risk factors, and natural history of peripheral arterial disease. Diabetes Obes Metab. 2002;4(Suppl 2):S1–6.
- 3. Park KB, Do YS, Kim DI, et al. The TransAtlantic InterSociety Consensus (TASC) classification system in iliac arterial stent placement: long-term patency and clinical limitations. J Vasc Interv Radiol. 2007;18:193–201.
- 4. Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–9.
- 5. Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–42.
- 6. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2016;42:4–15.
- 7. Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–9.
- 8. Gokce N, Keaney JF, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75.
- 9. Silvestro A, Scopacasa F, Ruocco A, et al. Inflammatory status and endothelial function in asymptomatic and symptomatic peripheral arterial disease. Vasc Med. 2003;8:225–32.
- 10. Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8.
- 11. Gnasso A, Carallo C, Irace C, et al. Association between wall shear stress and flow-mediated vasodilation in healthy men. Atherosclerosis. 2001;156:171–6.
- 12. Rother U, Krenz K, Lang W, et al. Immediate changes of angiosome perfusion during tibial angioplasty. J Vasc Surg. 2017;65:422–30.
- 13. Pawlaczyk K, Gabriel M, Urbanek T, et al. Changes in flow-mediated dilatation in patients with femoropopliteal occlusion receiving conservative and invasive treatment. Kardiol Pol. 2016;74:772–8.
- 14. Zhan LX, Bharara M, White M, et al. Comparison of initial hemodynamic response after endovascular therapy and open surgical bypass in patients with diabetes mellitus and critical limb ischemia. J Vasc Surg. 2012;56:380–6.
- 15. Husmann M, Dorffler-Melly J, Kalka C, Diehm N, Baumgartner I, Silvestro A. Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. J Vasc Surg. 2008;48:1211–6.
- 16. Savolainen H, Hansen A, Diehm N, et al. Small is beautiful: why profundaplasty should not be forgotten. World J Surg. 2007;31:2058–61.
- 17. Saucy F, Dischl B, Delachaux A, et al. Foot skin blood flow following infrainguinal revascularization for critical lower limb ischemia. Eur J Vasc Endovasc Surg. 2006;31:401–6.
- 18. Wagner H-J, Schmitz R, Alfke H, Klose K-J. Influence of percutaneous transluminal angioplasty on transcutaneous oxygen pressure in patients with peripheral arterial occlusive disease. Radiology. 2003;226:791–7.
- 19. Alback A, Biancari F, Schmidt S, et al. Haemodynamic results of femoropopliteal percutaneous transluminal angioplasty. Eur J Vasc Endovasc Surg. 1998;16:7–12.
- 20. Belkin M, Welch HJ, Mackey WC, O’Donnell TFJ. Clinical and hemodynamic results of bypass to isolated tibial artery segments for ischemic ulceration of the foot. Am J Surg. 1992;164:281–5.
- 21. Jacobs MJ, Beckers RC, Jorning PJ, Slaaf DW, Reneman RS. Microcirculatory haemodynamics before and after vascular surgery in severe limb ischaemia—the relation to post-operative oedema formation. Eur J Vasc Surg. 1990;4:525–9.
- 22. Osmundson PJ, Rooke TW, Hallett JW. Effect of arterial revascularization on transcutaneous oxygen tension of the ischemic extremity. Mayo Clin Proc. 1988;63:897–902.
- 23. Borozan PG, Schuler JJ, Spigos DG, Flanigan DP. Long-term hemodynamic evaluation of lower extremity percutaneous transluminal angioplasty. J Vasc Surg. 1985;2:785–93.
- 24. Flanigan PD, Schuler JJ, Spigos DG, Lim LT. Anatomic and hemodynamic evaluation of percutaneous transluminal angioplasty. Surg Gynecol Obstet. 1982;154:181–5.
- 25. Kim H-R, Han S-K, Rha S-W, Kim H-S, Kim W-K. Effect of percutaneous transluminal angioplasty on tissue oxygenation in ischemic diabetic feet. Wound Repair Regen. 2011;19:19–24.
- 26. Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002;35:501–5.
- 27. Midttun M, Sejrsen P, Paaske WP. Peripheral blood flow rates and microvascular responses to orthostatic pressure changes in claudicants before and after revascularisation. Eur J Vasc Endovasc Surg. 1999;17:225–9.
- 28. Unal O, Karatepe O, Ugurlucan M, Koc B, Filizcan U, Aksoy M. Effects of lower extremity revascularization on the endothelial functions measured with noninvasive brachial artery flow-mediated dilatation. Ann Vasc Surg. 2011;25:969–74.
- 29. Tsai P-H, Liu J-J, Chou S-Y, Chang Y-C, Yeh S-L. Effect of lower extremity bypass surgery on inflammatory reaction and endothelial dysfunction in type 2 diabetic patients. Mediators Inflamm. 2009:417301.
- 30. Danielsson P, Truedsson L, Norgren L. Systemic white blood and endothelial cell response after revascularization of critical limb ischemia is only influenced in case of ischemic ulcers. Int Angiol. 2006;25:310–5.
- 31. Komori K, Matsumoto T, Ishida M, et al. Enhancement of nitric oxide production after arterial reconstruction in patients with arteriosclerosis obliterans. J Vasc Surg. 1997;26:657–62.
- 32. Akamatsu D, Sato A, Goto H, et al. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17:1266–74.
- 33. Pellegrino T, Storto G, Filardi PP, et al. Relationship between brachial artery flow-mediated dilation and coronary flow reserve in patients with peripheral artery disease. J Nucl Med. 2005;46:1997–2002.
- 34. Chao CYL, Cheing GLY. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–14.
- 35. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25.
- 36. Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–47.