Introduction
Sickle cell disease (SCD) is a category of disease affecting hemoglobin molecule. Hemoglobin in SCD is called sickled hemoglobin or HbS, abnormal hemoglobin, because of which the shape of red blood cells (RBCs) becomes crescent or sickle-shaped. These sickled-shaped RBCs get stuck in the capillaries which leads to episodes of acute pain because vaso-occlusion gradually leads to organ damage over time. It is one of the world’s most typical severe monogenic diseases, most prevalent in wide regions of sub-Saharan Africa, the Mediterranean Basin, the Middle East, and India., SCD is characterized by single nucleotide substitution in the gene encoding hemoglobin subunit beta. Substitution of adenine to thymine results in the replacement of glutamic acid to valine. This mutation leads to the polymerization of hemoglobin molecules inside the RBCs, which leads to the crescent shape of RBCs. Patients with SCD have limited oxygen-carrying capacity because of low hemoglobin levels, which can be exacerbated by acute medical conditions like acute chest syndrome or vaso-occlusive crisis. RBCs in SCD patients are more sticky than normal RBCs, which can cause occlusion in blood vessels. Increased blood viscosity, related to HbS, may further restrict blood flow via constricted blood vessels or in normal brain capillaries.–
Neurological complications such as ischemic stroke, silent cerebral infarction (SCI), headache, and cognitive dysfunction are also very common in SCD. Cognitive abnormalities in SCD manifest in various aspects such as working memory, verbal learning, visuomotor function, inadequacies in general intellectual functioning, executive functions, language, and attention., Initially, overt stroke was regarded as the predominant cause of cognitive impairment in SCD. But latest evidence has shown that overt stroke and SCI are usually linked with cognitive decline. Recent data also revealed that neurocognitive abnormalities could occur in children with SCD even if there is no indication of a stroke on magnetic resonance imaging (MRI).,
In this review, we attempt to demystify all the vital areas of the brain responsible for cognitive processing and discuss them in light of alterations reported in SCD. We have included the majority of the imaging studies, associated neuromarkers/biomarkers, and their correlation with various aspects of cognition. Later, we also discussed enhancing the cognitive abilities of individuals with SCD through pharmacological and nonpharmacological interventions.
Neural Substrates of SCD Induced Hypoxic Brain Injury
Neuroimaging Studies
Neuroimaging studies are different imaging techniques that directly or indirectly examine the brain’s anatomy and functioning. It is a relatively recent field of study within medicine, neurology, and psychology. Out of the two broadly classified categories of neuroimaging techniques, positron emission tomography (PET), near-infrared spectroscopy (NIRS), transcranial Doppler (TCD) ultrasonography and functional magnetic resonance imaging (fMRI), etc. assesses the alteration in blood flow in relation to brain activity, while electroencephalography (EEG) based techniques work with the principle of measuring the electrical activity of the brain. Table 1 provides an outline of imaging studies done on SCD patients.
Transcranial Doppler (TCD) Ultrasonography
TCD ultrasonography has recently gained popularity in medical science as a noninvasive, low-cost, secure, and accessible method of assessing cerebrovascular function. It allows for consistent and bidirectional monitoring of cerebral hemodynamics, and pulsatility across the significant cerebral vasculature (central, frontal, posterior, and basilary arteries). It is also free of movement artifact and demonstrates high test-retest reproducibility. The distal internal cerebral artery (ICA) and the adjacent sections of the middle cerebral artery (MCA) and anterior cerebral artery (ACA) are involved in SCD., Studies on infants with sickle cell anemia reported higher cerebral blood flow velocities (systolic and mean) in the basilar artery (BA), MCA, and ICA, which was linked to a medium to a high probability of neurodevelopmental delays. Analysis revealed that children and adolescents with SCD with aberrant systolic velocities (maximum velocity more than 200 cm/s in the MCA and ICA) performed worse cognitively than those with SCD who had usual systolic velocities (maximum velocity 170–200 cm/s).– Kral et al. also discovered a link between cerebral hemodynamics and cognition in SCD patients. Children with SCD with higher and mid-range systolic velocities performed better in verbal recall tasks than those with SCD with normal TCD velocities. Krejza et al. used the Kaufman Brief Intelligence Test (K-BIT-IQ) and TCD to assess cerebral hemodynamics and pulsatility indexes (PI) in the middle cerebral arteries (MCA). Lower PI in the right MCA was linked to a lower K-BIT-IQ component and language scores in 46 SCD children. Furthermore, they discovered that interhemispheric disparities in PIs were much more significantly connected to neuropsychological ability, while flow velocities were unrelated to the K-BIT-IQ score. According to Strouse et al., cognitive function and cerebral blood flow (CBF) have a significant inverse association. Whereas, Onofri et al. and Aygun et al. found no association between mean velocities and cognitive performance., A recent Nigerian study suggests that SCD children with high TCD velocity are at risk for deficiencies in executive planning, specifically males with higher TCD velocity are at a higher risk for complications in auditory working memory. Apart from the above studies, adult SCD patients have lower TCD velocities than pediatric SCD patients.,
MRI/fMRI/MRA Studies
MRI may produce crisp, high-resolution images of the brain’s anatomical structure and identify abnormalities or lesions in the brain. To increase picture contrast, a dye or tracer, like gadolinium, may be injected into a vein in the arm. However, the use of gadolinium contrast agents is gradually reduced, primarily for SCD patients with renal disease, which can impair gadolinium clearance from the body. Variations in the intensity of the nuclear magnetic resonance signal retrieved from different sites in the brain can improve the quality of images. After the scanner’s pulse sequence, the relaxation periods, T1, T2, and fluid-attenuated inversion recovery (FLAIR), are assessed and selected to look at exact tissue inside the brain. Magnetic resonance angiography (MRA), on the other hand, gives crucial information on the state of the microvasculature and has largely supplanted intra-arterial catheter angiography as a precise and noninvasive method of identifying cerebral artery abnormalities.,
The relationship between MRI results and cognitive functioning was assessed by Kugler et al., and found that 50% of patients demonstrated progressive MRI abnormalities and had bad scores in one or more cognitive functioning domains. Armstrong et al. and Gold et al. found central nervous system (CNS) abnormalities on MRI in children with SCD having a clinical history of cerebrovascular accident (CVA). These SCD children had smaller frontal lobe infarcts and were considerably weakened on measures of intellect, memory, and frontal lobe function compared with standard MRI scans of sibling controls., Previously, quantitative MRI investigations in SCD children with cerebral infarction discovered a substantial link between the level of visible tissue injury and cognitive impairments. Schatz et al. discovered differences in left vs. right hemisphere damage in children with SCD stroke by conducting tests that assess particular visual-spatial skills. Further, T2-weighted MRI scan was done to assess midsagittal corpus callosum (CC) size and its relationship with cognitive functioning. Posterior CC size decreased among SCD children, but no association could be found with cognitive functioning because of less sample size., , Other studies also show that intracranial volume is smaller in SCD patients and has significantly more lacunae, mainly in the frontal lobe, parietal lobe, and basal ganglia. Lacunae are supposed to be caused by the obstruction of a penetrating branch of one of the main cerebral arteries. They are defined as a small cystic infarct in the cerebrum and brainstem’s deeper (noncortical) regions. The authors discovered an apparent association between lacunae and IQ, but after controlling for age, this link became nonsignificant., Individuals with SCD also had thinner frontal lobe cortex and smaller basal ganglia and thalamus sizes compared to healthy controls. The authors stated that cognitive impairment might be exacerbated by the reduced volume of the basal ganglia and aberrations in the thalamus.
In addition, resting-state fMRI analysis in SCD patients reported lower functional integration in the sensory-motor, auditory, salient, and subcortical networks when compared to controls, and a more significant proportion of white matter oxygen extraction was linked to reduced connectivity in these networks. These findings propose that increased oxygen extraction and impaired functional connectivity may serve as neuroimaging biomarkers for cognitive deficiency in SCD., According to Novelli et al., cerebral vascular anomalies have also been associated with cognitive decline in SCD patients. Patients with SCD had considerably lower long venule density and higher short venule density than controls, which is inversely associated with cognitive ability. Whole-brain examination of the amplitude of low-frequency fluctuations (ALFF) in resting-state fMRI revealed lower ALFF in the frontal lobe, cerebellum, and medial superior frontal gyrus, as well as existence of white matter hyperintensities which were related to decreased frontal and medial superior frontal gyri activity in SCD subjects. Reduced ALFF in the frontal lobe was associated with lower verbal fluency and cognitive control. Zou et al. employed blood oxygenation level-dependent (BOLD) and cerebral blood flow (CBF)-based fMRI to examine primary visual cortex reaction to visual stimulus in children with SCD and discovered that BOLD responses were decreased. Nocturnal hemoglobin oxygen desaturation and sleep fragmentation can also contribute to cognitive dysfunction in SCD patients. A study by Andreotti et al. proposes a link between cytokine levels and decision-making function in SCD children, implying that inflammatory processes may have a role in cognitive outcomes in these children. They examined verbal and nonverbal abilities, mental flexibility, inhibition, and verbal fluency, in relation to plasma levels of different cytokines like IL-4, 5, 8, and 13 and found that there were substantial negative correlations between cytokine levels and various measures of executive function skills.
PET Scan Studies
MRI provides a high-resolution anatomical description, whereas Positron emission tomography (PET) is the only imaging technique that can display tissue function and structure by utilizing a metabolically active tracer molecule labeled with a positron-emitting isotope. Although there is very scarce literature on PET and cognition in SCD, some data shows anomalies in glucose metabolism and microvascular blood circulation, notably in the frontal lobes. Further study requires a correlation between PET abnormalities and progressive neurologic dysfunction.,
Single-photon emission computed tomography (SPECT) study by Al-Kandari et al. revealed a perfusion deficiency mainly in the frontal lobe of SCD patients, either alone or in collaboration with the temporal and parietal lobes. The results suggest that SPECT was beneficial in the detection of brain perfusion deficiency in persons with SCD and that such primary identification may be potentially valuable in the future follow-up of such patients because cerebral perfusion deficiency is expected to develop silent infarction and overt stroke, that ultimately declines cognitive abilities.
EEG-ERP Studies
EEG is another brain imaging technique that primarily detects the currents which occur during synaptic excitations of several pyramidal neurons in the cerebral cortex and measures scalp electrical activity generated by brain regions. EEG is sensitive to various states, including stress, attentiveness, resting state, hypnosis, and sleep. Because the EEG technique is noninvasive and painless, it is commonly used to investigate the brain physiology of cognitive functions such as perceptions, memory, attention, language, and emotions in healthy individuals and infants. Another variant of EEG, i.e., ERPs, are extremely tiny voltages produced in brain areas in response to certain events or stimuli. EEG changes are time-locked to particular stimuli such as sensory, motor, or cognitive events. It offers a noninvasive and secure method for studying the psychophysiological aspects of brain function., EEG is widely used in the early diagnosis and management of neurological and cognitive involvement of SCD. Early case reports by L. Neidengard and E. Niedermeyer revealed a large amount of slow-wave activity in SCD patients. The degree of slowing in the EEG was much more significant than one would have expected from the clinical state. Resting-state EEG frequency analysis in SCD children showed a higher amount of slow-wave activity in occipital-parietal and temporal-frontal brain areas. Although the authors failed to suggest a strong reason behind that, it may be because of a lack of oxygen-carrying capacity or obstruction in blood flow in brain regions. Studies by Case et al. revealed strong evidence of EEG changes in pain processing regions in SCD. In comparison to controls, SCD patients showed higher theta and lowered beta power. Source localization demonstrated that locations with higher theta band activity were associated with pain processing. On EEG-fMRI data, spontaneous power and microstate analyses were also done. Independent component analysis revealed that patients had no activity in the default mode network (DMN) and executive control network (ECN) as compared to the control group.– EEG power measures are also associated with cognitive functions like global cognition, memory, language, and executive functioning. These EEG power measures might prove helpful in prospective studies to predict longitudinal cognitive decline. Downes et al. recorded auditory ERP in SCD children and compared it with age-matched controls. They observed more positive amplitudes by 100 ms in attended stimuli in healthy children but not in SCD children, indicating their attention deficits. Correct response negativity (CRN) and error-related negativity (ERN) were reduced in SCD patients with unilateral and bilateral frontal white matter injuries. Further, it was also observed that SCD patients show executive function deficits. Patients with SCD were found to have deteriorated cognitive abilities; cognitive responses to an auditory stimulus are delayed in SCD patients. The precuneus, which is interconnected to various cortical and subcortical areas of the brain and is involved in episodic memory, visual-spatial abilities, motor activity, coordination strategies, self-perception, executive and working memory, was not activated in SCD patients when compared to controls during stimulus presentation.
Neuromarkers and Biochemical Basis
Biomarkers are measurable biological characteristics that are an indicator of normal biological processes. Anemia is a major factor in the pathophysiology of SCD and might be a source of cognitive impairment. Hemoglobin deficiency is a sign of inadequate brain oxygenation, which might explain poor cognitive performance. A wide variety of studies have tried to associate neurocognition with markers of anemia, like hemoglobin or hematocrit., , , , , Ruffieux et al. disclosed a significant association between low hemoglobin F (HbF) levels with lower executive functioning. According to Boehme et al., inflammatory markers such as IL-22 were associated with neurocognitive dysfunction alone. In contrast, cytokine levels such as CXCL8, CXCL1, IFNg, TNFa, sFasL, IL18, IL22, sICAM1, and VEGFA were associated with abnormal TCD (or stroke) plus neurocognitive dysfunction. Cytokine levels were found to be inversely related to each of the conditions mentioned in all cases. Although hydroxyurea therapy is expected to improve neurocognition, studies have found no negative correlation between hydroxyurea administration and neurocognition., Glial fibrillary acidic protein (GFAP) is a brain-specific intermediate filament protein expressed in glial cells (astrocytes) and is a known biomarker of acute stroke and head trauma in adults. Savage et al. reported a negative correlation between GFAP levels and IQ in children with SCD.
Impact of SCD Induced Hypoxia on Neurocognition
Neural Substrates of Cognition
Neurocognition is the ability to relate and decipher the information, which encompasses various cognitive domains, e.g., working memory, speed of processing, attention, and/or executive functions. Various brain areas function in coordination for better cognitive outcomes., Worsening of working memory has been reported to be associated with derangement in cortical and subcortical structures at the distal distributions of the anterior and middle cerebral artery, which disturbs oxygen delivery to deep white matter, basal ganglia, middle and superior frontal gyrus, and dorsal parietal regions., , Besides, faster performers have efficient interactions between brain regions and increased neuronal activity in the prefrontal cortex (PFC) compared to slow performers for any executive function. Performance relating to general intelligence has been observed to be dependent upon the parieto-frontal structurally intact axonal fibers, which help in fast information processing. Thus, these facts delineate the dynamic interactions between various neural substrates for efficient cognitive outcomes.
SCD and Neurocognition: Cause and Effect Relationship
SCD is characterized by chronic and acute anemia, low baseline Hb (≤10 g/dL), hypoxia, and intracranial stenosis. Various studies showed that SCI is the primary risk factor for neurocognition deficits in SCD patients, including children, adolescents, and adults, compared to patients without SCI., There is increased cerebral blood flow, and decreased cerebrovascular reserve because of low baseline Hb, hypoxia caused by fever, and seizure, which leads to SCI, whose severity is increased by comorbidities like hypertension, diabetes, hyperlipidemia, and renal disease., The prevalence of SCI has been documented ≥ 20% of SCD children that showed an increasing trend with age., , The other risk factors for SCI are male gender, high SBP, and previous seizures.
SCIs are more in brain areas with low cerebral blood flow, hindering the proper oxygen supply in these patients. Reduced oxygen delivery was observed in white matter (WM), specifically in regions of high risk for silent strokes. The cerebral blood flow was found to be inversely related to cerebral infarcts density obtained through the Silent Infarct Transfusion (SIT) Trial Infarct density map of 286 SCD children. It was reported that in around 90% of children, the cerebral infarct density was more in the deep white matter of the frontal and parietal lobes with lower cerebral blood flow.
Pegelow et al., documented in SCD patients of 6 to 19 years that stroke occurred more frequently in the frontal lobe than the parietal lobe, followed by subcortical nuclei and temporal lobe, and a few lesions in the occipital lobe or cerebellum. In children with SCD, the frontal cortex has been observed to be affected with or without the manifestation of tissue injury., – The frontoparietal regions and the associated internal carotid artery, the middle and anterior cerebral arteries are injured by a stroke in SCD. Focal brain injuries such as clinical stroke and silent stroke result in small lesions in the brain that cause structural and volumetric changes in patients as reported through MRI in SCD.,
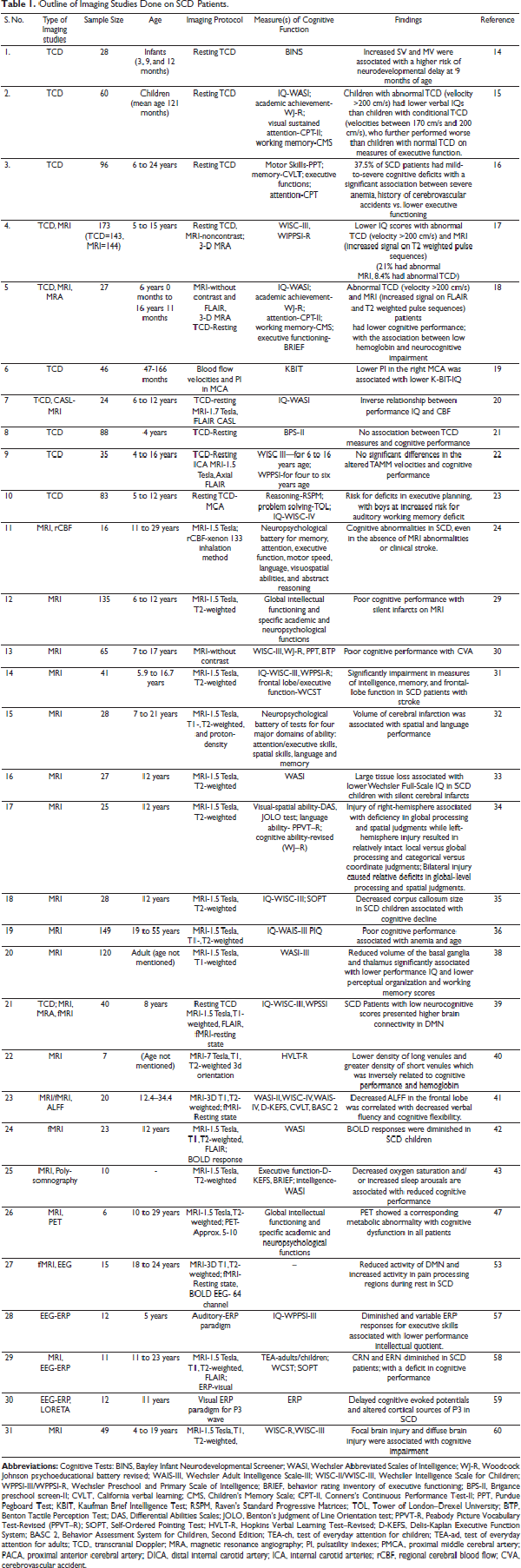
The occurrence/density of silent cerebral infarcts has been associated with cognitive impairment in SCD. The hemoglobin oxygen saturation levels and hematocrit are identified as biological factors linked to cognitive functions in children with SCD. Increased cerebral blood flow and oxygen extraction fraction have been positively associated with lower executive functions and increased silent cerebral infarcts in individuals with SCD. It has been examined that SCI-generated lesions in subcortical areas alter the functional memory status via deteriorating processing speed.Figure 1 depicts the mechanisms behind neurocognitive decline in SCD.
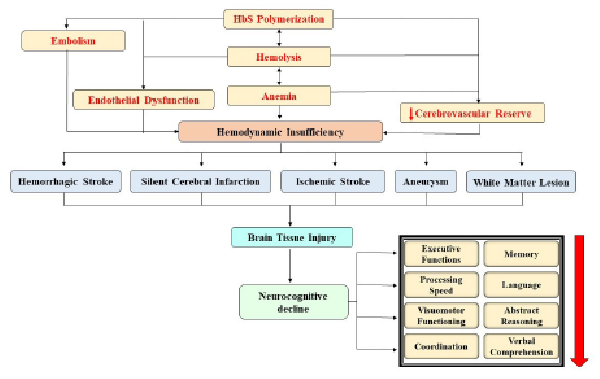
Mechanisms Behind Neurocognitive Decline in SCD.
Cognitive Domains and Associated Neural Substrates in SCD
Executive Functions
Existing reports on the cognitive attributes and their neural bases showed that executive function is the most explored cognitive domain in relation to SCD. This domain is predominantly affected as a consequence of disease.
Working memory, a widely studied executive function, is associated with the temporary storage and processing of information. The working memory performance is mediated by two attributes, viz. central executive function and processing speed. Thus, better central executive functions reflect better working memory as observed through prompt addition related problem-solving capacity. The Theta oscillations in prefrontal, parietal, temporal, and occipital cortical regions of the brain during the audiovisual working memory task show that multiple brain regions are associated with working memory.
DeBaun et al. reviewed that SCI in sickle cell patients is associated with worse cognitive performances in domains like executive functions (selective attention, card sorting, working memory), processing speed, visual-motor speed and coordination, vocabulary, visual memory, abstract reasoning and verbal comprehension. A reduced performance was also noted in an array of executive functions like nonverbal reasoning and visual-spatial skills, working memory, and processing speed in nonsymptomatic SCD adult patients., –, Lower intelligence quotient (IQ) level in SCD patients and deterioration in cognitive functions like memory, language, learning, attention, retrieval, and overall executive functioning visible through WAIS-III verbal IQ (VIQ), performance IQ (PIQ), and full-scale IQ (FSIQ) index in older patients has been linked to anemia. Children with SCD showed low IQ and dysfunctions in executive tasks such as visuospatial working memory, sustained attention, and planning capacity., The adolescent SCD patient also showed neurocognitive problems such as poor performance in verbal IQ scores, mathematical problem-solving capacity, and deteriorated visual-motor functions. Children with infarcts in the frontal cortex showed reduced working memory span compared to SCD children without infarcts. The visual-spatial skills, processing speed, and working memory have also been linked with the structural changes in the brain, including reduced volume in the basal ganglia and thalamus in SCD adults compared to non-SCD.
The cognitive impairment occurred more in individuals with damaged white matter., Thus, SCI damages the white matter and causes a reduction in global white matter volume in both the right and left cerebral hemispheres, specifically in the regions where anterior and middle cerebral artery distributed along with the corpus callosum, right brainstem, and right cerebellum as documented in adolescent and adult SCD patients compared to control. They also discovered that white matter injuries in the frontal, parietal, and temporal lobes were associated with low hemoglobin levels, platelet volume, chronic microvascular insufficiency, and hypoxia. Steen et al. found slow volumetric growth of brain gray matter in children compared to controls, affecting neurocognitive development. In individuals with SCD, there is increased cerebral blood flow; however, diminished blood supply is one of the prime causes of white matter loss–. The decreasing oxygen saturation has also been related to abnormal white matter in the corpus callosum in SCD, confirming that acute and chronic hypoxia negatively impact the neurocognition capacity. The severity of chronic anemia has been considered one of the most vital factors of hypoxic-ischemia-related damage or loss of white matter in SCD patients. The severity of anemia is the strongest predictor of whole-brain white matter volume loss.,
Craft et al. have alleged that lesion location is more related to attention and executive function problems than lesion volume. The overall IQ level of children with larger lesion volume showed more deterioration than children with less lesion volume. Further, children with left cortical infarct also reported impaired IQ on a full scale, verbal and performance scale (FSIQ, VIQ, and PIQ), while children with right cortical infarct were poor in FSIQ and PIQ only. The SCD children with clinical or silent infarct were more prone to make errors on a cancellation task. A meta-analysis study showed that SCD patients with a history of stroke have more cognitive deficits than those without infarcts, and SCD patients mainly have a problem with verbal reasoning, perceptual reasoning, and executive function.
Recently, tract-specific analysis and white matter tract studies revealed the microscopic injury in white matter associated with the deterioration in processing speed and response inhibition executive functions in SCD patients. Chai et al. found white matter damage in “genu of the corpus callosum, corticospinal tract, inferior frontal-occipital fasciculus, right inferior longitudinal fasciculus, superior longitudinal fasciculus, and left uncinate fasciculus.” Corpus callosum has been suggested to be essential for processing speed, working memory and executive functions related to cognitive skills. Reduced fractional anisotropy (FA) values and an increase in apparent diffusion coefficient values in the corpus callosum and corticospinal tracts also indicate structural changes in these regions in SCD patients, resulting in neurocognition performance deterioration. In SCD patients, SPO2 was found to correlate with cerebral blood flow velocity in the right and left middle cerebral arteries, which was found to be negatively associated with IQ; thus, chronic anemia, low hematocrit, and hypoxia cause chronic cerebral ischemia and, ultimately, impaired neurocognitive functions., , Diffuse brain injury and focal brain injuries such as lacunar infarction, encephalomalacia, or leukoencephalopathy have been shown to cause neurocognitive dysfunction in verbal intelligence quotient and verbal comprehension.
Memory
Memory seems to alter to a lesser degree in SCD patients., Cohen et al. examined that children diagnosed with left cortical infarct reported auditory-verbal memory and visual-spatial memory deficits; however, patients diagnosed with right cortical infarct were deficits only in visual-spatial memory. Children having anterior lobe infarcts also showed poor performance on both short- and long-delayed free recall tasks compared to children without cortical infarcts, though children with diffuse infarcts or their siblings without SCD showed similar cognitive performance.,
Visuomotor Functioning
SCD children have impaired visuomotor functioning compared to control, while with increasing age, visuomotor functioning improved. Visuomotor functioning-related cognitive abilities have been more susceptible to alteration because of disease compared to auditory-verbal skills in children with SCD. Schatz et al. reported that 30% of children with silent infarcts and 33% without infarcts displayed deficits in visuomotor functions compared to siblings without SCD.
Language
Children having clinical infarcts as detected in MRI committed more errors while doing the rapid naming test compared to children without MRI abnormalities for clinical infarcts. It has been suggested that language problems in SCD children are linked to the degree and lateralization of neurological injuries.
Methodological Constraints of the Reports
Methodological variations relate to subject selections of varied ages and are not gender matched. Siblings with similar family or developing environments are preferred as controls, since family environment, education, and economic status have an impact on cognitive development trajectories and functions. Ethnicity also influences cognitive functions. Most of the studies are conducted in the USA, a developed country with better education, health facilities, and economic standard; however, similar parallel studies in other countries also revealed other socio-economic factors impacting the cognitive functions in SCD children.
Interventions and Future Perspectives
The SIT Trial has been considered an effective blood transfusion therapy to prevent recurrent silent cerebral infarcts in participants with SCA. SIT Trial with brain MRIs in 169 children of the 5 to 14 years age group showed progressive brain volume changes in SCD children and no change in brain volume in children without SCD. Regular monthly blood transfusion therapy has been suggested as a promising intervention to maintain the optimal transcranial Doppler (TCD) velocities for cerebral blood flow in addition to hydroxyurea administration, specifically at an early age to prevent the risk of SCI and stroke. However, hydroxyurea, the approved therapy to prevent a secondary stroke and treat anemia in SCD, may increase SCI-related complications. Hematopoietic (blood-forming) stem cell transplantation (HSCT) has been purported to reduce the deformed RBC in children <16 years of age; and is the only known treatment for SCD that reduces or eliminates the sickling of RBC. Stem cell therapy could also suggest an effective way to alleviate the consequence of disease that enhances the cognitive status of SCD individuals.
Educational interventions such as training and a friendly and homely environment from childhood improve the cognitive-developmental trajectory. Cognitive-behavioral therapy can be used to improve any cognitive function by managing pain in childhood and preventing further cognitive decline in secondary cognitive processing, such as the central executive function, which is the component that primarily affects working memory in SCD children., Thus, facilitating central executive function could improve working memory status in SCD children. Slow and deep breathing techniques, meditation, and yoga practices are also helpful, acceptable, and feasible nonpharmacological interventions in enhancing cognitive abilities and pain management in patients with SCD., Figure 2 outlines the investigations done during different events of cerebral injury in SCD and various interventions for their management.
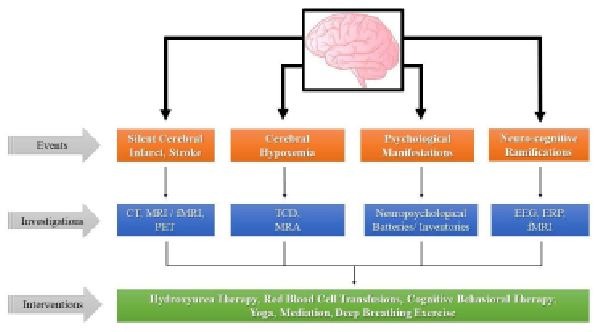
Different Events of Cerebral Injury, Their Investigations, and Interventions in SCD.
Summary
Various neurological complications, i.e., ischemic stroke, SCI, headache, and cognitive dysfunction, are commonly seen in SCD, which can be evaluated by different techniques like PET scan, NIRS, TCD, MRI, and EEG. SCD patients have multifaceted hemodynamic dysregulations, i.e., abnormal systolic velocities in the MCA and ICA in TCD, MRI abnormalities such as cerebrovascular accident, frontal-lobe infarcts, decreased CC size, and smaller intracranial volume with cognitive impairment. PET scan reveals anomalies in glucose metabolism and microvascular blood circulation in frontal lobes. Whereas, EEG-ERP studies show higher slow-wave activities and lesser positive amplitudes for auditory stimuli suggestive of cognitive decline in these patients. Further, SCD patients exhibit worse cognitive performances in domains like executive functions, processing speed, visual-motor speed and coordination, vocabulary, visual memory, abstract reasoning, and verbal comprehension. Inadequate brain oxygenation because of hemoglobin deficiency is believed to be linked to poor cognitive performance in these patients.
The primary risk factor for neurocognition deficits in SCD patients is SCI which causes a reduction in global white matter volume both in the right and left cerebral hemispheres, specifically in the regions of the distribution of anterior and middle cerebral artery, along with other areas such as corpus callosum, right brainstem, and right cerebellum. Also, low hemoglobin levels, platelet volume, chronic microvascular insufficiency, and hypoxia cause white matter injuries in the frontal, parietal, and temporal lobes. Besides, cerebral hemodynamic insufficiency (oxygen demand exceeds supply) and reduced oxygen saturation in SCD patients are other reasons for white matter loss. In addition, decreased size of the corpus callosum with damage of white matter in the genu of the corpus callosum, corticospinal tract, inferior frontal-occipital fasciculus, right inferior longitudinal fasciculus, superior longitudinal fasciculus, and left uncinate fasciculus are other causes for cognitive abnormalities in SCD patients.
The only known treatments for SCD are regular blood transfusion, hydroxyurea therapy, and hematopoietic stem cell transplantation that reduces or eliminates the sickling of RBC. Besides, nonpharmacological interventions, i.e., educational interventions, cognitive-behavioral therapy, slow and deep breathing techniques, meditation, and yoga practices, might also prove useful in pain management and enhancing cognitive abilities in SCD patients.
Conclusion
In conclusion, this review provides evidence that patients with SCD are at increased risk of neurocognitive abnormalities across multiple domains throughout their lifespan. These neurocognitive impairments are related to the degree of anemia, suggesting that decreased oxygen transport to the brain is a pathogenetic cause. The neurocognitive deficiencies may explain why these patients have such high rates of intellectual disability. The current findings highlight the significance of regular cognitive examinations, pharmacological and nonpharmacological interventions, and potential neurocognitive rehabilitation programs for persons with SCD.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Tarun Sahu
https://orcid.org/0000-0002-2721-7432
Babita Pande
https://orcid.org/0000-0002-0545-6002
Henu Kumar Verma
https://orcid.org/0000-0003-1130-8783
- 1. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med 2017; 376: 1561–1573.
- 2. Serjeant GR. Sickle-cell disease. Lancet 1997; 350: 725–730.
- 3. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376: 2018–2031.
- 4. Ware RE, de Montalembert M, Tshilolo L, . Sickle cell disease. Lancet 2017; 390: 311–323.
- 5. Verma HK, Ratre YK, Bhaskar LVKS, . Erythrocyte microRNAs: A tiny magic bullet with great potential for sickle cell disease therapy. Ann Hematol 2021; 100: 607–614.
- 6. Kato GJ, Piel FB, Reid CD, . Sickle cell disease. Nat Rev Dis Prim 2018; 4: 1–22.
- 7. Sahu T, Verma HK, Ganguly S, . Common, but neglected: A comprehensive review of leg ulcers in sickle cell disease. Adv Ski Wound Care 2021; 34: 423–431.
- 8. Castro IPS, Viana MB. Cognitive profile of children with sickle cell anemia compared to healthy controls. J Pediatr (Rio J) 2019; 95: 451–457.
- 9. Hijmans CT, Grootenhuis MA, Oosterlaan J, . Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr Blood Cancer 2011; 57: 297–302.
- 10. Stotesbury H, Kawadler JM, Hales PW, . Vascular instability and neurological morbidity in sickle cell disease: An integrative framework. Front Neurol 2019; 10: 1–21.
- 11. Farooq S, Testai FD. Neurologic complications of sickle cell disease. Curr Neurol Neurosci Rep; 19. Epub ahead of print 2019. DOI: 10.1007/s11910-019-0932-0.
- 12. Bakker MJ, Hofmann J, Churches OF, . Cerebrovascular function and cognition in childhood: A systematic review of transcranial Doppler studies. BMC Neurol 2014; 14: 1–12.
- 13. Adams RJ. Neurologic complications of sickle cell disease. 1st ed. Elsevier B.V. Epub ahead of print 2014. DOI: 10.1016/B978-0-7020-4087-0.00068-1.
- 14. Hogan AM, Kirkham FJ, Prengler M, . An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br J Haematol 2006; 132: 99–107.
- 15. Kral MC, Brown RT, Nietert PJ, . Transcranial Doppler ultrasonography and neurocognitive functioning in children with sickle cell disease. Pediatrics 2003; 112: 324–331.
- 16. Ruffieux N, Njamnshi AK, Wonkam A, . Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol J Norm Abnorm Dev Child Adolesc 2013; 19: 143–160.
- 17. Bernaudin F, Verlhac S, Fréard F, . Multicenter prospective study of children with sickle cell disease: Radiographic and psychometric correlation. J Child Neurol 2000; 15: 333–343.
- 18. Kral MC, Brown RT, Connelly M, . Radiographic predictors of neurocognitive functioning in pediatric sickle cell disease. J Child Neurol 2006; 21: 37–44.
- 19. Krejza J, Arkuszewski M, Radcliffe J, . Association of pulsatility index in the middle cerebral artery with intelligence quotient in children with sickle cell disease. Neuroradiol J 2012; 25: 351–359.
- 20. Strouse JJ, Cox CS, Melhem ER, . Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA). Blood 2006; 108: 379–381.
- 21. Aygun B, Parker J, Freeman MB, . Neurocognitive screening with the Brigance preschool screen-II in 3-year-old children with sickle cell disease. Pediatr Blood Cancer 2011; 56: 620–624.
- 22. Onofri A, Montanaro M, Rampazzo P, . Intellectual impairment and TCD evaluation in children with sickle cell disease and silent stroke. Perspect Med 2012; 1: 272–274.
- 23. Prussien KV, Salihu A, Abdullahi SU, . Associations of transcranial Doppler velocity, age, and gender with cognitive function in children with sickle cell anemia in Nigeria. Child Neuropsychol 2019; 25: 705–720.
- 24. Silva GS, Vicari P, Figueiredo MS, . Transcranial Doppler in adult patients with sickle cell disease. Cerebrovasc Dis 2006; 21: 38–41.
- 25. Valadi N, Silva GS, Bowman LS, . Transcranial Doppler ultrasonography in adults with sickle cell disease. Neurology 2006; 67: 572–574.
- 26. Kandeel AY, Zimmerman RA, Ohene-Frempong K. Comparison of magnetic resonance angiography and conventional angiography in sickle cell disease: Clinical significance and reliability. Neuroradiology 1996; 38: 409–416.
- 27. Jordan LC, DeBaun MR, Donahue MJ. Advances in neuroimaging to improve care in sickle cell disease. Lancet Neurol 2021; 20: 398–408.
- 28. Kugler S, Anderson B, Cross D, . Abnormal cranial magnetic resonance imaging scans in sickle-cell disease. Neurological correlates and clinical implications. Arch Neurol 1993; 50: 629–635.
- 29. Armstrong FD, Thompson RJJ, Wang W, . Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97: 864–870.
- 30. Gold JI, Johnson CB, Treadwell MJ, . Detection and assessment of stroke in patients with sickle cell disease: Neuropsychological functioning and magnetic resonance imaging. Pediatr Hematol Oncol 2008; 25: 409–421.
- 31. Watkins KE, Hewes DK, Connelly A, . Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev Med Child Neurol 1998; 40: 536–543.
- 32. Craft JSS, Koby M, Siegel MJ, . Neuropsychologic deficits in children with sickle cell disease and cerebral infarction: Role of lesion site and volume. Child Neuropsychol 1999; 5: 92–103.
- 33. Schatz J, White DA, Moinuddin A, . Lesion burden and cognitive morbidity in children with sickle cell disease. J Child Neurol 2002; 17: 890–894.
- 34. Schatz J, Craft S, Koby M, . Asymmetries in visual-spatial processing following childhood stroke. Neuropsychology 2004; 18: 340–352.
- 35. Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: Relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc 2006; 12: 24–33.
- 36. Vichinsky EP, Neumayr LD, Gold JI, . Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 2010; 303: 1823–1831.
- 37. van der Land V, Zwanenburg JJM, Fijnvandraat K, . Cerebral lesions on 7 Tesla MRI in patients with sickle cell anemia. Cerebrovasc Dis 2015; 39: 181–189.
- 38. Mackin RS, Insel P, Truran D, . Neuroimaging abnormalities in adults with sickle cell anemia: Associations with cognition. Neurology 2014; 82: 835–841.
- 39. Colombatti R, Lucchetta M, Montanaro M, . Cognition and the default mode network in children with sickle cell disease: A resting state functional MRI study. PLOS One 2016; 11: e0157090.
- 40. Fields ME, Mirro AE, Guilliams KP, . Functional connectivity decreases with metabolic stress in sickle cell disease. Ann Neurol 2020; 88: 995–1008.
- 41. Novelli EM, Elizabeth Sarles C, Jay Aizenstein H, . Brain venular pattern by 7T MRI correlates with memory and haemoglobin in sickle cell anaemia. Psychiatry Res 2015; 233: 18–22.
- 42. Coloigner J, Kim Y, Bush A, . Contrasting resting-state fMRI abnormalities from sickle and non-sickle anemia. PLOS One 2017; 12: e0184860.
- 43. Zou P, Helton KJ, Smeltzer M, . Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain Imaging Behav 2011; 5: 295–306.
- 44. Hollocks MJ, Kok TB, Kirkham FJ, . Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. J Int Neuropsychol Soc 2012; 18: 168–173.
- 45. Andreotti C, King AA, Macy E, . The association of cytokine levels with cognitive function in children with sickle cell disease and normal MRI studies of the brain. J Child Neurol 2015; 30: 1349–1353.
- 46. Powars DR, Conti PS, Wong WY, . Cerebral vasculopathy in sickle cell anemia: Diagnostic contribution of positron emission tomography. Blood 1999; 93: 71–79.
- 47. Reed W, Jagust W, Al-Mateen M, . Role of positron emission tomography in determining the extent of CNS ischemia in patients with sickle cell disease. Am J Hematol 1999; 60: 268–272.
- 48. Al-Kandari FA, Owunwanne A, Syed GM, . Regional cerebral blood flow in patients with sickle cell disease: Study with single photon emission computed tomography. Ann Nucl Med 2007; 21: 439–445.
- 49. lopes da Silva FH, Donald LS. Niedermeyer’s electroencephalography: Basic principles, clinical applications, and related fields. 7th ed. Oxford University Press, 2011.
- 50. Sur S, Sinha VK. Event-related potential: An overview. Ind Psychiatry J 2009; 18: 70–73.
- 51. Neidengard L, Niedermeyer E. The electroencephalogram in neurological complications of sickle cell anemia (SS-hemoglobinopathy). Clin EEG Neurosci 1975; 6: 68–74.
- 52. Gott PSP, Haywood LJM, Allen JPM, . Frequency analysis of the EEG in children with sickle cell disease. J Natl Med Assoc 1977; 69: 811–813.
- 53. Case M, Zhang H, Mundahl J, . Characterization of functional brain activity and connectivity using EEG and fMRI in patients with sickle cell disease. NeuroImage Clin 2017; 14: 1–17.
- 54. Case M, Shirinpour S, Zhang H, . Increased theta band EEG power in sickle. J Pain Res 2018; 67–76.
- 55. Case M, Shirinpour S, Vijayakumar V, . Graph theory analysis reveals how sickle cell disease impacts neural networks of patients with more severe disease. NeuroImage Clin 2019; 21: 101599.
- 56. van der Hiele K, Vein AA, Reijntjes RHAM, . EEG correlates in the spectrum of cognitive decline. Clin Neurophysiol 2007; 118: 1931–1939.
- 57. Downes M, Kirkham FJ, Telfer PT, . Altered neurophysiological processing of auditory attention in preschool children with sickle cell disease. J Pediatr Psychol 2018; 43: 856–869.
- 58. Hogan AM, Vargha-Khadem F, Saunders DE, . Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain 2006; 129: 2177–2188.
- 59. Colombatti R, Ermani M, Rampazzo P, . Cognitive evoked potentials and neural networks are abnormal in children with sickle cell disease and not related to the degree of anaemia, pain and silent infarcts. Br J Haematol 2015; 169: 597–600.
- 60. Steen RG, Miles MA, Helton KJ, . Cognitive impairment in children with hemoglobin SS sickle cell disease: Relationship to MR imaging findings and hematocrit. AJNR Am J Neuroradiol 2003; 24: 382–389.
- 61. Bills S, Taormina I, Meier ER, . Biomarkers of disease severity predict neurocognitive functioning in pediatric SCD. Blood 2016; 128: 248.
- 62. Boehme A, Ashrafi A, Idro R, . Association between Inflammatory markers and abnormal neurological, neurocognitive and magnetic resonance imaging (MRI) findings in children with sickle cell anemia in Uganda. Blood 2019; 134: 2300.
- 63. Santos E, Popovic M-B, Mayor C, . Neuropsychological impact of sickle-cell disease in children and adolescents. Mémoire de Maîtrise en médecine 2017; 1–32.
- 64. Merkhofer C, Sylvester S, Zmuda M, . The impact of cognitive function on adherence to hydroxyurea therapy in patients with sickle cell disease. Blood 2016; 128: 2493.
- 65. Savage WJ, Barron-Casella E, Fu Z, . Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am J Hematol 2011; 86: 427–429.
- 66. Nuechterlein KH, Barch DM, Gold JM, . Identification of separable cognitive factors in schizophrenia. Schizophr Res 2004; 72: 29–39.
- 67. Anderson V. Assessing executive functions in children: Biological, psychological, and developmental considerations. Pediatr Rehabil 2001; 4: 119–136.
- 68. Fuster JM. Frontal lobes. Curr Opin Neurobiol 1993; 3: 160–165.
- 69. Smith KE, Schatz J. Working memory in children with neurocognitive effects from sickle cell disease: Contributions of the central executive and processing speed. Dev Neuropsychol; 41. Epub ahead of print 2016. DOI: 10.1080/87565641.2016.1238474.
- 70. Pavlakis SG, Bello J, Prohovnik I, . Brain infarction in sickle cell anemia: Magnetic resonance imaging correlates. Ann Neurol; 23. Epub ahead of print 1988. DOI: 10.1002/ana.410230204.
- 71. Rypma B, Berger JS, Prabhakaran V, . Neural correlates of cognitive efficiency. Neuroimage; 33. Epub ahead of print 2006. DOI: 10.1016/j.neuroimage.2006.05.065.
- 72. Penke L, Maniega SM, Bastin ME, . Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry; 17. Epub ahead of print 2012. DOI: 10.1038/mp.2012.66.
- 73. Jordan LC, DeBaun MR. Cerebral hemodynamic assessment and neuroimaging across the lifespan in sickle cell disease. J Cereb Blood Flow Metab; 38. Epub ahead of print 2018. DOI: 10.1177/0271678X17701763.
- 74. Debaun MR, Armstrong FD, Mckinstry RC, . Silent cerebral infarcts: A review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Epub ahead of print 2012. DOI: 10.1182/blood-2011-02.
- 75. Kassim AA, Pruthi S, Day M, . Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood; 127. Epub ahead of print 2016. DOI: 10.1182/blood-2016-01-694562.
- 76. Debaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood; 127. Epub ahead of print 2016. DOI: 10.1182/blood-2015-09-618579.
- 77. Estcourt LJ, Fortin PM, Hopewell S, . Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochrane Database Syst Rev; 2017. Epub ahead of print 2017. DOI: 10.1002/14651858.CD012389.pub2.
- 78. Ohene-Frempong K, Weiner SJ, Sleeper LA, . Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood; 91. Epub ahead of print 1998. DOI: 10.1182/blood.V91.1.288.
- 79. DeBaun MR, Gordon M, McKinstry RC, . Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med; 371. Epub ahead of print 2014. DOI: 10.1056/nejmoa1401731.
- 80. Chai Y, Bush AM, Coloigner J, . White matter has impaired resting oxygen delivery in sickle cell patients. Am J Hematol; 94. Epub ahead of print 2019. DOI: 10.1002/ajh.25423.
- 81. Ford AL, Ragan DK, Fellah S, . Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood; 132. Epub ahead of print 2018. DOI: 10.1182/blood-2018-04-841247.
- 82. Pegelow CH, Macklin EA, Moser FG, . Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood; 99. Epub ahead of print 2002. DOI: 10.1182/blood.V99.8.3014.
- 83. Hogan AM, Telfer PT, Kirkham FJ, . Precursors of executive function in infants with sickle cell anemia. J Child Neurol; 28. Epub ahead of print 2013. DOI: 10.1177/0883073812453495.
- 84. Schatz J, Finke RL, Kellett JM, . Cognitive functioning in children with sickle cell disease: A meta-analysis. J Pediatr Psychol; 27. Epub ahead of print 2002. DOI: 10.1093/jpepsy/27.8.739.
- 85. Wang WC, Wynn LW, Rogers ZR, . A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr; 139. Epub ahead of print 2001. DOI: 10.1067/mpd.2001.119590.
- 86. van der Land V, Hijmans CT, de Ruiter M, . Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol; 168. Epub ahead of print 2015. DOI: 10.1111/bjh.13179.
- 87. King AA, Rodeghier MJ, Panepinto JA, . Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol; 89. Epub ahead of print 2014. DOI: 10.1002/ajh.23805.
- 88. Prussien K V, Compas BE, Siciliano RE, . Cerebral hemodynamics and executive function in sickle cell anemia. Stroke. Epub ahead of print 2021. DOI: 10.1161/STROKEAHA.120.032741.
- 89. DeBaun MR, Schatz J, Siegel MJ, . Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology; 50. Epub ahead of print 1998. DOI: 10.1212/WNL.50.6.1678.
- 90. Geary DC, Hoard MK, Nugent L. Independent contributions of the central executive, intelligence, and in-class attentive behavior to developmental change in the strategies used to solve addition problems. J Exp Child Psychol; 113. Epub ahead of print 2012. DOI: 10.1016/j.jecp.2012.03.003.
- 91. Xie Y, Li Y, Duan H, . Theta oscillations and source connectivity during complex audiovisual object encoding in working memory. Front Hum Neurosci; 15. Epub ahead of print 2021. DOI: 10.3389/fnhum.2021.614950.
- 92. Stotesbury H, Kirkham FJ, Kölbel M, . White matter integrity and processing speed in sickle cell anemia. Neurology; 90. Epub ahead of print 2018. DOI: 10.1212/WNL.0000000000005644.
- 93. Hijmans CT, Fijnvandraat K, Grootenhuis MA, . Neurocognitive deficits in children with sickle cell disease: A comprehensive profile. Pediatr Blood Cancer; 56. Epub ahead of print 2011. DOI: 10.1002/pbc.22879.
- 94. Berkelhammer LD, Williamson AL, Sanford SD, . Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Child Neuropsychology; 13. Epub ahead of print 2007. DOI: 10.1080/09297040600800956.
- 95. Prussien K V, Jordan LC, Debaun MR, . Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: A meta-analysis. J Pediatr Psychol; 44. Epub ahead of print 2019. DOI: 10.1093/jpepsy/jsz031.
- 96. Brandling-Bennett EM, White DA, Armstrong MM, . Patterns to verbal long-term and working memory performance reveal deficits in strategic processing in children with frontal infarcts related to sickle cell disease. Dev Neuropsychol; 24. Epub ahead of print 2003. DOI: 10.1207/S15326942DN2401_01.
- 97. Jacobs HIL, Leritz EC, Williams VJ, . Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum Brain Mapp; 34. Epub ahead of print 2013. DOI: 10.1002/hbm.21412.
- 98. Turken AU, Whitfield-Gabrieli S, Bammer R, . Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage; 42. Epub ahead of print 2008. DOI: 10.1016/j.neuroimage.2008.03.057.
- 99. Choi S, Bush AM, Borzage MT, . Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients. NeuroImage Clin; 15. Epub ahead of print 2017. DOI: 10.1016/j.nicl.2017.04.023.
- 100. Steen RG, Emudianughe T, Hunte M, . Brain volume in pediatric patients with sickle cell disease: Evidence of volumetric growth delay? Am J Neuroradiol 2005; 26(3): 455–462.
- 101. Borzage MT, Bush AM, Choi S, . Predictors of cerebral blood flow in patients with and without anemia. J Appl Physiol; 120. Epub ahead of print 2016. DOI: 10.1152/japplphysiol.00994.2015.
- 102. Bush AM, Borzage MT, Choi S, . Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol; 91. Epub ahead of print 2016. DOI: 10.1002/ajh.24441.
- 103. Prohovnik I, Hurlet-Jensen A, Adams R, . Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab; 29. Epub ahead of print 2009. DOI: 10.1038/jcbfm.2009.6.
- 104. Kawadler JM, Kirkham FJ, Clayden JD, . White matter damage relates to oxygen saturation in children with sickle cell anemia without silent cerebral infarcts. Stroke; 46. Epub ahead of print 2015. DOI: 10.1161/STROKEAHA.115.008721.
- 105. Brown RT, Davis PC, Lambert R, . Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. J Pediatr Psychol; 25. Epub ahead of print 2000. DOI: 10.1093/jpepsy/25.7.503.
- 106. Chai Y, Ji C, Coloigner J, . Tract-specific analysis and neurocognitive functioning in sickle cell patients without history of overt stroke. Brain Behav; 11. Epub ahead of print 2021. DOI: 10.1002/brb3.1978.
- 107. Balci A, Karazincir S, Beyoglu Y, . Quantitative brain diffusion-tensor MRI findings in patients with sickle cell disease. Am J Roentgenol; 198. Epub ahead of print 2012. DOI: 10.2214/AJR.11.7404.
- 108. Hogan AM, Pit-Ten Cate IM, Vargha-Khadem F, . Physiological correlates of intellectual function in children with sickle cell disease: Hypoxaemia, hyperaemia and brain infarction. Dev Sci; 9. Epub ahead of print 2006. DOI: 10.1111/j.1467-7687.2006.00503.x.
- 109. Quinn CT, Variste J, Dowling MM. Haemoglobin oxygen saturation is a determinant of cerebral artery blood flow velocity in children with sickle cell anaemia. Br J Haematol; 145. Epub ahead of print 2009. DOI: 10.1111/j.1365-2141.2009.07652.x.
- 110. Schatz J, Brown RT, Pascual JM, . Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology; 56. Epub ahead of print 2001. DOI: 10.1212/WNL.56.8.1109.
- 111. Cohen MJ, Branch WB, McKie VC, . Neuropsychological impairment in children with sickle cell anemia and cerebrovascular accidents. Clin Pediatr (Phila); 33. Epub ahead of print 1994. DOI: 10.1177/000992289403300902.
- 112. Craft S, Schatz J, Glauser TA, . Neuropsychologic effects of stroke in children with sickle cell anemia. J Pediatr; 123. Epub ahead of print 1993. DOI: 10.1016/S0022-3476(05)80844-3.
- 113. Eigbire-Molen O, Darbari DS, Ponisio MR, . Progressive loss of brain volume in children with sickle cell anemia: A report from the silent cerebral infarct transfusion trial cohort. Blood; 126. Epub ahead of print 2015. DOI: 10.1182/blood.v126.23.546.546.
- 114. Bernaudin F, Verlhac S, Arnaud C, . Chronic acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood; 125. Epub ahead of print 2015. DOI: 10.1182/blood-2014-09-599852.
- 115. Estcourt LJ, Kimber C, Hopewell S, . Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochrane Database Syst Rev; 2020. Epub ahead of print 2020. DOI: 10.1002/14651858.CD012389.pub3.
- 116. Walters MC. Stem cell therapy for sickle cell disease: transplantation and gene therapy. Hematology Am Soc Hematol Educ Program. Epub ahead of print 2005. DOI: 10.1182/asheducation-2005.1.66.
- 117. Lakkakula BVKS, Sahoo R, Verma H, . Pain management issues as part of the comprehensive care of patients with sickle cell disease. Pain Manag Nurs 2018; 19: 558–572.
- 118. Matthie N, Brewer CA, Moura VL, . Breathing exercises for inpatients with sickle cell disease. MEDSURG Nurs 2015; 24: 35–38.
- 119. Moody K, Abrahams B, Baker R, . A randomized trial of yoga for children hospitalized with sickle cell vaso-occlusive crisis. J Pain Symptom Manage 2017; 53: 1026–1034.