Introduction
Neonatal sepsis (NS) is a potentially life-threatening clinical condition. A population level estimate for neonatal sepsis of 2,202 per 100,000 live births was reported, with mortality between 11 and 19% []. Despite recent advances in the treatment of neonatal infection, mortality rates and associated comorbidities remain high []. The nonspecific nature of symptoms and rapid progression of this disease emphasize the importance of early recognition. Various biomarkers have been associated with NS, including C-reactive protein (CRP), procalcitonin, and interleukin-6 []. However, these markers differ when they rise and when they return to normal during sepsis [, ]. Therefore, it is essential to identify new biomarkers that can detect onset of sepsis as early as possible for timely antibiotic therapy.
Calcium is associated with bone formation and metabolism, and it participates in a range of physiological processes, including cell signaling, neurotransmission, and muscle contraction. It also acts as a cofactor of enzymatic reactions in the coagulation cascade []. Ionized calcium (iCa) is a biologically active form of calcium responsible for these physiological functions. Hypocalcemia can be characterized by serum iCa concentrations <4 mg/dL (1 mmol/L) [], and it has been identified as a risk factor of mortality in both adult patients and critically ill infants [-]. In NS, the levels of calcium correlate inversely with CRP and procalcitonin levels []. Hypocalcemia is often observed in neonates with sepsis and has been attributed to an increase in procalcitonin secretion, which is converted to calcitonin and in turn regulates calcium metabolism [, ].
In this retrospective study, we examined the clinicopathologic characteristics of neonatal patients with sepsis and evaluated the association between patient outcomes and pretreatment serum iCa levels. We also assessed the potential of serum iCa levels to predict mortality in neonatal sepsis patients.
Methods
Study Design and Patient Recruitment
Medical records maintained by the neonatal intensive care unit of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China, were retrospectively examined to identify cases of neonatal septicemia (ICD-10 code P36.901) registered between January 2010 and June 2016. For the purpose of this study, neonatal sepsis was defined as the growth of potentially pathogenic organisms (bacteria or fungi) in the blood or cerebrospinal fluid of patients whose clinicopathologic characteristics were consistent with infection []. Sepsis-related death was defined as death within 14 days after developing sepsis [].
All patients in the NS group fulfilled the diagnostic criteria for sepsis outlined in the “Protocol for diagnosis and treatment of neonatal septicemia” [], and sepsis in all cases was confirmed by culture. A control group of neonates, matched for gestational age (± 1 week) and birth weight (± 100 g), was selected at a ratio of 2:1 relative to the NS group from among the first compatible neonates born alive before and after each NS case []. Neonates with congenital abnormalities, central nervous system anomalies, hypercalcemia, and organ failure before sepsis diagnosis were excluded from both groups.
We reviewed and recorded relevant data on clinical symptoms and hematological parameters for all included patients. Blood samples from all NS patients were collected prior to the initiation of antimicrobial therapy. Newborns in both groups who required minimal enteral feeding were given parenteral nutrition using 3-in-1 solutions supplying calcium (0.6–0.8 mmol/kg/day) and vitamin D (0.8 μg/kg/day). Newborns who tolerated oral feeding were supplemented with vitamin D (400 IU/day) []. According to the neonatal intensive care unit-NICU-protocol, symptomatic hypocalcemia was treated with 10% solution of calcium gluconate at a dosage of 1–2 mL/kg intravenously at a rate of 1 min/mL.
Statistical Analyses
All statistical analyses were performed using SPSS 15.0 (IBM, Chicago, IL, USA). Normally distributed continuous variables were analyzed using the Student t test and expressed as means ± SD. Skewed continuous variables were analyzed using the Mann-Whitney U test and expressed as median values. Categorical variables were analyzed using Pearson’s χ2 test and expressed as numbers and percentages. Risk factors for predicting mortality were identified using a multivariate logistic regression analysis and expressed as odds ratios with 95% CIs. A p value <0.05 (2-sided) was considered statistically significant.
Based on the results of the multivariate analysis, the prognostic variables for sepsis-related mortality were determined by ROC curve analysis. A nomogram was further developed to predict mortality in neonates with sepsis. Each level within variables was assigned a score according to the point scale. By calculating the total score and locating it on the total point scale, a corresponding probability of sepsis-related death for each individual patient was determined.
Results
From a total of 7,021 case records of patients, 198 patients (2.82%) were diagnosed with culture-proven NS. Patients with congenital abnormalities (n = 6), organ failure prior to sepsis diagnosis (n = 10), endocrine diseases in mothers (n = 4), hypercalcemia (n = 5), and incomplete data (n = 4) were excluded. In the NS group, 134 cases were matched with 2 controls per case, while the remaining 35 cases were matched with only 1 control per case. The final analysis was performed using clinicopathologic data on 169 patients with sepsis and a control group of 303 neonates.
Characteristics of Neonates
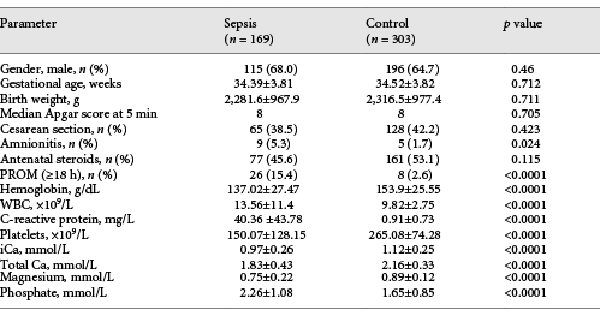
The clinical characteristics and laboratory findings for NS and control neonates are summarized in Table 1. No significant differences were observed between the groups with respect to sex, gestational age, birth weight, 5-min Apgar score, mode of delivery, or prenatal steroid usage. NS patients had higher white blood cell counts (13.56 vs. 9.82 × 109/L) and CRP levels (40.36 vs. 0.91 mg/L), but lower levels of platelets (150 vs. 265 × 109/L) and hemoglobin (137.02 vs. 153.9 g/dL). The NS group also showed lower serum total calcium (1.83 vs. 2.16 mmol/L), iCa (0.97 vs. 1.12 mmol/L), and magnesium (0.75 vs. 0.89 mmol/L), along with higher phosphate levels (2.26 vs. 1.65 mmol/L). Mothers of NS neonates showed significantly higher rates of premature membrane rupture (15.4 vs. 2.6%) and amnionitis (5.3 vs. 1.7%) than mothers of control neonates.
Comparison of NS Neonates with Hypocalcemia and Normal iCa Levels
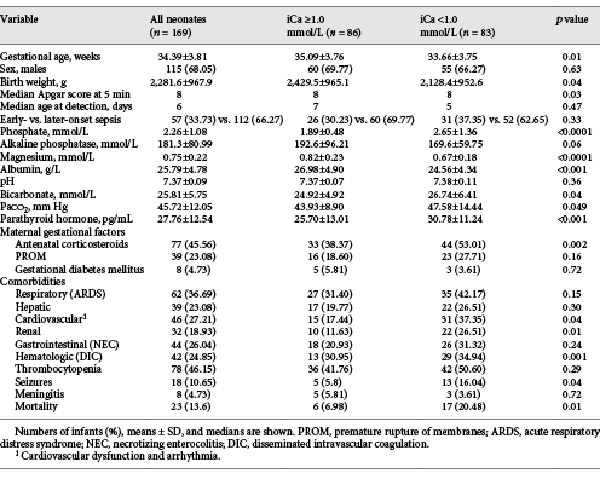
Among NS patients, hypocalcemia was present in 49.1% (83/169), and neonates with hypocalcemia had a lower gestational age (33.66 ± 3.75 vs. 35.09 ± 3.76 weeks), body weight (2,128.4 ± 952.6 vs. 2,429.5 ± 965.1 g), and 5-min Apgar scores than those with normal serum iCa level (Table 2). The 2 groups of NS neonates were similar in sex, maternal gestational factors, and timing of sepsis onset. The rate of treatment with antenatal corticosteroids was higher among NS neonates with hypocalcemia than those with normal iCa levels (53.01 vs. 38.37%).
Hypocalcemia in NS patients was associated with hypomagnesemia, hyperphosphatemia, hypoalbuminemia, high levels of bicarbonates, and high partial pressure of carbon dioxide (Table 2). We observed that neonates with hypocalcemia had higher parathyroid hormone (PTH) levels (30.78 ± 11.24 vs. 25.70 ± 13.01 pg/mL) as well as higher rates of cardiovascular complications (37.35 vs. 17.44%), renal complications (26.51 vs. 11.63%), disseminated intravascular coagulation (34.94 vs. 30.95%), and seizures (16.04 vs. 5.8%). The most frequent cardiovascular complications were arrhythmia and hypotension. In 4 neonates with severe hypocalcemia, we observed a 2:1 atrioventricular block and iCa <0.6 mmol/L. NS patients with and without hypocalcemia showed no differences in terms of the acute respiratory distress syndrome, hepatic dysfunction, necrotizing enterocolitis, thrombocytopenia, or meningitis.
Hypocalcemia as an Independent Predictor of Mortality
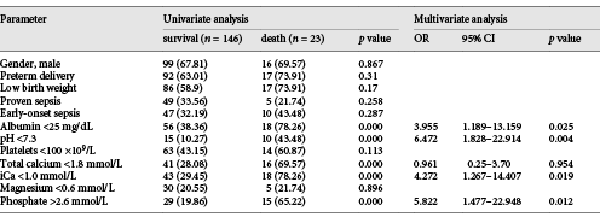
The overall sepsis-related mortality rate in the NS group was 13.61% (23/169), and the rate was higher among NS neonates with hypocalcemia (20.48%) than among those with normal iCa (6.98%; Table 2). Univariate analysis demonstrated an association between mortality and levels of serum albumin, total calcium, iCa, phosphate, and acidosis. Multivariate regression analysis identified the following independent risk factors of mortality: hypoalbuminemia (albumin <25 mg/dL) [], hypocalcemia (iCa <1.0 mmol/L), hyperphosphatemia (phosphate >2.6 mmol/L), and acidosis (pH <7.3) [, ] (Table 3).
The prognostic value of these serum biomarkers was evaluated by ROC curve analysis. In the analysis of the overall sample, the area under the ROC curve (AUC) was 0.70 (95% CI 0.624–0.768; p = 0.0004), 0.74 (95% CI 0.671–0.808; p < 0.0001), 0.73 (95% CI 0.653–0.792; p = 0.0002), and 0.67 (95% CI 0.59–0.737; p = 0.0154) for serum albumin, iCa, phosphate, and acidosis, respectively (online suppl. Fig. 1, for all online suppl. material, see http://www.karger.com/doi/10.1159/000508685). The sensitivities of albumin, iCa, phosphate, and acidosis were 78.26, 78.26, 65.22, and 43.48%; their specificities were 61.64, 70.55, 80.14, and 89.73%, respectively.
Albumin, iCa, phosphate, and acidosis were used to develop a nomogram (Fig. 1). The total score was 345:100 for acidosis, 93 for hyperphosphatemia, 78 for hypocalcemia, and 74 for hypoalbuminemia. The total score was 345 points. According to the formula: W = score (variable)/total score, the exact weight of each variable for sepsis-related mortality was acidosis (28.99%), hyperphosphatemia (26.96%), hypocalcemia (22.61%), and hypoalbuminemia (21.45%).
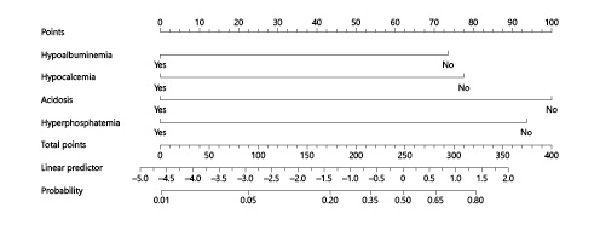
Fig. 1
Nomogram to predict mortality in neonates with sepsis. Variable values attributed to each individual patient are located on the different axes and a line is drawn upwards to determine the number of points received for each variable value. The sum of these numbers is located on the total point axis, and a line is then drawn downwards to the survival axis to determine the likelihood of sepsis-related mortality.
Discussion
This study examined clinicopathologic data collected from 472 neonates and analyzed the association of serum iCa with sepsis-related morbidity and mortality. Our results show that hypocalcemia is common in neonates with sepsis (49.1%) and can be used as an independent risk factor to predict sepsis-related mortality. In the ROC analysis, the AUC was 0.744 (95% CI 0.671–0.808). The sensitivity and specificity of hypocalcemia were 78.26 and 70.55%, respectively. We also developed a nomogram based on significant variables associated with mortality.
An increased risk of sepsis is known to be associated with low birth weight and gestational age [, ]. When we matched NS patients with controls based on birth weight and gestational age, we found associations of NS with amnionitis and premature rupture of the membrane. Additionally, NS patients with hypocalcemia had a higher risk of cardiovascular anomalies and organ dysfunction, and hypocalcemia could explain 22.61% of the overall sepsis-related mortality in our study.
Hypocalcemia in NS patients was associated with hypomagnesemia, hyperphosphatemia, hypoalbuminemia, high levels of bicarbonates, and high partial pressure of carbon dioxide. These findings corroborate previous results on critically ill pediatric or adult patients [, ]. Indeed, many critically ill patients show biochemical abnormalities, e,g., in calcium, magnesium, phosphate, sodium, potassium, and/or albumin levels []. About 90% of critically ill patients have low concentrations of total calcium, and 15–20% of these patients have hypocalcemia (based on iCa) []. Similarly, hypomagnesemia is common in critically ill patients with coexisting electrolyte abnormalities [], while hypo- and hyperphosphatemia are common in patients with severe sepsis and septic shock [].
A number of factors contribute to neonatal hypocalcemia, including gestational age, maternal and infant comorbidities, and perinatal factors []. Since calcium is actively transferred from the mother to the fetus during the third trimester of pregnancy, abrupt cessation of placental transfer of calcium after birth could lead to neonatal hypocalcemia []. Our results suggest that low birth weight, low gestational age, antenatal corticosteroids, and 5-min Apgar score <7 are associated with hypocalcemia in neonates with sepsis. Antenatal corticosteroids are widely used to manage lung immaturity in preterm neonates and may lead to hypocalcemia []. We found no difference in the risk of hypocalcemia in neonates born to mothers with or without gestational diabetes mellitus, consistent with previous work [].
Calcium derangements frequently occur during sepsis, and these alterations have been linked to bacteremia and the effects of inflammatory mediators on PTH secretion and function []. We found significantly higher PTH levels in patients with hypocalcemia, indicating increased PTH secretion by the parathyroid chief cells. Despite elevated PTH levels, there was a decrease in serum iCa in NS neonates, which is surprising since PTH is known to directly target the bones and kidneys to increase serum calcium levels. Hence, hypocalcemia may be due to an acquired defect in the ability of PTH to act on target tissue [].
The most important finding of our study is the higher mortality rate in NS patients with hypocalcemia, which may help address the controversy about a potential association between low iCa concentrations and sepsis-related mortality []. The decrease in serum iCa levels may reflect free calcium entry into cells, causing subsequent cellular injury []. In adult patients with sepsis, lower levels of iCa [] and extreme hypo- or hypercalcemia have been associated with mortality []. Hypocalcemia has also been associated with severe organ dysfunction []. The results of our study show that septic neonates with hypocalcemia face a higher risk of organ dysfunction, including cardiovascular and renal dysfunction, disseminated intravascular coagulation, or seizures, compared to neonates with normal calcium levels.
All these associations may provide clues into how hypocalcemia may increase the risk of mortality in NS neonates. Calcium homeostasis is essential for maintaining the normal myocardial contraction/relaxation cycle, which may help explain why cardiovascular comorbidities such as arrhythmia and hypotension were common in our NS patients with hypocalcemia (Table 2). Acute kidney injury is a common complication of sepsis and has a significant relationship with mortality []. Both PTH and vitamin D increase serum calcium levels by promoting the reabsorption of Ca2+ in renal tubules [, ]. However, in acute renal insufficiency, calcium excretion into the urine increases, adversely affecting other organs []. Since calcium is a key cofactor of the coagulation cascade, low calcium levels may be associated with coagulopathy, predisposing neonates with hypocalcemia to increased bleeding []. Hypocalcemia increases neuromuscular irritability and may result in paresthesia and tetany [, ], as well as electroencephalogram abnormalities [], which may help explain why seizures were more common among our neonates with hypocalcemia than among those with normal iCa levels. Indeed, neurological dysfunction appears to contribute to the mortality of pediatric patients with sepsis [].
Based on our multivariate regression analysis, acidosis, hypoalbuminemia, hypocalcemia, and hyperphosphatemia were predictors of sepsis-related mortality in our neonates. Using these results, we developed a nomogram that can be used for clinical predictions of mortality risk in neonates with sepsis. The factors in our nomogram should be confirmed in other patient samples, and the relative weighting of the predictor likely needs to be optimized for the particular patient population.
The results of this study should be interpreted with caution given our methodological limitations. Our study was retrospective and conducted at a single center. We sampled ionized calcium levels in blood only before antibiotic treatment against sepsis. Future research should focus on evaluating calcium concentrations and their effects on neonates throughout the course of the disease. We excluded neonates with hypercalcemia from the study, in agreement with the practice of similar studies [, ]. This may introduce selection bias, although hypercalcemia was present in only 5 of the otherwise eligible 198 neonates. Future work should include such neonates in order to ensure more accurate results.
Despite these limitations, our study suggests that hypocalcemia is commonly observed in neonates with sepsis and can be used as a risk factor to predict sepsis-related mortality.
Statement of Ethics
Written informed consent to participate in this study was provided by the participants’ legal guardian. The protocol for this study was approved by the Institutional Review Board of the Union Hospital (2016-S251).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This project was funded by the National Natural Science Foundation of China (Nos. 81601324 and 81300523).
Author Contributions
Y.L. designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Y. Chai. and Z.R. designed the data collection instruments, collected the data, and reviewed and revised the manuscript. Y. Chen. designed the study, coordinated, and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–30. 2213-2600
- 2. Saugstad OD. Reducing global neonatal mortality is possible. Neonatology. 2011;99(4):250–7. 1661-7800
- 3. Gkentzi D, Dimitriou G. Procalcitonin use for shorter courses of antibiotic therapy in suspected early-onset neonatal sepsis: are we getting there?J Thorac Dis. 2017;9(12):4899–902. 2072-1439
- 4. Celik IH, Demirel FG, Uras N, Oguz SS, Erdeve O, Biyikli Z, et al What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal. 2010;24(6):407–12. 0887-8013
- 5. Oncel MY, Ozdemir R, Yurttutan S, Canpolat FE, Erdeve O, Oguz SS, et al Mean platelet volume in neonatal sepsis. J Clin Lab Anal. 2012;26(6):493–6. 0887-8013
- 6. Aberegg SK. Ionized Calcium in the ICU: Should It Be Measured and Corrected?Chest. 2016;149(3):846–55. 0012-3692
- 7. Thomas TC, Smith JM, White PC, Adhikari S. Transient neonatal hypocalcemia: presentation and outcomes. Pediatrics. 2012;129(6):e1461–7. 0031-4005
- 8. Egi M, Kim I, Nichol A, Stachowski E, French CJ, Hart GK, et al Ionized calcium concentration and outcome in critical illness. Crit Care Med. 2011;39(2):314–21. 0090-3493
- 9. Magnotti LJ, Bradburn EH, Webb DL, Berry SD, Fischer PE, Zarzaur BL, et al Admission ionized calcium levels predict the need for multiple transfusions: a prospective study of 591 critically ill trauma patients. J Trauma. 2011;70(2):391–5. 0022-5282
- 10. Zhang Z, Xu X, Ni H, Deng H. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One. 2014;9(4):e95204. 1932-6203
- 11. Ahmad MS, Ahmad D, Medhat N, Zaidi SA, Farooq H, Tabraiz SA. Electrolyte Abnormalities in Neonates with Probable and Culture-Proven Sepsis and its Association with Neonatal Mortality. J Coll Physicians Surg Pak. 2018;28(3):206–9. 1022-386X
- 12. Kutílek Š, Vracovská M, Pečenková K, Brožíková H, Pikner R, Fejfarková Z. Calcemia and Inflammatory Markers in Early-Onset Neonatal Infection. Acta Med (Hradec Kralove). 2019;62(2):58–61. 1211-4286
- 13. Müller B, Becker KL, Kränzlin M, Schächinger H, Huber PR, Nylèn ES, et al Disordered calcium homeostasis of sepsis: association with calcitonin precursors. Eur J Clin Invest. 2000;30(9):823–31. 0014-2972
- 14. Subspecialty Group of Neonatology Pediatric Society Chinese Medical A. Editorial Board Chinese Journal of P[Protocol for diagnosis and treatment of neonatal septicemia]. Zhonghua Er Ke Za Zhi. 2003;41(12):897–9.0578-1310
- 15. Sano H, Kobayashi R, Iguchi A, Suzuki D, Kishimoto K, Yasuda K, et al Risk factors for sepsis-related death in children and adolescents with hematologic and malignant diseases. J Microbiol Immunol Infect. 2017;50(2):232–8. 1684-1182
- 16. Rong Z, Liu H, Xia S, Chang L. Risk and protective factors of intraventricular hemorrhage in preterm babies in Wuhan, China. Childs Nerv Syst. 2012;28(12):2077–84. 0256-7040
- 17. Working Group Of Pediatrics Chinese Society Of Parenteral And Enteral NutritionWorking Group Of Neonatology Chinese Society Of PediatricsWorking Group Of Neonatal Surgery Chinese Society Of Pediatric Surgery. CSPEN guidelines for nutrition support in neonates. Asia Pac J Clin Nutr. 2013;22(4):655–63.0964-7058
- 18. Yang C, Liu Z, Tian M, Xu P, Li B, Yang Q, et al Relationship Between Serum Albumin Levels and Infections in Newborn Late Preterm Infants. Med Sci Monit. 2016;22:92–8. 1234-1010
- 19. Chetta KE, Hair AB, Hawthorne KM, Abrams SA. Serum phosphorus levels in premature infants receiving a donor human milk derived fortifier. Nutrients. 2015;7(4):2562–73. 2072-6643
- 20. Kahvecioğlu D, Çakır U, Yıldız D, Alan S, Erdeve Ö, Atasay B, et al Transient tachypnea of the newborn: are there bedside clues for predicting the need of ventilation support? Turk J Pediatr. 2016;58(4):400–5. 0041-4301
- 21. Kim JK, Chang YS, Sung S, Ahn SY, Park WS. Trends in the incidence and associated factors of late-onset sepsis associated with improved survival in extremely preterm infants born at 23-26 weeks’ gestation: a retrospective study. BMC Pediatr. 2018;18(1):172. 1471-2431
- 22. Baizat M, Zaharie G, Iancu M, Muresan D, Hă[Latin Small Letter s with comma below]mă[Latin Small Letter s with comma below]anu M, Procopciuc LM. Potential Clinical Predictors of Suspected Early and Late Onset Sepsis (EOS and LOS) in Preterm Newborns: a Single Tertiary Center Retrospective Study. Clin Lab. 2019;65(7). 1433-6510
- 23. Steele T, Kolamunnage-Dona R, Downey C, Toh CH, Welters I. Assessment and clinical course of hypocalcemia in critical illness. Crit Care. 2013;17(3):R106. 1364-8535
- 24. Tan SC, Freebairn R. Electrolyte disorders in the critically ill. Anaesth Intensive Care Med. 2017;18(3):133–7. 1472-0299
- 25. Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992;20(2):251–62. 0090-3493
- 26. Hansen BA, Bruserud Ø. Hypomagnesemia in critically ill patients. J Intensive Care. 2018;6(1):21. 2052-0492
- 27. Miller CJ, Doepker BA, Springer AN, Exline MC, Phillips G, Murphy CV. Impact of Serum Phosphate in Mechanically Ventilated Patients With Severe Sepsis and Septic Shock. J Intensive Care Med. 2018;•••:885066618762753.0885-0666
- 28. Cho WI, Yu HW, Chung HR, Shin CH, Yang SW, Choi CW, et al Clinical and laboratory characteristics of neonatal hypocalcemia. Ann Pediatr Endocrinol Metab. 2015;20(2):86–91. 2287-1012
- 29. Schäffer L, Luzi F, Burkhardt T, Rauh M, Beinder E. Antenatal betamethasone administration alters stress physiology in healthy neonates. Obstet Gynecol. 2009;113(5):1082–8. 0029-7844
- 30. Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36(6 Pt 2):617–27. 1262-3636
- 31. Song SK, Karl IE, Ackerman JJ, Hotchkiss RS. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis?Proc Natl Acad Sci USA. 1993;90(9):3933–7. 0027-8424
- 32. Dias CR, Leite HP, Nogueira PC, Brunow de Carvalho W. Ionized hypocalcemia is an early event and is associated with organ dysfunction in children admitted to the intensive care unit. J Crit Care. 2013;28(5):810–5. 0883-9441
- 33. Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, et al Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation. 2019;26(2):e12483. 1073-9688
- 34. Cipriani C, Pepe J, Clementelli C, Manai R, Colangelo L, Fassino V, et al Effect of a single intravenous zoledronic acid administration on biomarkers of acute kidney injury (AKI) in patients with osteoporosis: a pilot study. Br J Clin Pharmacol. 2017;83(10):2266–73. 0306-5251
- 35. Morotti A, Charidimou A, Phuah CL, Jessel MJ, Schwab K, Ayres AM, et al Association Between Serum Calcium Level and Extent of Bleeding in Patients With Intracerebral Hemorrhage. JAMA Neurol. 2016;73(11):1285–90. 2168-6149
- 36. Lin MC, Chiu NC, Chi H, Ho CS, Huang FY. Evolving trends of neonatal and childhood bacterial meningitis in northern Taiwan. J Microbiol Immunol Infect. 2015;48(3):296–301. 1684-1182