Introduction
Autoantibodies, as detected by the indirect immunofluorescence assay (IIFA) on HEp-2 cells (IIFA HEp-2), are recognised as important diagnostic markers in a plethora of autoimmune diseases, in particular the systemic autoimmune rheumatic diseases (SARD). Although somewhat dated by today’s standards, members of the American College of Rheumatology (ACR) prepared an evidence-based guideline for the usefulness of the HEp-2 IIFA results for diagnostic and prognostic purposes and also for meeting diagnostic criteria. That guideline was based on reactivity with nuclear antigens as detected by IIFA on rodent tissue or HEp-2 cells. More recently, the IIFA on HEp-2 cells was reinforced as the gold standard for autoantibody screening in SARD.
Interestingly, the HEp-2 IIFA test reveals much more information than the mere absence or presence of autoantibodies, that is, the level of antibody as well as the HEp-2 IIFA pattern. Based on titration or appropriate evaluation of the fluorescence intensity, the antibody level can be determined and this information has general concordance with the clinical relevance of the test result. Indeed, higher antibody levels are better associated with SARD and have an increased likelihood to identify the autoantigen in follow-up testing. The importance of the level of autoantibodies is also recognised in the ACR guideline as well as by the recommendations issued by the European Autoimmunity Standardization Initiative (EASI) and the International Union of Immunologic Societies (IUIS) Autoantibody Standardization Subcommittee.
The HEp-2 IIFA pattern may also reveal clinically relevant information. This information is not restricted to giving direction to follow-up testing for antigen-specificity, but, for instance, the centromere pattern is included in the classification criteria for systemic sclerosis, while the nuclear dense fine speckled pattern is reported to be more prevalent in apparently healthy individuals as compared with patients with SARD. To harmonise the names and descriptions of the distinct HEp-2 IIFA patterns, an ordered classification taxonomy was proposed. This proposal was subsequently elaborated on by the International Consensus on ANA Patterns (ICAP), initiated in parallel to the 12th International Workshop on Autoantibodies and Autoimmunity (2014) held in Sao Paulo, Brazil. During this workshop, a consensus was reached on the nomenclature and definitions of 28 HEp-2 IIFA patterns. Each HEp-2 IIFA pattern was ascribed an alphanumeric code from AC-1 to AC-28. The consensus nomenclature for each pattern and representative images were also made available online at the ICAP website (http://www.ANApatterns.org).
In addition to the nuclear patterns, important cytoplasmic and mitotic patterns may also be observed in HEp-2 IIFA analysis. Although reporting non-nuclear patterns is considered clinically relevant, for various jurisdictional reasons there is no clear-cut consensus viewpoint on reporting non-nuclear patterns as a negative or positive test. With the understanding that the term ‘Antinuclear antibody (ANA) test’ may be inappropriate to designate a test that also addresses autoantibodies to antigens in the cytoplasm and mitotic apparatus, an alternative name, anticellular antibodies, was suggested in the EASI/IUIS recommendations. Recent publications from ICAP have preferred the term HEp-2 IIFA as it covers the whole spectrum of patterns that can be observed when using the HEp-2 cells as substrate.
Originally, the HEp-2 IIFA patterns were associated with diseases, but it was anticipated that many of these associations are only valid if the antigen-specificity was confirmed by follow-up testing. In subsequent ICAP workshops, it was agreed that the disease associations should be replaced by clinical relevance. In this current paper, we present the consensus on the clinical relevance of the distinct HEp-2 IIFA patterns as achieved by consecutive workshops and discussions among the executive ICAP members.
Materials and methods
For discussion about the structure of clinical relevance templates were prepared for AC-2 (LECA), AC-3 (JD) and AC-5 (MS). This formed the basis of a guideline for description of each AC pattern (EC). Of highest importance, it was agreed that the information should be objective and helpful for the clinician, the pattern–antigen associations should be put in the right clinical context and information should be evidence-based.
In preparation for the third ICAP workshop in Kyoto (2016), composition of the clinical relevance documents was started for the nuclear patterns (JD, LECA, MS), cytoplasmic patterns (CAvM, EKLC) and mitotic patterns (MH, TM). As far as already available, the documents were commented on by the ICAP executive board and, after appropriate adjustment, discussed with the workshop participants. The feedback from participants mainly focused on the structure of the information provided, on the required level of detail and the format of recommended follow-up testing.
In anticipation of the fourth ICAP workshop in Dresden (2017), the set of clinical relevance documents was completed for all patterns. Further comments from the ICAP executive board were included. The resulting documents were individually discussed with the workshop participants for nuclear (JD), cytoplasmic (CAvM) and mitotic (MH) patterns. Besides several substantive comments, there was general agreement that the information should be provided in tabular format at two distinct levels. The first level should contain information on relevant follow-up testing in the respective clinical context, the recommended follow-up tests should be commercially available and detailed test characteristics should not be given because of potential geographic and jurisdictional differences. Information based on case reports or small patient cohorts, as well as information on possible follow-up testing that is only available in specialised research laboratories, should only be provided in the second level information.
Tables for nuclear, cytoplasmic and mitotic patterns were prepared for first and second level information (JD). These tables were commented by the ICAP executive board and finalised by JD. Of note, since the starting point of the tables on clinical relevance is the HEp-2 IIFA pattern and not the clinically suspected disease, the tables do not list all autoantibodies related to the respective disease.
Results
Nuclear HEp-2 IIFA patterns
To date, a total of 15 nuclear HEp-2 IIFA patterns have been described, that is, AC-1–AC-14 and AC-29. Table 1 summarises the clinical relevance of these patterns. Since AC-29 was only recently described, the advice for follow-up testing for autoantibodies to topoisomerase I (Scl-70) in case of clinical suspicion of systemic sclerosis is also added as a note to the clinical relevance of AC-1. In particular, disease-specific immunoassays, like autoimmune liver disease profile, inflammatory myopathy profile, systemic sclerosis profile, are often only available in specialty clinical laboratories.
For six nuclear HEp-2 IIFA patterns (AC-3, 5, 7, 8, 12 and 13), additional information about clinical relevance is summarised in online supplementary table S1. Although some assays for anti-CENP-A antibodies are commercially available, these antibodies are included in online supplementary table S1 because the majority of sera revealing the AC-3 pattern are also reactive with CENP-B. In contrast to CENP-A, CENP-B is included in many routine extractable nuclear antigens profiles.
Cytoplasmic HEp-2 IIFA patterns
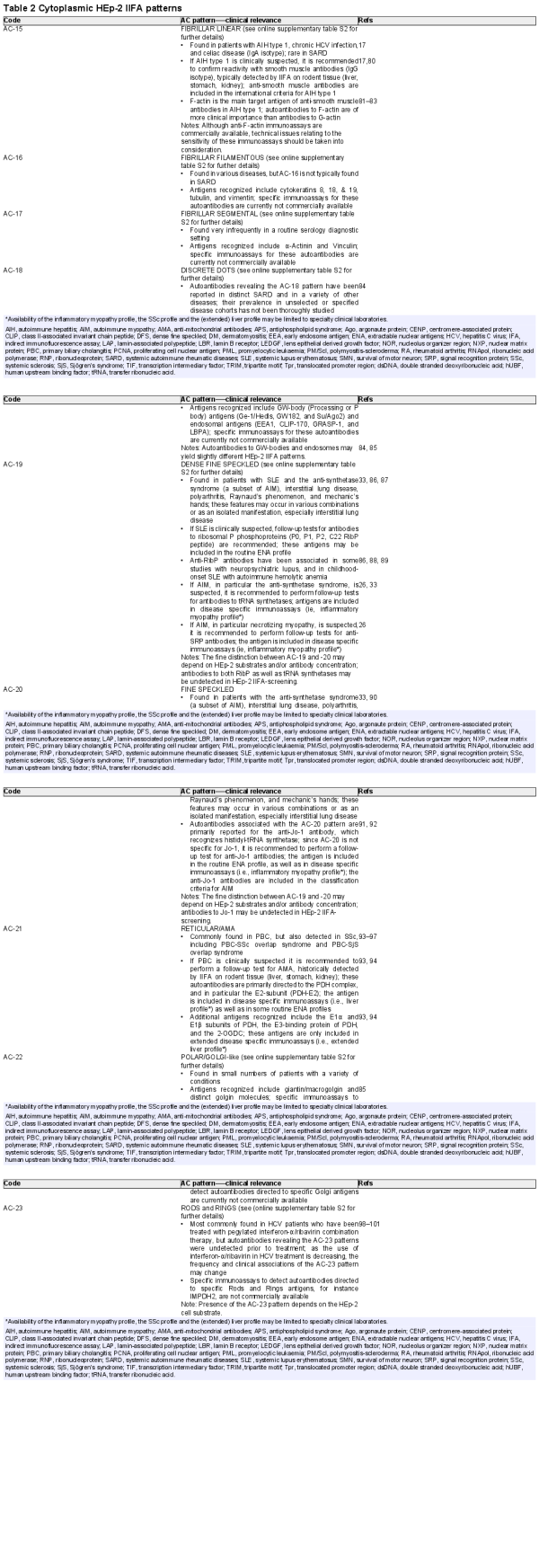
Table 2 summarises the clinical relevance of the nine cytoplasmic HEp-2 IIFA patterns, that is, AC-15–AC-23. It is recognised that the distinction between AC-19 (dense fine speckled) and AC-20 (fine speckled) can be challenging. Moreover, within the spectrum of anti-tRNA synthetase antibodies, not all produce an HEp-2 IIFA pattern and only some anti-Jo-1 antibodies are considered to give the AC-20 pattern, while the other anti-tRNA synthetase antibodies (EJ, KS, OJ, PL-7 and PL-12) are more likely to reveal the AC-19 pattern. Solid information on the pattern of two additional anti-tRNA synthetase antibodies (Ha and Zo) is lacking. Overall, the relation between these two cytoplasmic HEp-2 IIFA patterns and the distinct anti-tRNA synthetase antibodies is subject to further discussion. In clinical practice, the complete spectrum of the anti-tRNA synthetase antibodies should be determined irrespective of the subtype of cytoplasmic speckled pattern, that is, AC-19 or AC-20.
For seven cytoplasmic HEp-2 IIFA patterns (AC-15–AC-19, AC-22 and AC-23), more detailed information is provided in online supplementary table S2. In particular, for AC-16–AC-18, the clinical associations are quite diverse, depending on the antigen recognised. Overall, the clinical associations provided are primarily based on antigen-specific immunoassays and not on the HEp-2 IIFA pattern as such.
Mitotic HEp-2 IIFA patterns
The clinical relevance of the five mitotic patterns is summarised in table 3, with more detailed information in online supplementary table S3. As for the cytoplasmic patterns, clinical associations for the mitotic patterns are primarily based on antigen-specific immunoassays and not on the HEp-2 IIFA pattern as such.
Discussion
In the current paper, we present the ICAP consensus on the clinical relevance of 29 HEp-2 IIFA patterns defined by ICAP. The consensus on clinical relevance is defined in the clinical context of the patient, that is, suspected disease, and includes recommended follow-up testing within the spectrum of antigen-specificities that are commercially available. Obviously, if follow-up testing identifies the antigen, the clinical relevance can be further refined.
Defining the clinical relevance of HEp-2 IIFA patterns in the context of disease manifestations is meant to be an important tool for the clinician in the diagnostic work-up of patients suspected of SARD. Unfortunately, good data on the association between HEp-2 IIFA patterns and the distinct diseases are lacking, probably due to reasons summarised below. There are several reasons for not finding a perfect association between HEp-2 IIFA patterns and diseases. First, pattern assignment in clinical laboratories is rather inconsistent as shown by external quality assessments. This is exactly the reason why ICAP was initiated: the consensus on nomenclature and definitions of HEp-2 IIFA patterns allows to align pattern description across laboratories. Also, the integration of computer-aided immunofluorescence microscopy (CAIFM) may further improve the consistency in pattern assignments. As such, it is promising that several companies involved in CAIFM have declared their intention to accommodate to the ICAP classification. Second, even apparently healthy individuals may have autoantibodies as detected by the HEp-2 IIFA. Such autoantibodies, being either innocent bystander antibodies or predictive antibodies, may still be present on development of SARD and interfere with the SARD-related pattern. Interestingly, the pattern best associated with apparently healthy individuals is the nuclear dense fine speckled pattern (AC-2), but this association only holds if the specificity is confirmed as monospecific for DFS70. Third, the HEp-2 IIFA patterns may slightly differ depending on the cellular substrate used. For this reason, the ICAP website contains for each pattern multiple pictures taken from different brands of HEp-2 slides. Fourth, diseases like systemic lupus erythematosus and autoimmune inflammatory myopathies may be associated with distinct autoantibodies, each associated with a distinct HEp-2 IIFA pattern. If the autoantigens are ill defined, as is the case, for instance, in autoimmune hepatitis, only the most prevalent patterns are included. Altogether, it is evident that, with the exception of the centromere pattern (AC-3), all patterns are to be confirmed by antigen-specific immunoassay for a solid association with the respective autoimmune diseases.
While consensus statements have been generated for all 29 HEp-2 IIFA patterns, and it is highly recommended to report patterns, it is anticipated that laboratories may restrict their reports to the so-called ‘competent level’ patterns (http://www.ANApatterns.org). Although, for instance, the nucleolar patterns may not be reported as distinct entities (AC-8, AC-9 and AC-10), all three subtypes represent autoantibodies reactive with antigens associated with systemic sclerosis, either alone or in combination with autoimmune inflammatory myopathies. Follow-up testing, therefore, anyhow involves the systemic sclerosis multiparameter assay including all the relevant autoantibodies. Traditionally, only nuclear HEp-2 IIFA patterns have been considered as a true positive HEp-2 IIFA test, and this is most likely related to the time-honoured terminology ‘Antinuclear Antibody Test’, but it is evident from this report that even for nuclear HEp-2 IIFA patterns, the clinical associations are quite diverse. In particular, the nuclear dense fine speckled pattern (AC-2) seems to have an inverse association with SARD. On the other hand, the cytoplasmic HEp-2 IIFA patterns, and to a lesser extent the mitotic patterns, are also clinically relevant and may demand dedicated follow-up testing in daily clinical practice. Therefore, the ICAP executive board advocates that information on HEp-2 IIFA patterns should be reported to the clinician and should also be incorporated in diagnostic and classification criteria instead of the simple assignment ‘ANA-positive’.
Although the HEp-2 IIFA has been considered the gold standard for autoantibody detection in SARD, the limitations of this assay are understood. Indeed, up to 35% of healthy controls may be positive if a screening dilution of 1/40 is used. Therefore, in the EASI/IUIS recommendations, it is advocated that each laboratory verifies that the screening dilution is defined by a cut-off set at the 95th percentile. However, by taking into account that the HEp-2 IIFA nowadays is ordered by a wide spectrum of clinical disciplines, the number of clinically unexpected positive results, that is, positive test results with no clinical evidence of an associated autoimmune disease, is ever increasing and may even equal the likelihood of a clinically true-positive result. A study performed in a community setting concluded that many patients with a positive ANA test are incorrectly given a diagnosis of systemic lupus erythermatosus and sometimes even treated with toxic medications. These arguments are used to introduce a gating strategy in order to restrict test-ordering to those cases that have a sufficiently high pretest probability for having a SARD. However, it can also be argued that patients with a low pretest probability should be tested using the HEp-2 IIFA in order to prevent true cases, especially those with very early disease manifestations, from being missed. This is a paradigm shift to disease prediction and prevention. In this strategy, the HEp-2 IIFA could be integrated in multianalyte ‘omic’ profiles for case finding and establishing an early diagnosis and preventing severe complications. Obviously, it is anticipated that the added value of the HEp-2 IIFA in this approach can be increased by incorporating information on both patterns as well as titres in combination with well-directed advices on follow-up testing.
Although the current consensus on the clinical relevance of HEp-2 IIFA patterns has come across after extensive discussion and debate within the ICAP executive board as well as with the workshop participants, the information provided is not based on a systematic review or meta-analysis of the existing literature. Because of the short history of ICAP, being founded in 2014, inclusion of older literature might have been hampered by potential differences in pattern nomenclature and definitions. For instance, the nuclear dense fine speckled (AC-2) and topo I-like (AC-29) patterns were previously often considered homogeneous, speckled or even mixed patterns. The centromere pattern (AC-3) or the cytoplasmic reticular/AMA (AC-21) patterns, on the other hand, are examples that probably have been less prone to change in pattern definition over time. The universal use of the ICAP nomenclature and pattern definitions, both in daily clinical practice as well as in the scientific literature, may enable systematic reviews in the future, and may well fine-tune current consensus based on expert opinions only.
In conclusion, the consensus statements on clinical relevance should be readily available to clinicians and this will enable further harmonisation of test-result interpretation with respect to HEp-2 IIFA patterns. Obviously, clinicians should be aware of the clinical suspicion for the respective patient, and therefore should order specific tests accordingly, also taking into account the anticipation of prevalence of HEp-2 IIFA negative (AC-0) results in SARD. The information on clinical relevance of HEp-2 IIFA patterns is intended to support the decision strategy of the clinician. Information presented in the online supplementary tables 1–3 is primarily intended to be used for complex cases in the consultation of the laboratory specialist by the clinician. Depending on various jurisdictional regulations, follow-up testing can be automated in predefined algorithms which eventually will shorten the diagnostic delay. Eventually, appropriate integration of HEp-2 IIFA pattern information may help to better define disease criteria and even enable a paradigm shift in the pretest probability paradox.
We thank all the workshop participants for their constructive comments and fruitful discussions.
Handling editor Josef S Smolen
Contributors All authors actively participated in the respective workshops in Kyoto and Dresden. They also participated in the discussions of the executive ICAP committee. The draft of the manuscript was made by JD and was commented on by all authors. Final discussions have taken place at the international autoimmunity meeting in Lisbon. Required amendments were made by JD and approved by all authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests The ICAP committee is funded by unrestricted educational grants by several in vitro diagnostics companies (for details see www.anapatterns.org/sponsors.php). JD has received lecture fees from Euroimmun and Thermo Fisher. MJF is a consultant to Inova Diagnostics and Werfen International; none of the other authors declare any competing interest.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.
References
- 1. Mahler M, Meroni P-L, Bossuyt X, et al. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J Immunol Res 2014;2014:1–18.doi:10.1155/2014/315179
- 2. Solomon DH, Kavanaugh AJ, Schur PH, et al. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002;47:434–44.doi:10.1002/art.10561
- 3. Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis 2010;69:1420–2.doi:10.1136/ard.2009.127100
- 4. Damoiseaux JGMC, Cohen Tervaert JW. From ANA to ena: how to proceed? Autoimmun Rev 2006;5:10–17.doi:10.1016/j.autrev.2005.05.007
- 5. Op De Beeck K, Vermeersch P, Verschueren P, et al. Detection of antinuclear antibodies by indirect immunofluorescence and by solid phase assay. Autoimmun Rev 2011;10:801–8.doi:10.1016/j.autrev.2011.06.005
- 6. Oyaert M, Bossuyt X, Ravelingien I, et al. Added value of indirect immunofluorescence intensity of automated antinuclear antibody testing in a secondary hospital setting. Clin Chem Lab Med 2016;54:e63–6.doi:10.1515/cclm-2015-0887
- 7. Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23.doi:10.1136/annrheumdis-2013-203863
- 8. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55.doi:10.1136/annrheumdis-2013-204424
- 9. Mariz HA, Sato EI, Barbosa SH, et al. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis & Rheumatism 2011;63:191–200.doi:10.1002/art.30084
- 10. Wiik AS, Høier-Madsen M, Forslid J, et al. Antinuclear antibodies: a contemporary nomenclature using HEp-2 cells. J Autoimmun 2010;35:276–90.doi:10.1016/j.jaut.2010.06.019
- 11. Chan EKL, Damoiseaux J, Carballo OG, et al. Report of the first international consensus on standardized Nomenclature of antinuclear antibody HEp-2 cell patterns 2014–2015. Front Immunol 2015;6.doi:10.3389/fimmu.2015.00412
- 12. Damoiseaux J, von Mühlen CA, Garcia-De La Torre I, Torre G-dela, et al. International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Autoimmun Highlights 2016;7.doi:10.1007/s13317-016-0075-0
- 13. Herold M, Klotz W, Andrade LEC, et al. International consensus on antinuclear antibody patterns: defining negative results and reporting unidentified patterns. Clin Chem Lab Med 2018;56:1799–802.doi:10.1515/cclm-2018-0052
- 14. Andrade LEC, Klotz W, Herold M, et al. International consensus on antinuclear antibody patterns: definition of the ac-29 pattern associated with antibodies to DNA topoisomerase I. Clin Chem Lab Med 2018;56:1783–8.doi:10.1515/cclm-2018-0188
- 15. Conrad K, Schössler W, Hiepe F. Autoantibodies in systemic autoimmune diseases: a diagnostic reference. 2. 3th edn. Autoantigens autoantibodies autoimmunity, 2015.
- 16. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86.doi:10.1002/art.34473
- 17. Conrad K, Schössler W, Hiepe F. Autoantibodies in organ specific autoimmune diseases. a diagnostic reference. 8. 2th edn. Autoantigens autoantibodies autoimmunity, 2017.
- 18. Dellavance A, Gallindo C, Soares MG, et al. Redefining the Scl-70 indirect immunofluorescence pattern: autoantibodies to DNA topoisomerase I yield a specific compound immunofluorescence pattern. Rheumatology 2009;48:632–7.doi:10.1093/rheumatology/kep070
- 19. European Association for the Study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971–1004.doi:10.1016/j.jhep.2015.06.030
- 20. Watanabe A, Kodera M, Sugiura K, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum 2004;50:892–900.doi:10.1002/art.20096
- 21. Mahler M, Parker T, Peebles CL, et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol 2012;39:2104–10.doi:10.3899/jrheum.120598
- 22. Ochs RL, Mahler M, Basu A, et al. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med 2016;16:273–93.doi:10.1007/s10238-015-0367-0
- 23. Johnson SR, Fransen J, Khanna D, et al. Validation of potential classification criteria for systemic sclerosis. Arthritis Care Res 2012;64:358–67.doi:10.1002/acr.20684
- 24. Mehra S, Walker J, Patterson K, et al. Autoantibodies in systemic sclerosis. Autoimmun Rev 2013;12:340–54.doi:10.1016/j.autrev.2012.05.011
- 25. Shiboski CH, Shiboski SC, Seror R, et al. American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2016;2017:9–16.
- 26. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med 2016;280:8–23.doi:10.1111/joim.12451
- 27. Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 2012;64:523–32.doi:10.1002/art.33379
- 28. Bossuyt X, Frans J, Hendrickx A, et al. Detection of anti-SSA antibodies by indirect immunofluorescence. Clin Chem 2004;50:2361–9.doi:10.1373/clinchem.2004.035964
- 29. Northway JD, Tan EM. Differentiation of antinuclear antibodies giving Speckled staining patterns in immunofluorescence. Clin Immunol Immunopathol 1972;1:140–54.doi:10.1016/0090-1229(72)90013-X
- 30. Satoh M, Fritzler MJ, Chan EKL. Antihistone and antispliceosomalantibodies. In: Lahita RG, Tsokos G, Buyon JP, et al, eds. Systemic lupus erythematosus. San Diego: Academic Press, 2011: 275–92.
- 31. Satoh M, Vázquez-Del Mercado M, Chan EK. Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol 2009;19:219–28.doi:10.3109/s10165-009-0155-3
- 32. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med 1972;52:148–59.doi:10.1016/0002-9343(72)90064-2
- 33. Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol 2017;52:1–19.doi:10.1007/s12016-015-8510-y
- 34. Cozzani E, Drosera M, Riva S, et al. Analysis of a multiple nuclear dots pattern in a large cohort of dermatological patients. Clin Lab 2012;58:329–32.
- 35. Hu SL, Zhao FR, Hu Q, et al. Meta-analysis assessment of gp210 and Sp100 for the diagnosis of primary biliary cirrhosis. PLoS One 2014;9:e101916.doi:10.1371/journal.pone.0101916
- 36. Granito A, Yang WH, Muratori L, et al. PML nuclear body component SP140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol 2010;105:125–31.doi:10.1038/ajg.2009.596
- 37. Ichimura Y, Matsushita T, Hamaguchi Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis 2012;71:710–3.doi:10.1136/annrheumdis-2011-200697
- 38. Benveniste O, Stenzel W, Allenbach Y. Advances in serological diagnostics of inflammatory myopathies. Curr Opin Neurol 2016;29:662–73.doi:10.1097/WCO.0000000000000376
- 39. Ceribelli A, Fredi M, Taraborelli M, et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther 2012;14.doi:10.1186/ar3822
- 40. Onouchi H, Muro Y, Tomita Y. Clinical features and IgG subclass distribution of anti-p80 coilin antibodies. J Autoimmun 1999;13:225–32.doi:10.1006/jaut.1999.0318
- 41. Fujimoto M, Kikuchi K, Tamaki T, et al. Distribution of anti-p80-coilin autoantibody in collagen diseases and various skin diseases. Br J Dermatol 1997;137:916–20.doi:10.1111/j.1365-2133.1997.tb01551.x
- 42. Andrade LE, Chan EK, Raska I, et al. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med 1991;173:1407–19.doi:10.1084/jem.173.6.1407
- 43. Satoh M, Chan JY, Ross SJ, et al. Autoantibodies to survival of motor neuron complex in patients with polymyositis: immunoprecipitation of D, E, F, and G proteins without other components of small nuclear ribonucleoproteins. Arthritis Rheum 2011;63:1972–8.doi:10.1002/art.30349
- 44. Mahler M, Fritzler MJ, Satoh M. Autoantibodies to the mitochondrial RNA processing (MRP) complex also known as Th/To autoantigen. Autoimmun Rev 2015;14:254–7.doi:10.1016/j.autrev.2014.11.007
- 45. Ceribelli A, Cavazzana I, Franceschini F, et al. Anti-Th/To are common antinucleolar autoantibodies in Italian patients with scleroderma. J Rheumatol 2010;37:2071–5.doi:10.3899/jrheum.100316
- 46. Mahler M, Raijmakers R. Novel aspects of autoantibodies to the PM/Scl complex: clinical, genetic and diagnostic insights. Autoimmun Rev 2007;6:432–7.doi:10.1016/j.autrev.2007.01.013
- 47. Mahler M, Gascon C, Patel S, et al. Rpp25 is a major target of autoantibodies to the Th/To complex as measured by a novel chemiluminescent assay. Arthritis Res Ther 2013;15.doi:10.1186/ar4210
- 48. Okano Y, Steen VD, Medsger TA. Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis. Arthritis Rheum 1992;35:95–100.doi:10.1002/art.1780350114
- 49. Arnett FC, Reveille JD, Goldstein R, et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum 1996;39:1151–60.doi:10.1002/art.1780390712
- 50. Tormey VJ, Bunn CC, Denton CP, et al. Anti-fibrillarin antibodies in systemic sclerosis. Rheumatology 2001;40:1157–62.doi:10.1093/rheumatology/40.10.1157
- 51. Nandiwada SL, Peterson LK, Mayes MD, et al. Ethnic differences in autoantibody diversity and hierarchy: More clues from a US cohort of patients with systemic sclerosis. J Rheumatol 2016;43:1816–24.doi:10.3899/jrheum.160106
- 52. Reimer G, Rose KM, Scheer U, et al. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest 1987;79:65–72.doi:10.1172/JCI112809
- 53. Kuwana M, Kaburaki J, Mimori T, et al. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest 1993;91:1399–404.doi:10.1172/JCI116343
- 54. Fritzler MJ, von Muhlen CA, Toffoli SM, et al. Autoantibodies to the nucleolar organizer antigen NOR-90 in children with systemic rheumatic diseases. J Rheumatol 1995;22:521–4.
- 55. Fujii T, Mimori T, Akizuki M. Detection of autoantibodies to nucleolar transcription factor nor 90/hUBF in sera of patients with rheumatic diseases, by recombinant autoantigen-based assays. Arthritis Rheum 1996;39:1313–8.doi:10.1002/art.1780390808
- 56. Imai H, Ochs RL, Kiyosawa K, et al. Nucleolar antigens and autoantibodies in hepatocellular carcinoma and other malignancies. Am J Pathol 1992;140:859–70.
- 57. Yamasaki Y, Honkanen-Scott M, Hernandez L, et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum 2006;54:3051–6.doi:10.1002/art.22043
- 58. Coppo P, Clauvel JP, Bengoufa D, et al. Autoimmune cytopenias associated with autoantibodies to nuclear envelope polypeptides. Am J Hematol 2004;77:241–9.doi:10.1002/ajh.20188
- 59. Konstantinov K, Foisner R, Byrd D, et al. Integral membrane proteins associated with the nuclear lamina are novel autoimmune antigens of the nuclear envelope. Clin Immunol Immunopathol 1995;74:89–99.doi:10.1006/clin.1995.1013
- 60. Reeves WH, Chaudhary N, Salerno A, et al. Lamin B autoantibodies in sera of certain patients with systemic lupus erythematosus. J Exp Med 1987;165:750–62.doi:10.1084/jem.165.3.750
- 61. Miyachi K, Hankins RW, Matsushima H, et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmun 2003;20:247–54.doi:10.1016/S0896-8411(03)00033-7
- 62. Courvalin JC, Lassoued K, Bartnik E, et al. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest 1990;86:279–85.doi:10.1172/JCI114696
- 63. Nickowitz RE, Worman HJ. Autoantibodies from patients with primary biliary cirrhosis recognize a restricted region within the cytoplasmic tail of nuclear pore membrane glycoprotein gp210. J Exp Med 1993;178:2237–42.doi:10.1084/jem.178.6.2237
- 64. Bandin O, Courvalin JC, Poupon R, et al. Specificity and sensitivity of gp210 autoantibodies detected using an enzyme-linked immunosorbent assay and a synthetic polypeptide in the diagnosis of primary biliary cirrhosis. Hepatology 1996;23:1020–4.doi:10.1002/hep.510230512
- 65. Wesierska-Gadek J, Klima A, Komina O, et al. Characterization of autoantibodies against components of the nuclear pore complexes: high frequency of anti-p62 nucleoporin antibodies. Ann N Y Acad Sci 2007;1109:519–30.doi:10.1196/annals.1398.058
- 66. Kraemer DM, Tony HP. Nuclear pore protein p62 autoantibodies in systemic lupus erythematosus. Open Rheumatol J 2010;4:24–7.doi:10.2174/1874312901004010024
- 67. Courvalin JC, Lassoued K, Worman HJ, et al. Identification and characterization of autoantibodies against the nuclear envelope lamin B receptor from patients with primary biliary cirrhosis. J Exp Med 1990;172:961–7.doi:10.1084/jem.172.3.961
- 68. Ou Y, Enarson P, Rattner JB, et al. The nuclear pore complex protein TPR is a common autoantigen in sera that demonstrate nuclear envelope staining by indirect immunofluorescence. Clin Exp Immunol 2004;136:379–87.doi:10.1111/j.1365-2249.2004.02432.x
- 69. Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol 1978;121:2228–34.
- 70. Vermeersch P, De Beeck KO, Lauwerys BR, et al. Antinuclear antibodies directed against proliferating cell nuclear antigen are not specifically associated with systemic lupus erythematosus. Ann Rheum Dis 2009;68:1791–3.doi:10.1136/ard.2008.104190
- 71. Mahler M, Burlingame RW, Wu J, et al. Serological and clinical associations of anti-PCNA antibodies in systemic lupus erythematosus detected by a novel chemiluminescence assay. Arthritis Rheum 2011;63.
- 72. Mahler M, Miyachi K, Peebles C, et al. The clinical significance of autoantibodies to the proliferating cell nuclear antigen (PCNA). Autoimmun Rev 2012;11:771–5.doi:10.1016/j.autrev.2012.02.012
- 73. Hsu TC, Tsay GJ, Chen TY, et al. Anti-PCNA autoantibodies preferentially recognize C-terminal of PCNA in patients with chronic hepatitis B virus infection. Clin Exp Immunol 2006;144:110–6.doi:10.1111/j.1365-2249.2006.03046.x
- 74. Casiano CA, Landberg G, Ochs RL, et al. Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci 1993;106:1045–56.
- 75. Casiano CA, Humbel RL, Peebles C, et al. Autoimmunity to the cell cycle-dependent centromere protein p330d/CENP-F in disorders associated with cell proliferation. J Autoimmun 1995;8:575–86.doi:10.1016/0896-8411(95)90009-8
- 76. Rattner JB, Rees J, Whitehead CM, et al. High frequency of neoplasia in patients with autoantibodies to centromere protein CENP-F. Clin Invest Med 1997;20:308–19.
- 77. Bencimon C, Salles G, Moreira A, et al. Prevalence of anticentromere F protein autoantibodies in 347 patients with non-Hodgkin's lymphoma. Ann N Y Acad Sci 2005;1050:319–26.doi:10.1196/annals.1313.034
- 78. Welner S, Trier NH, Frisch M, et al. Correlation between centromere protein-F autoantibodies and cancer analyzed by enzyme-linked immunosorbent assay. Mol Cancer 2013;12.doi:10.1186/1476-4598-12-95
- 79. Basu D, Reveille JD. Anti-scl-70. Autoimmunity 2005;38:65–72.doi:10.1080/08916930400022947
- 80. Liberal R, Grant CR, Longhi MS, et al. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev 2014;13:435–40.doi:10.1016/j.autrev.2013.11.009
- 81. Lidman K, Biberfeld G, Fagraeus A, et al. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol 1976;24:266–72.
- 82. Fusconi M, Cassani F, Zauli D, et al. Anti-actin antibodies: a new test for an old problem. J Immunol Methods 1990;130:1–8.doi:10.1016/0022-1759(90)90291-3
- 83. Chretien-Leprince P, Ballot E, Andre C, et al. Diagnostic value of anti-F-actin antibodies in a French multicenter study. Ann N Y Acad Sci 2005;1050:266–73.doi:10.1196/annals.1313.028
- 84. Bhanji RA, Eystathioy T, Chan EK, et al. Clinical and serological features of patients with autoantibodies to GW/P bodies. Clin Immunol 2007;125:247–56.doi:10.1016/j.clim.2007.07.016
- 85. Stinton LM, Eystathioy T, Selak S, et al. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes, and GW bodies. Clin Immunol 2004;110:30–44.doi:10.1016/j.clim.2003.10.005
- 86. Sciascia S, Bertolaccini ML, Roccatello D, et al. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol 2014;261:1706–14.doi:10.1007/s00415-014-7406-8
- 87. Yura H, Sakamoto N, Satoh M, et al. Clinical characteristics of patients with anti-aminoacyl-tRNA synthetase antibody positive idiopathic interstitial pneumonia. Respir Med 2017;132:189–94.doi:10.1016/j.rmed.2017.10.020
- 88. Valões CC, Molinari BC, Pitta AC, et al. Anti-ribosomal P antibody: a multicenter study in childhood-onset systemic lupus erythematosus patients. Lupus 2017;26:484–9.doi:10.1177/0961203316676386
- 89. Mahler M, Kessenbrock K, Szmyrka M, et al. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clin Vaccine Immunol 2006;13:77–83.doi:10.1128/CVI.13.1.77-83.2006
- 90. Marie I, Hatron PY, Cherin P, et al. Functional outcome and prognostic factors in anti-Jo1 patients with antisynthetase syndrome. Arthritis Res Ther 2013;15.doi:10.1186/ar4332
- 91. Fritzler MJ, Choi MY, Mahler M. The antinuclear antibody test in the diagnosis of antisynthetase syndrome and other autoimmune myopathies. J Rheumatol 2018;45:444.1–5.doi:10.3899/jrheum.170258
- 92. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European league against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64.doi:10.1136/annrheumdis-2017-211468
- 93. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72.doi:10.1016/j.jhep.2017.03.022
- 94. Shuai Z, Wang J, Badamagunta M, et al. The fingerprint of antimitochondrial antibodies and the etiology of primary biliary cholangitis. Hepatology 2017;65:1670–82.doi:10.1002/hep.29059
- 95. Skare TL, Nisihara RM, Haider O, et al. Liver autoantibodies in patients with scleroderma. Clin Rheumatol 2011;30:129–32.doi:10.1007/s10067-010-1586-0
- 96. Zheng B, Vincent C, Fritzler MJ, et al. Prevalence of systemic sclerosis in primary biliary cholangitis using the new ACR/EULAR classification criteria. J Rheumatol 2017;44:33–9.doi:10.3899/jrheum.160243
- 97. Nardi N, Brito-Zerón P, Ramos-Casals M, et al. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren's syndrome: prevalence and clinical significance in 335 patients. Clin Rheumatol 2006;25:341–6.doi:10.1007/s10067-005-0059-3
- 98. Covini G, Carcamo WC, Bredi E, et al. Cytoplasmic rods and rings autoantibodies developed during pegylated interferon and ribavirin therapy in patients with chronic hepatitis C. Antivir Ther 2012;17:805–11.doi:10.3851/IMP1993
- 99. Calise SJ, Keppeke GD, Andrade LE, et al. Anti-rods/rings: a human model of drug-induced autoantibody generation. Front Immunol 2015;6.doi:10.3389/fimmu.2015.00041
- 100. Novembrino C, Aghemo A, Ferraris Fusarini C, et al. Interferon-ribavirin therapy induces serum antibodies determining 'rods and rings' pattern in hepatitis C patients. J Viral Hepat 2014;21:944–9.doi:10.1111/jvh.12281
- 101. Keppeke GD, Calise SJ, Chan EK, et al. Anti-rods/rings autoantibody generation in hepatitis C patients during interferon-α/ribavirin therapy. World J Gastroenterol 2016;22:1966–74.doi:10.3748/wjg.v22.i6.1966
- 102. Takahashi T, Asano Y, Hirakawa M, et al. Linear scleroderma with prominent multiple lymphadenopathy followed by the development of polymyositis: a case report and review of published work. J Dermatol 2016;43:1224–7.doi:10.1111/1346-8138.13424
- 103. Howng SL, Chou AK, Lin CC, et al. Autoimmunity against hNinein, a human centrosomal protein, in patients with rheumatoid arthritis and systemic lupus erythematosus. Mol Med Rep 2011;4:825–30.doi:10.3892/mmr.2011.505
- 104. Hamaguchi Y, Matsushita T, Hasegawa M, et al. High incidence of pulmonary arterial hypertension in systemic sclerosis patients with anti-centriole autoantibodies. Mod Rheumatol 2015;25:798–801.doi:10.3109/14397595.2013.844296
- 105. Gavanescu I, Vazquez-Abad D, McCauley J, et al. Centrosome proteins: a major class of autoantigens in scleroderma. J Clin Immunol 1999;19:166–71.doi:10.1023/A:1020551610319
- 106. Fritzler MJ, Zhang M, Stinton LM, et al. Spectrum of centrosome autoantibodies in childhood varicella and post-varicella acute cerebellar ataxia. BMC Pediatr 2003;3.doi:10.1186/1471-2431-3-11
- 107. Rattner JB, Martin L, Waisman DM, et al. Autoantibodies to the centrosome (centriole) react with determinants present in the glycolytic enzyme enolase. J Immunol 1991;146:2341–4.
- 108. Mack GJ, Rees J, Sandblom O, et al. Autoantibodies to a group of centrosomal proteins in human autoimmune sera reactive with the centrosome. Arthritis Rheum 1998;41:551–8.doi:10.1002/1529-0131(199803)41:3<551::AID-ART22>3.0.CO;2-X
- 109. Mozo L, Gutiérrez C, Gómez J, Gómez Jesús. Antibodies to mitotic spindle apparatus: clinical significance of NuMA and HsEg5 autoantibodies. J Clin Immunol 2008;28:285–90.doi:10.1007/s10875-008-9170-y
- 110. Whitehead CM, Winkfein RJ, Fritzler MJ, et al. The spindle kinesin-like protein HsEg5 is an autoantigen in systemic lupus erythematosus. Arthritis Rheum 1996;39:1635–42.doi:10.1002/art.1780391005
- 111. Szalat R, Ghillani-Dalbin P, Jallouli M, et al. Anti-NuMA1 and anti-NuMA2 (anti-HsEg5) antibodies: clinical and Immunological features: a propos of 40 new cases and review of the literature. Autoimmun Rev 2010;9:652–6.doi:10.1016/j.autrev.2010.05.001
- 112. Grypiotis P, Ruffatti A, Tonello M, et al. [Clinical significance of fluoroscopic patterns specific for the mitotic spindle in patients with rheumatic diseases]. Reumatismo 2002;54:232–7.
- 113. Price CM, McCarty GA, Pettijohn DE. NuMA protein is a human autoantigen. Arthritis Rheum 1984;27:774–9.doi:10.1002/art.1780270708
- 114. Bonaci-Nikolic B, Andrejevic S, Bukilica M, et al. Autoantibodies to mitotic apparatus: association with other autoantibodies and their clinical significance. J Clin Immunol 2006;26:438–46.doi:10.1007/s10875-006-9038-y
- 115. Compton DA, Szilak I, Cleveland DW. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol 1992;116:1395–408.doi:10.1083/jcb.116.6.1395
- 116. Rattner JB, Mack GJ, Fritzler MJ. Autoantibodies to components of the mitotic apparatus. Mol Biol Rep 1998;25:143–55.doi:10.1023/A:1016523013819
- 117. Vermeersch P, Bossuyt X. Prevalence and clinical significance of rare antinuclear antibody patterns. Autoimmun Rev 2013;12:998–1003.doi:10.1016/j.autrev.2013.03.014
- 118. Humbel RL Conrad K, ed. Autoantibodies to mitotic cells. in Dresden autoantibody symposium. Dresden Germany: Pabst Science, 2013: 327–39.
- 119. RodrÃguez-Bayona B, Ruchaud S, RodrÃguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis 2007;4.doi:10.1186/1740-2557-4-1
- 120. Gitlits VM, Macaulay SL, Toh BH, et al. Novel human autoantibodies to phosphoepitopes on mitotic chromosomal autoantigens (MCAs). J Investig Med 2000;48:172–82.
- 121. Blaschek M, Muller S, Youinou P. Anti-"dividing cell antigen" autoantibody: a novel antinuclear antibody pattern related to histones in systemic lupus erythematosus. J Clin Immunol 1993;13:329–38.doi:10.1007/BF00920241
- 122. Rayzman VM, Sentry JW. MCA1 detection of histone H3 serine 10 phosphorylation, a novel biomarker for determination of mitotic indices. Hum Antibodies 2006;15:71–80.doi:10.3233/HAB-2006-15302
- 123. Didier K, Bolko L, Giusti D, et al. Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol 2018;9.doi:10.3389/fimmu.2018.00541
- 124. Pham BN, Albarede S, Guyard A, et al. Impact of external quality assessment on antinuclear antibody detection performance. Lupus 2005;14:113–9.doi:10.1191/0961203305lu2069oa
- 125. Van Blerk M, Van Campenhout C, Bossuyt X, et al. Current practices in antinuclear antibody testing: results from the Belgian external quality assessment scheme. Clin Chem Lab Med 2009;47:102–8.doi:10.1515/CCLM.2009.021
- 126. Foggia P, Percannella G, Soda P, et al. Benchmarking HEp-2 cells classification methods. IEEE Trans Med Imaging 2013;32:1878–89.doi:10.1109/TMI.2013.2268163
- 127. Bizzaro N, Antico A, Platzgummer S, et al. Automated antinuclear immunofluorescence antibody screening: a comparative study of six computer-aided diagnostic systems. Autoimmun Rev 2014;13:292–8.doi:10.1016/j.autrev.2013.10.015
- 128. Hobson P, Lovell BC, Percannella G, et al. Benchmarking human epithelial type 2 interphase cells classification methods on a very large dataset. Artif Intell Med 2015;65:239–50.doi:10.1016/j.artmed.2015.08.001
- 129. Hobson P, Lovell BC, Percannella G, et al. Computer aided diagnosis for anti-nuclear antibodies HEp-2 images: progress and challenges. Pattern Recognit Lett 2016;82:3–11.doi:10.1016/j.patrec.2016.06.013
- 130. Hobson P, Lovell BC, Percannella G, et al. HEp-2 staining pattern recognition at cell and specimen levels: datasets, algorithms and results. Pattern Recognit Lett 2016;82:12–22.doi:10.1016/j.patrec.2016.07.013
- 131. van Beers JJBC, Hahn M, Fraune J, et al. Performance analysis of automated evaluation of antinuclear antibody indirect immunofluorescent tests in a routine setting. Autoimmun Highlights 2018;9.doi:10.1007/s13317-018-0108-y
- 132. Infantino M, Shovman O, Pérez D, Pérez D, et al. A better definition of the anti-DFS70 antibody screening by IIF methods. J Immunol Methods 2018;461:110–6.doi:10.1016/j.jim.2018.07.001
- 133. Chan EK, Damoiseaux J, de Melo Cruvinel W, et al. Report on the second international consensus on ANA pattern (ICAP) workshop in Dresden 2015. Lupus 2016;25:797–804.doi:10.1177/0961203316640920
- 134. Mahler M, Meroni PL, Andrade LE, et al. Towards a better understanding of the clinical association of anti-DFS70 autoantibodies. Autoimmun Rev 2016;15:198–201.doi:10.1016/j.autrev.2015.11.006
- 135. Meroni PL, Chan EK, Damoiseaux J, et al. Unending story of the indirect immunofluorescence assay on HEp-2 cells: old problems and new solutions? Ann Rheum Dis 2019;78:e46.doi:10.1136/annrheumdis-2018-213440
- 136. Fritzler MJ. The antinuclear antibody test: last or lasting GASP? Arthritis Rheum 2011;63:19–22.doi:10.1002/art.30078
- 137. Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol 2017;13:495–502.doi:10.1038/nrrheum.2017.74
- 138. Pérez D, Gilburd B, Azoulay D, et al. Antinuclear antibodies: is the indirect immunofluorescence still the gold standard or should be replaced by solid phase assays? Autoimmun Rev 2018;17:548–52.doi:10.1016/j.autrev.2017.12.008
- 139. Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum 1997;40:1601–11.doi:10.1002/art.1780400909
- 140. Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med 2013;126:342–8.doi:10.1016/j.amjmed.2012.09.014
- 141. Avery TY, van de Cruys M, Austen J, et al. Anti-nuclear antibodies in daily clinical practice: prevalence in primary, secondary, and tertiary care. J Immunol Res 2014;2014:1–8.doi:10.1155/2014/401739
- 142. Narain S, Richards HB, Satoh M, et al. Diagnostic accuracy for lupus and other systemic autoimmune diseases in the community setting. Arch Intern Med 2004;164:2435–41.doi:10.1001/archinte.164.22.2435
- 143. Fritzler MJ, Martinez-Prat L, Choi MY, et al. The utilization of autoantibodies in approaches to precision health. Front Immunol 2018;9.doi:10.3389/fimmu.2018.02682
- 144. Fritzler MJ. Choosing wisely: review and commentary on anti-nuclear antibody (ANA) testing. Autoimmun Rev 2016;15:272–80.doi:10.1016/j.autrev.2015.12.002