Introduction
Gout is a common inflammatory joint disease characterized by monosodium urate crystal deposition in joints and connective tissue [Hainer et al., 2014). The term tophus is employed to describe macroscopic deposits of urate crystals, typically in periarticular and subcutaneous tissues [Chhana and Dalbeth, 2015]. Head and neck manifestations of gout are uncommon and can include tophaceous deposits in the auricular helix and larynx, as well as gouty arthritis of the sternoclavicular, temporomandibular and cricoarytenoid joints [Stark and Hirokawa, 1982; Guttenplan et al., 1991; Tsikoudas et al., 2002]. Tophaceous gout of the middle ear is rare, with only 3 cases published in the English literature to date [Saliba et al., 2003; Reineke et al., 2009; Mutlu et al., 2016]. In all cases, the tophi were initially misdiagnosed for cholesteatoma or tympanosclerosis, and the final diagnosis was only established after histopathological analysis of the surgically resected specimen. While infrequent, gouty involvement of the middle ear can lead to clinically significant disease, hence its relevance for the otolaryngologist. This report describes 2 patients with middle ear gouty tophi who presented with a white-colored middle ear mass associated with conductive hearing loss that was initially mistaken for another entity. Both patients underwent surgery, and histopathological analysis revealed a diagnosis of gout. A review of the literature is also presented.
Case Report
Case 1
An 83-year-old female was evaluated for a progressive decline in her right hearing associated with a middle ear mass. She reported decreased benefit of her right hearing aid over the past few years. She denied any other otological symptoms. Her medical history was relevant for dyslipidemia, hypertension, coronary artery disease and gastroesophageal reflux. Her medications included aspirin (81 mg daily dose), atenolol, atorvastatin, omeprazole as well as calcium and vitamin C and D supplementation. She did not report any typical gout symptoms at the time of evaluation, and there were no laboratory signs of hyperuricemia. Micro-otoscopy revealed a white mass in the anterior middle ear medial to an otherwise intact tympanic membrane. Tuning fork examination was consistent with a right conductive hearing loss. Audiological evaluation demonstrated right low- to high-frequency downsloping moderate-to-severe mixed hearing loss with an air-bone gap of 30 dBs. The speech discrimination score was normal at 88%. Tympanograms showed a right As-type curve. A noncontrast high-resolution CT scan demonstrated a 4.5-mm heterogeneously hyperdense lesion in the mesotympanum between the tensor tympani and anteromedial aspect of the malleus neck and manubrium (Fig. 1). Her images were reviewed with our institution’s neuroradiologist, and the differential diagnosis included a cholesteatoma or a calcified chondroma. Surgical excision was recommended. Intraoperatively, a granular white mass anterior to the malleus and extending up to the tensor tympani tendon was noted: it was granular in consistency, and total removal was possible utilizing microinstruments and an argon laser without disruption of the ossicular chain or tympanic membrane (Fig. 2). The diagnosis of gouty tophus was revealed on final histopathological examination with the identification of negatively birefringent monosodium urate crystals under polarized light (Fig. 3). Near-total closure of the air-bone gap was noted on the postoperative audiogram. A serum uric acid level assessment was then ordered and shown to be within the normal range. She was referred to her primary care provider who initiated allopurinol treatment.
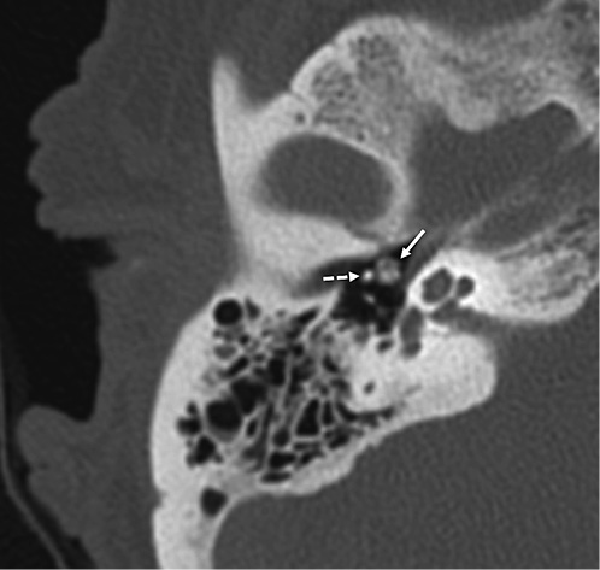
Fig. 1
Noncontrast high-resolution CT scan of the right temporal bone (axial view), demonstrating a heterogeneously hyperdense lesion in the middle ear (solid arrow) immediately anteromedial to the malleus neck (dashed arrow). The superior extent of the lesion was also abutting the lateral aspect of the tympanic segment of the facial nerve (slice not shown).
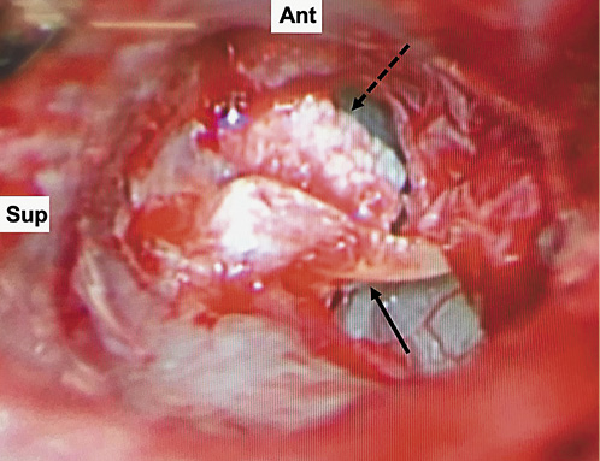
Fig. 2
Intraoperative image (right ear) showing a granular white-colored mass within the middle ear (dashed arrow) anterior to the malleus (solid arrow). The tympanic membrane was dissected off the lateral process and part of the manubrium of the malleus and reflected anteroinferiorly.
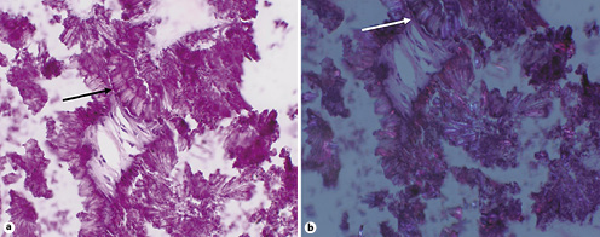
Fig. 3
High-power, hematoxylin and eosin stain. a Embedded in calcifications are needle-shaped crystals (black arrow). b The same crystals demonstrate negative birefringence on polarized light, suggestive of gout (white arrow).
Case 2
A 67-year-old male was referred to our otology clinic for a left middle ear osteoma associated with progressive conductive hearing loss for a few years. He denied any other otological symptoms. His past medical history was unremarkable. He did not report any typical gout symptoms at the time of evaluation, nor did he have any relevant biochemical workup. Micro-otoscopy revealed a thick white-colored polypoid plaque covering the superior aspect of the left eardrum and surrounding the malleus neck, lateral process and part of the manubrium. Tuning fork examination was compatible with a conductive hearing loss on that side. A noncontrast high-resolution CT scan of the temporal bones done at an outside institution revealed a heterogeneously hyperdense 8.2-mm (longest dimension) lesion along the superior aspect of the annular ring, abutting the malleus posteriorly and extending into the mesotympanum inferiorly towards the Eustachian tube (Fig. 4). Audiological evaluation confirmed a moderate-to-severe flat conductive hearing loss with a near maximal air-bone gap (45 dB) but excellent speech discrimination scores (96%). With a provisional diagnosis of middle ear osteoma, the patient underwent middle ear exploration with resection of the lesion. Intraoperatively, the white mass was of chalky consistency and was removed in fragments, unlike osteomas. The ossicular chain and tympanic membrane remained intact. The diagnosis of gouty tophus was revealed on the final histopathological examination with the identification of a foreign body giant cell reaction and negatively birefringent monosodium urate crystals under polarized light. Postoperatively, his eardrum healed appropriately with complete closure of his air-bone gap. A uric acid level was not obtained. He was referred to his primary care provider for further metabolic workup.
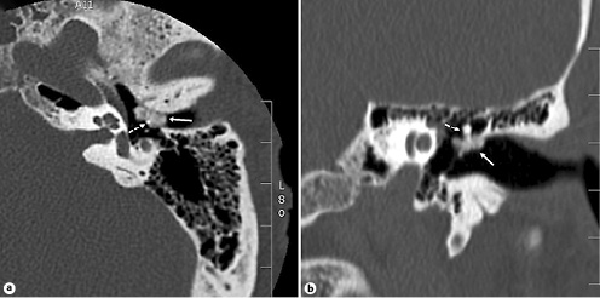
Fig. 4
Noncontrast high-resolution CT scan of the left temporal bone. a Axial view: a heterogeneously hyperdense lobular lesion is seen at the superior aspect of the annular ring (solid arrow), surrounding the neck of the malleus (dashed arrow). b Coronal view: the same lesion demonstrates extension into the mesotympanum.
Discussion
This report described 2 cases of tophaceous gout of the middle ear initially misdiagnosed as a cholesteatoma (or calcified chondroma) and an osteoma, respectively. In both cases, the final diagnosis was only revealed after histopathological analysis of the surgically resected specimens. Owing to their soft granular consistency, middle ear gouty tophi seem to carry a favorable surgical prognosis, as total removal without disruption of the ossicular chain was possible in 4 out of the 5 reported cases (including ours).
Gouty tophi presenting as middle ear masses are rare and often mistaken for other more common entities. Even in hindsight, the only atypical finding potentially clueing to the correct diagnosis was the heterogeneous hyperdense appearance on the CT images, unlike what is typically seen in osteomas, cholesteatomas or tympanosclerosis. This heterogeneity was likely caused by the punctate calcifications seen in gout [Hainer et al., 2014]. In addition, these lesions may not be recognized as tophi because the clinical diagnosis of gout may not have been established at the time of evaluation. In fact, a few reports in the literature describe patients with normal serum uric acid whose first manifestation of gout was multiple subcutaneous tophi [Koley et al., 2010; Gupta et al., 2009]. Likewise, only 3 cases of middle ear gouty tophi have been previously reported in the English literature (a fourth case, published in German, was not included in this review), and none demonstrated hyperuricemia or clinical manifestations of gout [Tausch-Treml and Berghaus, 1990]. The first report by Saliba et al. [2003] described an otherwise healthy middle-aged man with aural heaviness and a white mass in the middle ear posterior to the malleus. Reineke et al. [2009] then reported a postmenopausal woman with mixed hearing loss and a white sclerotic plaque under a thickened tympanic membrane, initially diagnosed as cholesteatoma. Lastly, Mutlu et al. [2016] described a 37-year-old male with recurrent otorrhea and white middle ear plaques presumed to be foci of tympanosclerosis, visible under a chronically perforated eardrum. Similar to our cases, the diagnosis of gout in these reports was only revealed after the histopathological analysis of the resected specimens. In light of these findings, the benefit of a systemic workup for hyperuricemia is debatable in these patients.
While uncommon, tophus deposition in the head and neck has been described in a number of sites outside of the ear. In the larynx, tophaceous lesions of the hyoid bone, arytenoid cartilages, thyroid lamina, false vocal cords and subglottis were reported [Stark and Hirokawa, 1982; Guttenplan et al., 1991; Habermann et al., 2001; Tsikoudas et al., 2002]. The involved cartilages were often irregularly expanded or destructed. Nasal gouty tophus has also been described, with deposits presenting as dorsal nasal lumps displacing the normal anatomy [Hughes et al., 2005]. Tophaceous gout of the temporomandibular joint was associated with preauricular firm swelling and trismus [Guerlain et al., 2015]. The temporomandibular joint is also a common location for tophaceous pseudogout – a related disorder characterized by calcium pyrophosphate crystal deposition – with 19 reported cases in the literature [Jordan et al., 1998]. Large tophaceous deposits of the temporomandibular joint were noted to extend into the infratemporal fossa and even involve the middle fossa floor, resulting in significant destruction of the temporal bone and masquerading as chondrosarcomas [Jordan et al., 1998; Li-Yu et al., 2000; Saliba et al., 2003; Zweifel et al., 2012]. Tophaceous involvement of the skull base was also described, with a lesion occupying the cavernous sinus and pterygopalatine fossa and manifesting as ipsilateral abducens palsy and diplopia [Reinard et al., 2015]. Regardless of their specific location, head and neck tophi were generally associated with local inflammation and tissue disruption, mimicking infectious or neoplastic processes. When possible, fine-needle aspiration and identification of crystals were helpful in establishing the correct diagnosis and avoiding unnecessarily aggressive procedures.
The occurrence of tophi at classic sites such as the olecranon process or Achilles tendon was postulated to be caused by precipitation of uric acid crystals in cooler parts of the body [McCarty, 1994]. However, the pathophysiology underlying middle ear tophaceous deposits remains unclear. Their location – usually in close relation with the tympanic membrane – suggests a possible relation with the rich blood supply in this area: branches from the deep auricular, stylomastoid and anterior tympanic arteries converge into two vascular rings on each side of the eardrum. This hypothesis remains to be explored.
Conclusion
Middle ear gouty tophi are uncommon and tend to present as white-colored granular masses. They are frequently mistaken for cholesteatoma or tympanosclerosis in patients who otherwise do not manifest any clinical or biochemical signs of gout. Surgical resection in the present 2 cases was associated with favorable outcomes.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
Joe Saliba: study design, data collection, manuscript writing; Hitomi Sakano: data collection, manuscript writing; Rick A. Friedman: study design, manuscript writing and critical review; Jeffrey P. Harris: study design, manuscript writing and critical review.
References
- 1. Chhana A, Dalbeth N. The gouty tophus: a review. Curr Rheumatol Rep. Springer US. 2015;17(3):19.
- 2. Guerlain J, Goudot P, Schouman T. A Big Toe in the Ear? Two Cases of Gouty Tophi Located in the Temporomandibular Joint. J Rheumatol. 2015;42(7):1261–2.
- 3. Gupta A, Rai S, Sinha R, Achar C. Tophi as an initial manifestation of gout. Journal of cytology. Medknow Publications Pvt Ltd; 26(4):165–6.
- 4. Guttenplan MD, Hendrix RA, Townsend MJ, Balsara G. Laryngeal manifestations of gout. Ann Otol Rhinol Laryngol. 1991;100(11):899–902.
- 5. Habermann W, Kiesler K, Eherer A, Beham A, Friedrich G. Laryngeal manifestation of gout: a case report of a subglottic gout tophus. Auris Nasus Larynx. 2001;28(3):265–7.
- 6. Hainer BL, Matheson E, Wilkes RT. Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90(12):831–6.
- 7. Hughes JP, Di Palma S, Rowe-Jones J. Tophaceous gout presenting as a dorsal nasal lump. JLO. Cambridge University Press; 2005 Jun;119(6):492–4.
- 8. Jordan JA, Roland P, Lindberg G, Mendelsohn D. Calcium pyrophosphate deposition disease of the temporal bone. Ann Otol Rhinol Laryngol. 1998;107(11 Pt 1):912–6.
- 9. Koley S, Salodkar A, Choudhary S, Bhake A, Singhania K, Choudhury M. Tophi as first manifestation of gout. Indian J Dermatol Venereol Leprol. 2010;76(4):393–6.
- 10. Li-Yu J, Schumacher HR Jr, Gratwick G. Invasive tophaceous pseudogout in the temporomandibular joint: misdiagnosis as tumor: case report and review of the literature. J Clin Rheumatol. 2000;6(5):272–7.
- 11. McCarty DJ. Gout without hyperuricemia. JAMA. 1994;271(4):302–3.
- 12. Mutlu A, Dündar E, İşeri M, Erçin C, Cefle A. An Unusual Presentation of Gout: Tophi in the Middle Ear. J Int Adv Otol. 2016;12(2):216–8.
- 13. Reinard KA, Felicella MM, Zakaria HM, Rock JP. Intradural Tophaceous Gout of the Cavernous Sinus and Spine: Case Report and Review of Literature. J Neurol Disord. 2015;3(3).
- 14. Reineke U, Ebmeyer J, Schütte F, Upile T, Sudhoff HH. Tophaceous gout of the middle ear. Otol Neurotol. 2009;30(1):127–8.
- 15. Saliba I, Bouthiller A, Desrochers P, Berthlet F, Dufour JJ. Tophaceous gout and pseudogout of the middle ear and the infratemporal fossa: case report and review of the literature. J Otolaryngol. 2003;32(4):269–72.
- 16. Stark TW, Hirokawa RH. Gout and its manifestations in the head and neck. Otolaryngol Clin North Am. 1982;15(3):659–64.
- 17. Tausch-Treml R, Berghaus A. [Gout tophus of the middle ear]. HNO. 1990;38(12):465–7.
- 18. Tsikoudas A, Coatesworth AP, Martin-Hirsch DP. Laryngeal gout. J Laryngol Otol. 2002;116(2):140–2.
- 19. Zweifel D, Ettlin D, Schuknecht B, Obwegeser J. Tophaceuos calcium pyrophosphate dihydrate deposition disease of the temporomandibular joint: the preferential site?J Oral Maxillofac Surg. 2012;70(1):60–7.
This work was not previously presented.
