Introduction
The epididymis, a highly coiled epithelial duct, constitutes a sperm transport route; testicular spermatozoa enter into the epididymis from the efferent duct and go out through the vas deferens. The spermatozoa become functionally mature and fully fertile in the epididymis [, ]. In rodents, the initial segment (IS) is the most proximal region of the epididymis which is characterized by the pseudostratified tall epithelial cells. The IS plays a critical role in sperm maturation by expressing specific proteins; disrupting the expression of such proteins causes male infertility [, ].
One of such regulatory mechanisms recently characterized is lumicrine signaling, in which testis secreted factors released into the lumen of seminiferous tubules go through luminal space via an efferent duct and act on the IS epithelium to influence its development and function []. There are several molecules identified to be included in lumicrine signaling. Neural epidermal growth factor–like like 2 (NELL2) and NELL2-interacting cofactor for lumicrine signaling (NICOL) are the molecular entities of testis-derived lumicrine factors [, ]. NELL2 and NICOL constitute together a molecular complex and activate cell surface receptor tyrosine kinase ROS1 which is expressed in the IS of epididymis. Mice lacking NEL-like 2 (Nell2), predicted gene 1673 (Gm1673 or Nicol), or Ros1 proto-oncogene (Ros1) exhibit IS differentiation failures and are completely male infertile because the epididymal spermatozoa remain immature and the ejaculated spermatozoa are unable to migrate from the uterus into the oviduct in the female reproductive tract [, , ]. Upon these findings, the increasing significance is to ask how the lumicrine regulation of IS differentiation is characterized at the molecular level and whether there are more factors associated with lumicrine signaling.
Adhesion G protein-coupled receptor G2 (ADGRG2) is expressed in the male reproductive tract including efferent duct and caput epididymis []. Structurally, ADGRG2 belongs to the adhesion G protein-coupled receptor (GPCR) family []. The extracellular region of adhesion GPCRs is exceptionally large and contains the membrane-proximal GPCR autoproteolysis-inducing domain. Dysfunction of ADGRG2 results in male infertility. In humans, a hemizygous loss-of-function mutation in ADGRG2 causes congenital bilateral absence of the vas deferens []. In mice, targeted inactivation of adhesion G protein-coupled receptor G2 (Adgrg2) caused a dysregulated fluid reabsorption within the efferent ducts leading to a backup of fluid accumulation in the testis and a subsequent stasis of spermatozoa within the efferent ducts [].
It would be beneficial to understand the function of ADGRG2 to examine how ADGRG2 transmits ligand-induced signals. ADGRG2 protein is abundantly localized on the apical surface of the ducts [], implying that ADGRG2 binds its ligands coming from the male reproductive tract luminal space as in lumicrine signaling. The association of ADGRG2 with lumicrine signaling is also implied from its regulation of gene expression in the epididymis; upon Adgrg2 ablation, the expressions of many genes are downregulated in the caput epididymis [] and several of such genes are known to be regulated by lumicrine signaling []. These findings raise a possibility that ADGRG2 is included in lumicrine signaling and lumicrine-mediated gene expression, although it has never been fully explored yet. In the present study, the association of ADGRG2 in lumicrine signaling was investigated based on the histological and transcriptomic studies of Adgrg2-null mice generated by CRISPR/CAS9-mediated genome editing.
Materials and methods
Animals
B6D2F1 mice and W/Wv mice were purchased from Japan SLC, Inc. Nell2 (or Ros1) knockout (KO) mice were obtained previously []. For efferent duct ligation, the efferent ducts of 10-week-old B6D2F1 males were unilaterally ligated and the ipsilateral epididymis was excised 4 weeks after ligation. Adgrg2 KO mice (B6D2-Adgrg2<em1Osb>) were generated on a B6D2F1 background using CRISPR/CAS9-mediated genome editing. Briefly, crispr (cr) RNA#1 and crRNA#2 (Sigma, custom synthesis), SygRNA SpCas9 tracrRNA (Sigma, #TRACRRNA05N-5NMOL), and TrueCut Cas9 Protein v2 (ThermoFisher, #A36496) were injected into fertilized eggs obtained by mating C57BL/6J females with DBA males. The treated eggs were implanted into pseudopregnant ICR females to obtain F0 individuals. The wild-type B6D2F1 females were mated with the F0 males to obtain F1 Adgrg2+/− females. The resulting F1 Adgrg2+/− females were mated with B6D2F1 wild-type males to obtain Adgrg2−/Y KO F2 males and Adgrg2+/Y wild-type control littermate males. This F2 generation individuals were used for the experiment. The crRNA sequences and genotyping primer sequences and the number of target sites of 12mer + PAM and 8mer + PAM are summarized in Supplementary Table S1. The number of 20mer + PAM target sites for both crRNAs in mouse genome is 1; there are no potential off-target sites. The potential 12mer + PAM off-target sites for each crRNA are summarized in Supplementary Table S2. The genotyping primer sequences are listed in Supplementary Table S3. The mouse line generated in this study will be deposited as frozen sperm at the RIKEN BioResource Research Center (BRC) and Center for Animal Resources and Development (CARD) at Kumatomo University. The BRC and CARD repository IDs for B6D2-Adgrg2<em1Osb> will be assigned soon and then the mouse line will be made available to all researchers. All experiments involving animals were approved by the Institutional Animal Care and Use Committees of Osaka University (Osaka, Japan) and were conducted in compliance with the university guidelines and regulations for animal experimentation.
Epididymal anatomy
In this study, the epididymal anatomy followed Johnston et al. [] in which the IS is included in the caput epididymis. Because of the difficulty in dissecting IS separately from caput epididymis especially in mice in which IS differentiation is ablated, the IS was dissected together with the caput and such a tissue dissection was indicated by the description “IS-caput.”
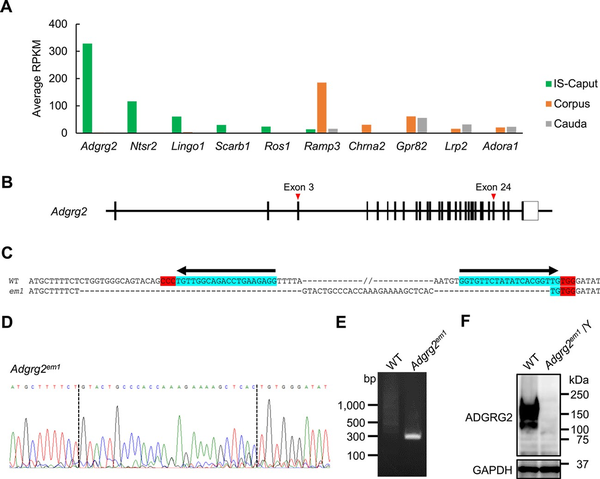
Figure 1
Generation of Adgrg2-null mice. (A) The expression of genes encoding cell surface receptors expressed in IS-caput, corpus, and cauda epididymis. The expression levels are represented in RPKM. (B) A schematic representation of mouse Adgrg2 gene. (C) The DNA sequence of Adgrg2 WT and em1 alleles. Locations of guide RNA target sites PAM sequences are also shown. (D) The electropherogram of Adgrg2em1 allele DNA sequencing. (E) Genomic PCR to detect Adgrg2em1 allele. (F) Immunoblot analysis of ADGRG2 protein expression in the IS epididymis of WT and Adgrg2em1/Y mice. Immunoblot detection of GAPDH is also shown as an internal control.
Antibodies
The following commercially available antibodies were used: rabbit monoclonal anti-ERK1/2 (#4695) and anti-phospho-ERK1/2 (#4370) (Cell Signaling Technology), rabbit polyclonal anti-ADGRG2 (#HPA050029, Atlas Antibodies), rabbit polyclonal anti-ADAM28 (#22234-1-AP, Proteintech), mouse monoclonal anti-ACTIN (#sc-53014, Santa Cruz), mouse monoclonal anti-GAPDH (#sc-32233, Santa Cruz), mouse monoclonal anti-ADAM3 (#sc-365288, Santa Cruz), mouse monoclonal anti-ADAM2 (#MAB19292, Merck Millipore), peroxidase-conjugated goat polyclonal anti-rabbit IgG (#111-036-045, Jackson Immunoresearch), and goat polyclonal anti-mouse IgG (#115-036-062, Jackson Immunoresearch). Rabbit polyclonal antibody against OVCH2 was obtained as described previously []. The dilution conditions of antibodies are summarized in Supplementary Table S4.
Transcript analyses
The epididymis was dissected into three parts, i.e., IS-caput, corpus, and cauda. Total RNA was isolated from mouse tissues using an RNeasy mini kit (Qiagen, #74104). The ages of mice at tissue sampling were as follows: 8-week-olds for Adgrg2−/Y and its control WT, and 14-week-olds for W/Wv, efferent duct-ligated WT, and their control non-treated WT. RNA-seq of IS-caput epididymal transcripts was performed as follows: libraries for sequencing were prepared from isolated RNAs using a TruSeq stranded mRNA sample prep kit (Illumina, #20020594) and sequenced on a NovaSeq6000 (Illumina) using 101 bp single-ended mode. The obtained sequence reads were mapped onto a mouse reference genome (mm10) using TopHat ver. 2.1.1 []. Fragments per kilobase of exon per million mapped reads (FPKM) values were calculated for each gene using Cufflinks ver. 2.2.1 []. The obtained RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE232896 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE232896) for Adgrg2−/Y mice and GSE232898 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE232898) for EDL and W/Wv mice. The RNA-seq data of Nell2 KO IS-caput epididymis used for the comparative transcriptome study was obtained from NCBI GEO: GSE133920 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE133920). The RNA-seq data of WT IS-caput, corpus, and cauda epididymis used for the comparative transcriptome study was also obtained from NCBI GEO: GSE138517 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138517).
Protein analyses
The 8-week-old tissues were homogenized in lysis buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% Triton X-100) containing protease inhibitor cocktail (Nacalai Tesque, Japan, #25955-24) and phosphatase inhibitor cocktail (Nacalai Tesque, #07575-51) and centrifuged at 12,000×g at 4°C for 15 min. The resulting supernatants were then recovered as crude tissue protein extracts. The extracted proteins were separated by SDS-PAGE under reducing conditions using e-PAGEL precast gel (Atto, Japan, #E-T/R/D520L). As a molecular weight standard, Precision Plus Protein Dual Color Standards (Bio-Rad, #1610374) was used. The separated proteins were electrotransferred onto Immobilon-P polyvinylidene difluoride membranes (Merck, #IPVH00010) and the membranes were blocked with 3% bovine serum albumin (BSA)/TBST. The membranes were incubated with primary antibodies overnight. The bound antibodies were detected by following incubation with peroxidase-conjugated secondary antibodies and chemiluminescent reaction using Chemi-Lumi One Super (Nacalai Tesque, #02230). The chemiluminescent signals were detected using Amersham ImageQuant 800 (Cytiva).
Histology
The epididymides fixed with 4% formaldehyde/PBS overnight were immersed in paraffin and sectioned at 5 μm using a microtome. The sections were stained with hematoxylin and eosin (HE) and photographed using a system microscope (BX53; Olympus).
Statistical analysis
All experiments were repeated biologically at least three times and similar results were obtained. Statistical analyses were performed using Student’s t-tests with Microsoft Excel 2019 (Microsoft).
Results
Targeted deletion of Adgrg2 gene in mice
The epididymal transcriptome confirmed that Adgrg2 is most abundantly and exclusively expressed in the caput epididymis including the IS among genes encoding cell surface receptors including Ros1 (Figure 1A). Adgrg2 gene was targeted by CRISPR/CAS9-mediated genome editing; two crRNAs targeting around exons 3 and 24 of the Adgrg2 gene on chromosome X were introduced together with tracrRNA and CAS9 protein into fertilized eggs by electroporation to expect the deletion of the sandwiched region (Figure 1B). Finally, a mutant allele Adgrg2em1 consisting of 50 518 bp deletion and 26 bp insertion was obtained (Figure 1C–E). After confirmation of inheritance of the mutant allele, Adgrg2 ablation was also examined at the protein level. When the IS-caput protein lysates were analyzed by immunoblotting, anti-ADGRG2 immunoreactivity was detected at around 150 kDa in WT protein extract but it completely disappeared in Adgrg2em1/Y one (Figure 1F). There results indicate that the generated Adgrg2em1 allele is an Adgrg2-null allele and hereafter described as Adgrg2−.
Histological and biochemical characterization of Adgrg2 knockout IS-caput epididymis
The postnatal development of Adgrg2−/Y (Adgrg2 KO) male IS was examined histologically. The postnatal differentiation of the epididymal initial segment starts at around postnatal day 19 under the influence of testicular lumicrine factor []. In the control WT animals, the thickened luminal epithelium characteristic of differentiated epididymal IS was not observed at 2 weeks old but was apparent at after 4 weeks old (Figure 2A–C) The differentiation of the IS epithelium was also observed in Adgrg2 KO epididymis and there was no prominent histological abnormality observed (Figure 2D–F).
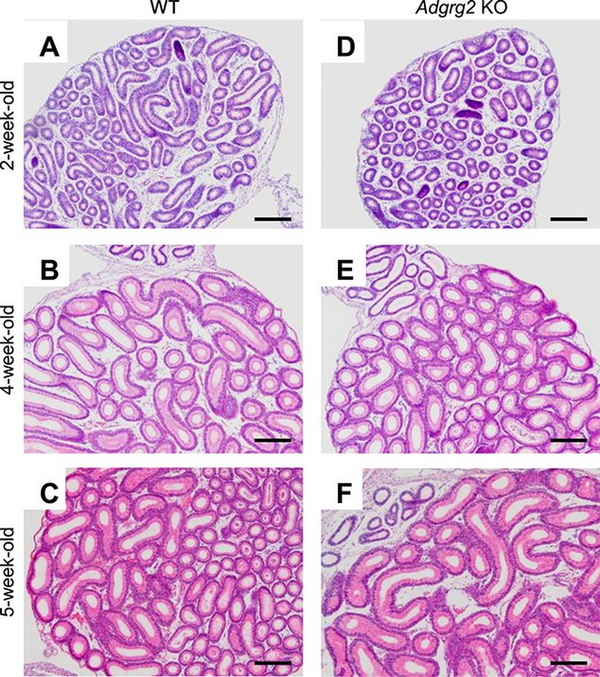
Figure 2
Histology of Adgrg2 KO IS-caput epididymides. (A–F) HE-stained sections of WT (A–C) and Adgrg2 KO (D–F) IS-caput epididymides at 2 weeks (A, D), 4 weeks (B, E), and 5 weeks (C, F) old. Bars, 200 μm.
Differentiation of the IS epithelium is regulated by NELL2/NICOL-ROS1-mediated lumicrine signaling [, , ]; lumicrine factors NELL2 and NICOL are secreted from testicular germ cells along spermatogenesis and act on epididymis to trigger IS differentiation. The 8-week-old epididymal IS of Nell2−/− (Nell2 KO) males significantly degenerated when compared with that of WT (Figure 3A–D). The IS luminal epithelium of 8-week-old Adgrg2 KO males was not critically affected (Figure 3E and F).
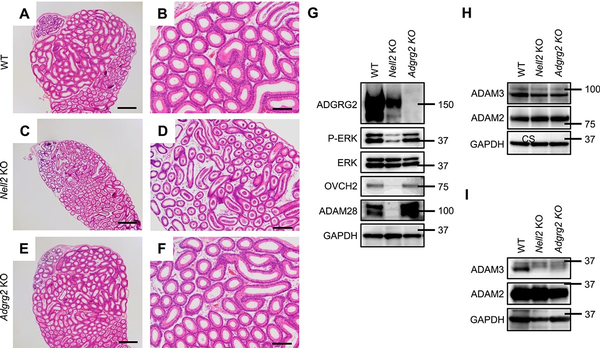
Figure 3
Comparative histology of Adgrg2 KO and Nell2 KO IS-caput epididymides. (A–F) HE-stained sections of WT (A, B), Nell2 KO (C, D), and Adgrg2 KO (E, F) IS-caput epididymides at 8 weeks old. Bars, 200 μm (A, C, E). (G) Immunoblot analyses of IS differentiation-associated proteins (P-ERK, OVCH2, and ADAM28) in 8-week-old WT, Nell2 KO, and Adgrg2 KO IS-caput epididymal protein lysates. ERK and GAPDH immunoblots are also shown as internal controls. (H, I) Immunoblot analyses of sperm proteins (ADAM3 and ADAM2) in 8-week-old WT, Nell2 KO, and Adgrg2 KO testis (H) and cauda epididymal sperm (I) protein lysates. GAPDH immunoblots are also shown as internal controls.
Along with the IS differentiation, the ROS1 kinase is activated and the phosphorylation level of extracellular signal-regulated kinase (ERK) increases []. The differentiating epididymal IS specifically expresses various proteins in a lumicrine-dependent manner [, ]. Therefore, the expression and phosphorylation levels of specific proteins are indicative of lumicrine-mediated IS differentiation. When examined by immunoblotting, the level of phosphorylated ERK was significantly decreased in the Nell2 KO IS-caput epididymis but not critically affected in Adgrg2 KO one (Figure 3G). The secreted proteins ovochymase 2 (OVCH2) and a disintegrin and metalloproteinase domain-containing protein 28 (ADAM28) are abundantly expressed in a lumicrine-dependent manner in the epididymal IS [, ]. The expression levels of these secreted proteins were significantly diminished in the Nell2 KO IS-caput epididymis mice but not in Adgrg2 KO ones (Figure 3G). The amount of processed form of ADAM3, which is initially expressed as a precursor in testis, decreased in the spermatozoa isolated from Adgrg2 KO cauda epididymis (Figure 3H and I). Collectively, these results indicate that lumicrine-mediated IS differentiation is not prominently affected in Adgrg2 KO epididymis at both the histological and protein expression levels.
Transcriptome analysis of Adgrg2 KO epididymis
The association of ADGRG2 in lumicrine signaling was investigated further by epididymal RNA-seq analysis. The transcriptome analysis of the Nell2 KO mouse IS-caput epididymis identified the downregulated expression of a group of genes []. The lumicrine signaling can also be interfered with by efferent duct ligation (EDL) which disconnects the luminal communication between the testis and epididymis or by depleting testicular germ cells as in Kit mutant W/Wv mice []. To evaluate the transcriptome of Adgrg2 KO IS-caput epididymis from the viewpoint of lumicrine signaling, RNA-seq was also performed for the IS-caput epididymides of EDL and W/Wv mice. The obtained RNA-seq results are summarized in Supplementary Data File S1.
As observed in Nell2 KO mice, the expressions of many genes were statistically significantly downregulated in the EDL and W/Wv IS-caput epididymides compared with their control WT ones (Figure 4A–C). There were also statistically significant decreases in gene expression in Adgrg2 KO IS-caput epididymis (Figure 4D), but the number of such affected genes was apparently small compared with those observed in Nell2 KO, EDL, and W/Wv IS-caput epididymides (Figure 4E).
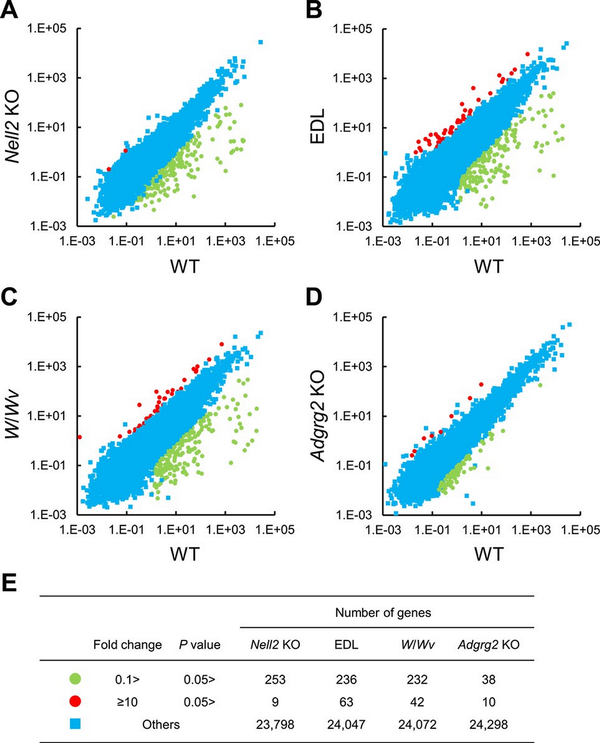
Figure 4
RNA-seq analyses of lumicrine-deficient and Adgrg2 KO IS-caput epididymis. (A–D), RNA-seq of WT vs Nell2 KO (A), WT vs EDL (B), WT vs W/Wv (C), and WT vs Adgrg2 KO IS-caput epididymis (D). RPKM (A) or FPKM (B–D) values are plotted. (E) Summary of comparative RNA-seq analyses of Nell2 KO, EDL, W/Wv, and Adgrg2 KO IS-caput epididymides. Graphic symbols correspond to those in panels A–D.
The transcriptome of Adgrg2 KO epididymis was examined further. Eighty-one genes significantly downregulated (1/10 > downregulation, the read number in WT > 10, and t-test P value < 0.05) in Nell2 KO IS-caput epididymis were selected and compared between Nell2 KO, EDL, W/Wv, and Adgrg2 KO IS-caput epididymides. When the expressions of these 81 genes were compared, they were similarly downregulated in Nell2 KO, EDL, and W/Wv IS-caput epididymides (Figure 5A and B). Most of these genes were also downregulated in Adgrg2 KO IS-caput epididymis, but the extent of such downregulations was quite moderate in Adgrg2 KO IS-caput epididymis compared with those in Nell2 KO, EDL, and W/Wv ones (Figure 5A and B). Collectively, although Adgrg2 ablation affected the expression of lumicrine-regulated genes, the IS-caput transcriptome of Adgrg2 KO mice did not reproduce those characteristics of lumicrine-deficient mice.
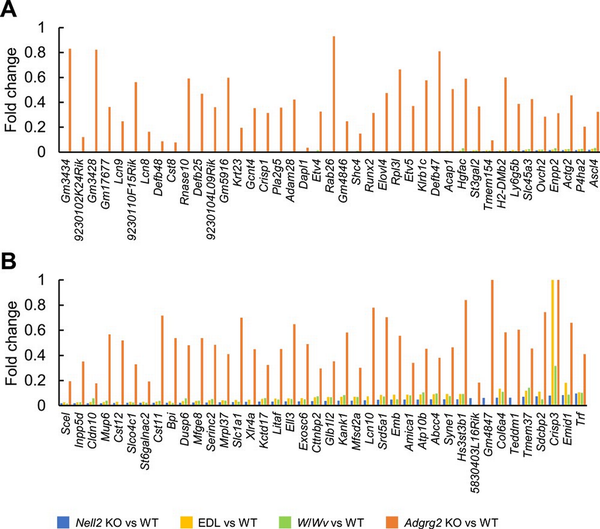
Figure 5
Comparative representation of genes downregulated in lumicrine-deficient mouse IS-caput epididymides. (A, B) Fold change of gene expressions in Nell2 KO, EDL, W/Wv, and Adgrg2 KO IS-caput epididymides compared with their control WT ones. Only genes downregulated in Nell2 KO IS-caput epididymis (>10 RPKM in WT, fold change < 0.1, and t-test P < 0.05) are selected.
Discussion
Adgrg2 is expressed in both efferent duct and epididymal IS. As major defects of Adgrg2 ablation appear only in the efferent duct [], the function of ADGRG2 is less characterized in IS epididymis than in the efferent duct. The epididymis IS is often evaluated by histology because it is characterized by the highly differentiated tall luminal epithelial cells and its defective differentiation is characterized by a decrease in the height of epithelial cells. However, histological analysis is not sufficiently informative to assess the function of proteins in lumicrine signaling at the molecular level. In the preceding study, the IS gene expression of Adgrg2 KO mice was investigated by using a microarray platform and finally, approximately 3300 genes were detected []. In the present study, the RNA-seq of Adgrg2 KO IS-caput epididymis was performed and the read numbers were determined on 24 346 genes. To fully characterize ADGRG2 association with lumicrine signaling, the IS-caput RNA-seq was also performed on lumicrine-deficient EDL and W/Wv mice. The total number of genes whose expressions were characterized is seven times larger in the present study than that in the previous microarray study and seems sufficient to characterize the regulatory mechanism of epididymal IS-caput gene expression by ADGRG2.
A possibility that ADGRG2 function as a lumicrine receptor has been implicated by its abundant expression in the IS and subcellular localization on the apical surface of the luminal epithelial cells. In lumicrine-deficient mice, the amount of processed ADAM3 decreases in the cauda epididymal spermatozoa [, ]. Decreased amount of processed ADAM3 in the spermatozoa of Adgrg2 KO cauda epididymis observed in the present study also implies the association of ADGRG2 in lumicrine signaling. However, unlike the lumicrine-deficient Nell2, Nicol, and Ros1 KO mice [], apparent histological abnormalities were not observed in the IS of Adgrg2 KO mice generated in the present study, as in another Adgrg2 mutant mouse line []. In addition, the expression levels of proteins regulated by lumicrine signaling did not alter significantly in Adgrg2 KO IS epididymis. Adgrg2 association with the lumicrine signaling was also examined by transcriptome analyses. The IS-caput epididymal transcriptome of the EDL and W/Wv mice was similar to that Nell2 KO mice, indicating that the Nell2 KO epididymal transcriptome reflects that characteristic of defective lumicrine signaling. The RNA-seq of Adgrg2 KO IS-caput epididymis also detected the downregulation of genes whose expression was decreased in Nell2 KO IS-caput epididymis. However, the extent of such downregulation in Adgrg2 KO mice appeared considerably moderate compared with those observed in lumicrine-deficient animals such as Nell2 KO, EDL, or W/Wv mice. Collectively, these results indicate that ADGRG2 is dispensable for lumicrine signaling. ADGRG2 may indirectly regulate, if any, lumicrine signaling. One possibility is that ADGRG2 function in the efferent duct secondary affects the IS epididymis gene expression as Adgrg2 ablation causes defects in the efferent duct, which is located upstream of the epididymis in the male reproductive tract. Another possibility is that a crosstalk between the signaling pathways mediated by ROS1 and ADGRG2 occurs in the IS epididymis, as such a signaling crosstalk between receptor tyrosine kinases and GPCRs is known []. It is reported that in the efferent duct the pharmacological activation of angiotensin II receptor type 2, a GPCR expressed, can rescue fluid reabsorption defect caused by Adgrg2 ablation []. Since in IS-caput region of epididymis neurotensin receptor type 2 (NTSR2), another GPCR encoded by neurotensin receptor 2 (Ntsr2), is also expressed (Figure 1A), it might be interesting to examine whether the activation of NTSR2 rescue IS-caput gene expression altered by Adgrg2 ablation. Recently, steroids dehydroepiandrosterone, dehydroepiandrosterone sulfate, and deoxycorticosterone were identified as potential ligands for ADGRG2 []. It is of interest whether such steroid derivatives act on ADGRG2 in a manner similar to lumicrine as ADGRG2 is apically expressed.
Increasing evidences indicate the pivotal role of epididymal IS in sperm maturation. Further studies on the regulatory mechanism of IS differentiation and gene expression will contribute to a better understanding of sperm maturation at the molecular level and to the development of novel male contraceptives targeting sperm maturation.
Acknowledgment
We thank the NGS core facility of the Genome Information Research Center at the Research Institute for Microbial Diseases of Osaka University for support with RNA sequencing and data analysis and Mr Kaito Yamamoto of the Department of Experimental Genome Research at the Research Institute for Microbial Diseases of Osaka University for supporting the generation of gene-modified animals.
References
- 1. Bedford JM. Effects of duct ligation on the fertilizing ability of spermatozoa from different regions of the rabbit epididymis. J Exp Zool 1967; 166:271–281.
- 2. Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature 1967; 216:816–818.
- 3. Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for posttesticular sperm maturation and male fertility. FASEB J 2012; 26:4198–4209.
- 4. Kiyozumi D, Noda T, Yamaguchi R, Tobita T, Matsumura T, Shimada K, Kodani M, Kohda T, Fujihara Y, Ozawa M, Yu Z, Miklossy G, et al NELL2-mediated lumicrine signaling through OVCH2 is required for male fertility. Science 2020; 368:1132–1135.
- 5. Moniem KA, Glover TD, Lubicz-Nawrocki CW. Effects of duct ligation and orchidectomy on histochemical reactions in the hamster epididymis. J Reprod Fertil 1978; 54:173–176.
- 6. Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod 1979; 20:162–181.
- 7. Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl 1998; 53:47–57.
- 8. Kiyozumi D, Shimada K, Chalick M, Emori C, Kodani M, Oura S, Noda T, Endo T, Matzuk MM, Wreschner DH, Ikawa M. A small secreted protein NICOL regulates lumicrine-mediated sperm maturation and male fertility. Nat Commun 2023; 14:2354.
- 9. Yeung CH, Wagenfeld A, Nieschlag E, Cooper TG. The cause of infertility of male c-ros tyrosine kinase receptor knockout mice. Biol Reprod 2000; 63:612–618.
- 10. Obermann H, Samalecos A, Osterhoff C, Schröder B, Heller R, Kirchhoff C. HE6, a two-subunit heptahelical receptor associated with apical membranes of efferent and epididymal duct epithelia. Mol Reprod Dev 2003; 64:13–26.
- 11. Liebscher I, Cevheroğlu O, Hsiao C-C, Maia AF, Schihada H, Scholz N, Soave M, Spiess K, Trajković K, Kosloff M, Prömel S. A guide to adhesion GPCR research. FEBS J 2022; 289:7610–7630.
- 12. Patat O, Pagin A, Siegfried A, Mitchell V, Chassaing N, Faguer S, Monteil L, Gaston V, Bujan L, Courtade-Saïdi M, Marcelli F, Lalau G, et al Truncating mutations in the adhesion G protein-coupled receptor G2 gene ADGRG2 cause an X-linked congenital bilateral absence of vas deferens. Am J Hum Genet 2016; 99:437–442.
- 13. Davies B, Baumann C, Kirchhoff C, Ivell R, Nubbemeyer R, Habenicht U-F, Theuring F, Gottwald U. Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol Cell Biol 2004; 24:8642–8648.
- 14. Davies B, Behnen M, Cappallo-Obermann H, Spiess A-N, Theuring F, Kirchhoff C. Novel epididymis-specific mRNAs downregulated by HE6/Gpr64 receptor gene disruption. Mol Reprod Dev 2007; 74:539–553.
- 15. Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod 2005; 73:404–413.
- 16. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009; 25:1105–1111.
- 17. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010; 28:511–515.
- 18. Xu B, Washington AM, Hinton BT. Initial segment differentiation begins during a critical window and is dependent upon lumicrine factors and SRC proto-oncogene (SRC) in the mouse. Biol Reprod 2016; 95:15–11.
- 19. Kiyozumi D. The molecular mechanisms of mammalian sperm maturation regulated by NELL2-ROS1 lumicrine signaling. J Biochem 2022; 172:341–346.
- 20. Jun HJ, Roy J, Smith TB, Wood LB, Lane K, Woolfenden S, Punko D, Bronson RT, Haigis KM, Breton S, Charest A. ROS1 signaling regulates epithelial differentiation in the epididymis. Endocrinology 2014; 155:3661–3673.
- 21. Cooper TG, Wagenfeld A, Cornwall GA, Hsia N, Chu ST, Orgebin-Crist MC, Drevet J, Vernet P, Avram C, Nieschlag E, Yeung CH. Gene and protein expression in the epididymis of infertile c-ros receptor tyrosine kinase-deficient mice. Biol Reprod 2003; 69:1750–1762.
- 22. Sipila P, Sipilä P, Pujianto DA, Shariatmadari R, Nikkilä J, Lehtoranta M, Huhtaniemi IT, Poutanen M, Sipilä P, Pujianto DA, Shariatmadari R, Nikkilä J, et al Differential endocrine regulation of genes enriched in initial segment and distal caput of the mouse epididymis as revealed by genome-wide expression profiling. Biol Reprod 2006; 75:240–251.
- 23. Di Liberto V, Mudò G, Belluardo N. Crosstalk between receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCR) in the brain: focus on heteroreceptor complexes and related functional neurotrophic effects. Neuropharmacology 2019; 152:67–77.
- 24. Zhang D, Sun Y, Ma M, Wang Y, Lin H, Li R-R, Liang Z, Gao Y, Yang Z, He D, Lin A, Mo H, et al Gq activity- and β-arrestin-1 scaffolding-mediated ADGRG2/CFTR coupling are required for male fertility. Elife 2018; 7:1–39.
- 25. Lin H, Xiao P, Bu RQ, Guo S, Yang Z, Yuan D, Zhu ZL, Zhang CX, He QT, Zhang C, Ping YQ, Zhao RJ, et al Structures of the ADGRG2–Gs complex in apo and ligand-bound forms. Nat Chem Biol 2022; 18:1196–1203.
