Introduction
Pain and depression represent two highly prevalent and deleterious disorders with high comorbidity rates (from 18% to 85%). Hence, pain is a major risk factor for depression, and depression can exacerbate chronic pain and obstruct effective therapies, but the mechanisms underlying the overlap between these conditions remain unclear.
When pain induces depression, we speculate that the balance between the recruitment of restorative analgesia and harmful pronociceptive mechanisms determines whether pain following injury resolves or if it persists and becomes chronic, with the ensuing appearance of comorbid anxiodepressive symptoms. The noradrenergic locus coeruleus (LC) in the pontine brainstem is one of the brain regions that may mediate this neuroplasticity. Indeed, while descending inhibition of acute pain by the LC has been well established,, in the past decade there have been several reports of its role in facilitating or inhibiting chronic pain. Furthermore, the role of the LC circuits in depression is still elusive, despite the many changes in the LC reported in animal models, depressed patients, and its key role in the action of antidepressants., Therefore, here we will address how depression arises after the induction of pain to understand the mechanisms involved in pain–depression comorbidity. Specifically, we will focus on the pain caused by nerve injury (neuropathic pain), not least because neuropathic pain is a debilitating condition affecting around 7–10% of the general population and it is notoriously resistant to currently available analgesic treatments.,
Here, we explored the time-dependent LC neural network reconfiguration in a rat model of neuropathic pain induced by chronic constriction injury (CCI), assessing the changes in the ipsilateral (LCipsi) or contralateral (LCcontra) pathways at three time points after nerve injury induction: short (CCI-ST, 2 days), mid (CCI-MT, 7 days) and long term (CCI-LT, 30–35 days, 4–5 weeks). Furthermore, as the LC is apparently composed of modules that might produce targeted neuromodulation,, we assessed the effects induced by the LC as a whole, as well as its specific efferent pathways to areas critical for sensory and unpleasant emotional experiences: the spinal cord and rostral anterior cingulate cortex (rACC).,
Materials and methods
A detailed description of the experimental procedure is provided in the Supplementary material.
Animals and pain model
Male transgenic Long–Evans tyrosine hydroxylase:Cre (TH:Cre) and wild-type Long–Evans rats (350–450 g) were produced and maintained under standard laboratory conditions. CCI was used as a model of neuropathic pain.,
Virus and tracer injection
Rats were injected with a designer receptor exclusively activated by designer drugs (DREADD) virus [hM4D(Gi)-DREADD, rM3D(Gs)-DREADD or control-DREADD] into the LC (AP: −3.2 mm, ML: ±1.3 mm, DV: 6.2 mm) and Fluoro-Gold tracer into rACC (AP: +3.0 mm, ML: ±1.0 mm and DV: 1.2 mm) or spinal cord (L4–L6 segments). See methodological validation of DREADD approach in Llorca-Torralba et al. and Supplementary Figs. 1, 2 and 11.
Behavioural assessment
Acetone test
Cold hypersensitivity was evaluated using the acetone test. Animals were placed individually into Plexiglas chambers on a metal grid, and a drop of acetone (100 µl) was applied to the centre of the ipsilateral and contralateral hind paw with a pipette. The acetone was applied four times to each hind paw alternately at 5-min intervals and the responses were recorded over 1 min according to the following scale: 0, no response; 1, quick withdrawal, flick or stamping of the paw; 2, prolonged withdrawal or repeated flicking of the paw; and 3, repeated flicking of the paw with persistent licking directed at the ventral side of the paw. The cumulative score for each rat was obtained by summing the score and dividing it by the number of assays.
Von Frey test
Mechanical hypersensitivity was measured with the von Frey test (Dynamic Plantar Aesthesiometer, Ugo Basile), applying a vertical force to the ipsilateral or contralateral hind paw that was increased from 0 to 50 g over a period of 20 s. Mechanical hypersensitivity was indicated by a reduction in the force that provokes paw withdrawal.
Cold plate test
Cold hypersensitivity was measured using the cold plate test. Animals were placed on a cold metal plate maintained at 4 ± 1°C (Panlab S.L.) and the number of times the animal briskly lifted its ipsilateral hind paw was measured over a period of 2 min.
Forced swimming test
Depressive-like behaviour was evaluated in the forced swimming test (FST) over two different sessions, a 15-min pretest was followed by a 5-min test performed 24 h later. The predominant behaviours were recorded (climbing, swimming, or immobility), and scored in each 5-s period of the 300-s test session.,
Locomotor spontaneous activity test
The total distance travelled (arbitrary units, AU, %) was measured as an indicator of locomotor activity.
Immunohistochemistry and histology
Immunohistochemistry was performed to evaluate the expression of DREADDs-mCherry, Fluoro-Gold tracer and c-fos in the LC, as well as dopamine beta-hydroxylase (DBH) in the spinal cord. The cannula placement in the rACC and spinal cord was also verified.,
Western blotting
Western blotting was performed to evaluate the expression of pCREB and TH proteins in the LC, which it was normalized to the level of β-actin.
Statistical analysis
Results are presented as the means ± standard error of the mean (SEM). An unpaired Student’s t-test was used to compare the values between the two groups. One- or two-way or repeated measures ANOVA followed by Newman–Keuls test were used to compare between more than two groups. P < 0.05 was considered significant.
Data availability
Data are available on request to the corresponding author.
Results
Analgesia is driven by the activation of noradrenergic-LCipsi neurons
Sensorial hypersensitivity was evaluated through the acetone test and as expected, it appears immediately in the ipsilateral hindpaw after nerve damage and it remained constant over time (ST, MT and LT: P < 0.001 versus sham; Fig. 1). By contrast, depressive-like behaviour was only observed in CCI-LT animals (immobility and climbing: P < 0.01 and P < 0.05 versus sham; Fig. 1). This finding indicates that pain-induced depression evolves after pain has lasted at least 4–5 weeks.,,,,, To assess the pontine mechanisms underlying the time-dependence of this phenotype further, we selectively expressed hM4D(Gi)- or rM3D(Gs)-DREADD in the LCipsi or LCcontra to selectively manipulate LC activity at different time points from nerve injury. In agreement with our recent work, selective expression of hM4D(Gi)- or rM3D(Gs)-DREADD was achieved in noradrenergic-LC neurons (Fig. 2A and Supplementary Fig. 1A–H). Thus, we assessed how hM4D(Gi)-DREADD-mediated LC inhibition affected sensorial hypersensitivity after systemic clozapine N-oxide (CNO) administration. LCipsi blockade only significantly enhanced hypersensitivity in the ipsilateral hindpaw in the CCI-ST animals (acetone test: P < 0.001 versus the CCI-ST-saline; Fig. 2B and Supplementary Fig. 2). Furthermore, this effect of CNO was also evident when exploring the number of lifts of the ipsilateral hindpaw in a different pain test (cold plate test: P < 0.001; Fig. 1C). Enhancement of the cold plate response disappeared completely after 360 min (Fig. 1C), in line with the duration of the inactivation produced by CNO previously. Surprisingly, no significant effect on acetone test was seen at any time point post-injury when the LCcontra was chemogenetically blocked (Fig. 1D).
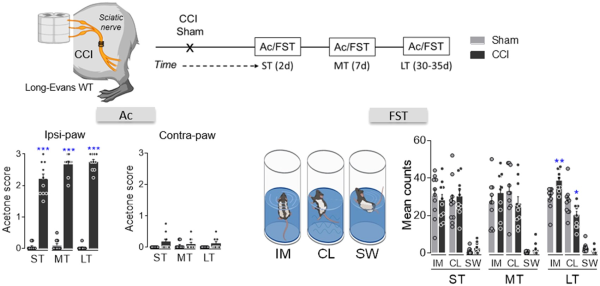
Figure 1
Sensory and affective characterization of the development of neuropathic pain. Scheme of the CCI model, timeline and graphs showing the response of the ipsilateral and contralateral paw in the acetone test and the predominant behaviour (immobility, IM; climbing, CL; swimming, SW) in the FST in the short (ST), mid (MT) and long term (LT) after CCI in wild-type rats (n = 10 animals/group): *P < 0.05, **P < 0.01, ***P < 0.001 versus sham, Student’s t-test for each time point (ST, MT and LT). Ac = acetone; d = days; WT = wild-type.
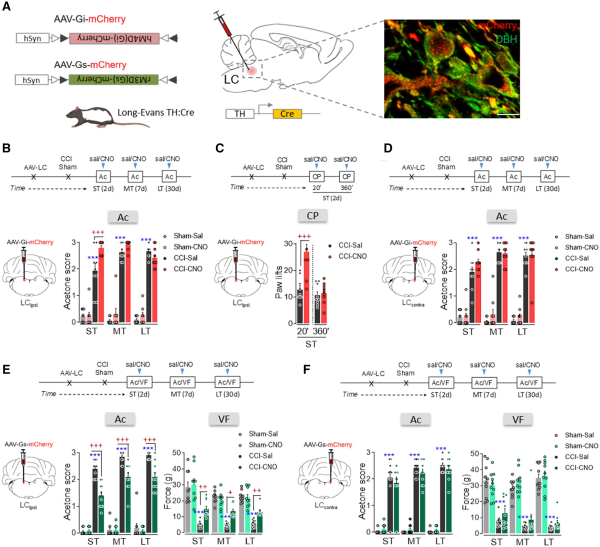
Figure 2
Effect of chemogenetic inhibition and activation of ipsilateral and contralateral LC on nociceptive behaviour after nerve injury. (A) Scheme of noradrenergic-LC inhibition or activation using the DREADD strategy in TH:Cre rats and representative immunohistofluorescence image (scale bar = 10 µm) of mCherry expression in noradrenergic-LC neurons (red = mCherry, green = DBH). (B) Response of the ipsilateral paw of CCI-ST, -MT and -LT rats in the acetone test after ipsilateral LC (LCipsi) noradrenergic inhibition by CNO (1 mg/kg, i.p.) (n = 8–10 animals/group: ***P < 0.001 versus sham-sal; +++P < 0.01 versus CCI-sal). (C) Response of the ipsilateral paw to thermal stimulus 20 and 360 min after LCipsi inhibition by CNO (1 mg/kg, i.p.) (n = 9 animals/group: +++P < 0.001 versus CCI-sal). (D) Response of the ipsilateral paw of CCI-ST, -MT and -LT rats in the acetone test after noradrenergic contralateral LC (LCcontra) inhibition by CNO (1 mg/kg, i.p.) (n = 9–10 animals/group: ***P < 0.001 versus sham-sal). (E) Response of the ipsilateral paw of CCI-ST, -MT and -LT rats in the acetone and von Frey tests after noradrenergic-LCipsi activation by CNO (1 mg/kg, i.p.) (n = 9–10 animals/group: ***P < 0.001 versus sham-sal; +P < 0.05, ++P < 0.01, +++P < 0.001 versus CCI-sal). (F) Response of the ipsilateral paw of CCI-ST, -MT and -LT rats in the acetone and von Frey tests after noradrenergic LCcontra activation by CNO (1 mg/kg, i.p.) (n = 10 animals/group: ***P < 0.001 versus sham-sal). One- or two-way ANOVA with repeated measures, Newman–Keuls post hoc test. Ac = acetone test; CP = cold plate test; d = days; i.p. = intraperitoneal; VF = von Frey test.
As previous studies have shown that the pain threshold increases following acute electrical or chemogenetic stimulation of the LC,, we explored the effect of global noradrenergic-LC activation using rM3D(Gs)-DREADDs at various phases of the development of neuropathy (Fig. 1E and F and Supplementary Fig. 1E–H). CNO activation of the LCipsi significantly reduced CCI evoked thermal and mechanical hypersensitivity in the ST, MT and LT animals relative to their respective CCI-saline controls (acetone test: ST, MD and LT, P < 0.001; von Frey test: ST P < 0.01, MT P < 0.05, and LT P < 0.01; Fig. 2E). By contrast, CNO-mediated activation of LCcontra neurons did not significantly modify CCI-induced pain at any time point (Fig. 2F). Furthermore, chemogenetic LCipsi/contra inhibition/activation did not significantly modify the sensorial responses in the sham group or in the contralateral hindpaws of the CCI group (Fig. 2B–F and Supplementary Fig. 3A–D).
Chemogenetic blockade of either the LCipsi or LCcontra neurons relieves pain-induced depression
As long-term pain induces depressive-like behaviour (Fig. 1), we used the same chemogenetic approaches to explore the effect of silencing or activating the LCipsi or LCcontra in depressive CCI-LT rats. Unlike the sensory tests, chemogenetic silencing the LCipsi or LCcontra had a marked effect on emotional behaviour, reflected by the significant decrease in immobility (LCipsi and LCcontraP < 0.001) and the increased climbing (LCipsiP < 0.01 and LCcontraP < 0.001) behaviour of these animals (Fig. 3A and B). Alternatively, blocking either LCipsi or LCcontra had no effect on sham animals (Fig. 3A and B), although significant depressive behaviour was triggered in sham animals by rM3D(Gs)-DREADD LC activation (LCipsiP < 0.01 and LCcontraP < 0.001 versus sham-saline; Fig. 3C). Furthermore, LC activation in CCI-LT rats did not further augment the already elevated immobility time in the FST (Fig. 3C). None of the behaviours evaluated were affected by any locomotor dysfunction (Supplementary Fig. 4 A and B). In addition, a bilateral activation of the LC was evident through the increase in the rate-limiting enzyme in noradrenaline biosynthesis, TH (LCipsi and LCcontraP < 0.05 versus sham), and that of phosphorylated CREB (LCipsiP = 0.07 and LCcontraP < 0.01 versus sham; Supplementary Fig. 5).
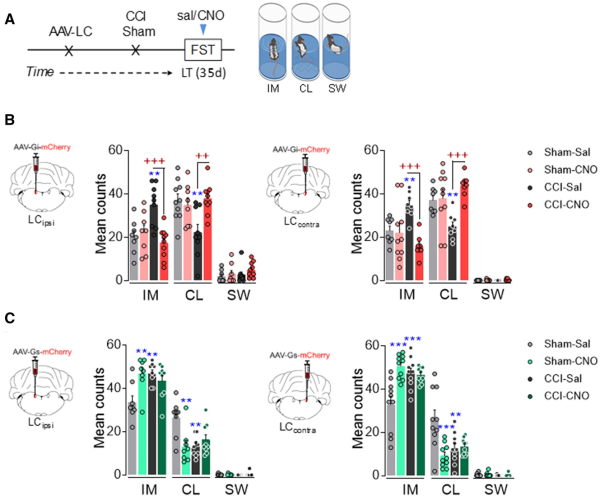
Figure 3
Effect of chemogenetic inhibition and activation of ipsilateral and contralateral LC on depressive-like behaviour after long-term neuropathy. (A) Timeline of noradrenergic-LC inhibition or activation using the DREADD strategy in TH:Cre rats. (B) Representative image of noradrenergic-LCipsi or LCcontra inhibition and predominant behaviour in the FST after CNO (1 mg/kg, i.p.) (n = 8–10 animals/group: **P < 0.001 versus sham-sal; ++P < 0.01, +++P < 0.001 versus CCI-sal). (C) Representative image of noradrenergic-LCipsi or LCcontra activation and predominant behaviour in the FST after CNO (1 mg/kg, i.p.) (n = 8–10 animals/group: **P < 0.001, ***P < 0.001 versus sham-sal). Two-way ANOVA, Newman–Keuls post hoc test. CL = climbing; d = days; IM = immobility; i.p. = intraperitoneal; sal = saline; SW = swimming.
Activation of the noradrenergic-LCipsi→spinal cord pathway relieves pain
To study the specific afferent pathways that modulate the evolution of neuropathy, we explored how the descending noradrenergic-LC pathway to the spinal cord influences the pain-related phenotype. Unilateral administration of the retrograde fluorescent tracer Fluoro-Gold into the superficial dorsal horn of the ipsilateral spinal cord labelled a similar number of neurons in the LCipsi and LCcontra along the rostro-caudal axis, these neurons predominantly located in the central LC (Fig. 4A and B and Supplementary Fig. 6A). Hence, the LC appears to project bilaterally to the spinal cord and most of the spinally projecting neurons (around 80%) are situated in the ventral aspect of the nucleus (Fig. 4C).
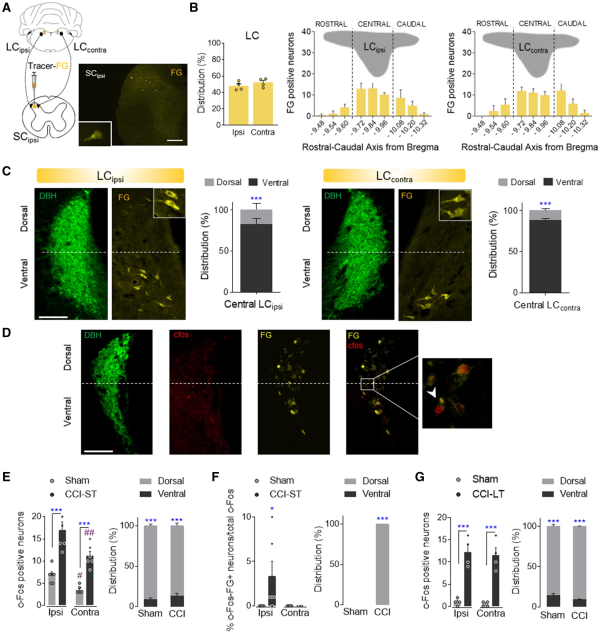
Figure 4
Study of the LC projections to the spinal cord. (A and B) Retrograde Fluoro-Gold tracer strategy and representative image (scale bar = 500 μm) to target LC neurons that project to the ipsilateral spinal cord in wild-type rats. Ipsilateral and contralateral distribution of Fluoro-Gold (FG) labelled LC neurons and Fluoro-Gold-positive neurons along the rostral-caudal axis of the ipsilateral (LCipsi) and contralateral (LCcontra) LC (mean + SEM of the number of neurons per slice, n = 4 animals: Student’s t-test and one-way ANOVA, Newman–Keuls post hoc test). (C) Representative immunofluorescence of the central LCipsi and LCcontra showing the dorsoventral aspect (half height relative to DBH expression) (scale bar = 200 µm). The graphs show the dorso-ventral distribution of the central LCipsi and LCcontra projections to the ipsilateral spinal cord (SCipsi) (n = 4 animals: ***P < 0.001 versus ventral LC, Student’s t-test). (D) Representative immunofluorescence of the central LC showing the DBH, c-Fos, Fluoro-Gold expression and merged image. The inset shows an example of c-Fos+FG-positive neurons (scale bar = 100 µm). (E and F) Numbers of c-Fos and c-Fos+FG-positive neurons of central LCipsi and LCcontra, as well as the dorsoventral distribution in sham and CCI-ST rats (mean + SEM of the number of neurons per slice, n = 6 animals/group: *P < 0.05, ***P < 0.001 versus Sham; #P < 0.05, ##P < 0.01 versus LCipsi, two-way ANOVA, Newman–Keuls post hoc test and ***P < 0.001 versus ventral LC, Student’s t-test). (G) Numbers of c-Fos positive neurons of central LCipsi and LCcontra, as well as the dorsoventral distribution in sham and CCI-LT rats (mean + SEM neurons per slice, n = 4 animals/group: ***P < 0.001 versus sham, two-way ANOVA, Newman–Keuls post hoc test and ***P < 0.001 versus ventral LC, Student’s t-test). c-Fos (red); DBH = dopamine beta-hydroxylase (green); FG = Fluoro-Gold (yellow).
In another experiment set, Fluoro-Gold was injected unilaterally into the ipsilateral spinal cord of CCI-ST and CCI-LT rats, and the expression of c-Fos in the LC was assessed (Fig. 4D–G). Nerve injury significantly increased the number of c-Fos labelled neurons in LC of CCI-ST rats bilaterally relative to the sham-ST rats (LCipsi: P < 0.001 and LCcontra: P < 0.001; Fig. 4E). Importantly, LCipsi c-Fos expression was significantly higher than that in the LCcontra of CCI-ST animals (P < 0.01, Fig. 4E). This lateralized LC c-Fos expression was also evident in sham animals (P < 0.05, Fig. 4E), suggesting an acute LC activation related to skin and muscle injury in sham animals. Furthermore, these c-Fos labelled neurons were preferentially located in the dorsal pole of the LC (∼90%, Fig. 4E). When exploring which c-Fos neurons also expressed Fluoro-Gold, we found that there was only a small population in the dorsal LCipsi pole of the CCI-ST rats (P < 0.05 versus sham; Fig. 4F). However, c-Fos/Fluoro-Gold colabelling was not found in either sham-LCipsi/LCcontra or CCI-ST LCcontra (Fig. 4F). Regarding long-term pain, significantly stronger c-Fos expression was evident in the dorsal LCipsi and LCcontra of CCI-LT animals relative to the respective LC area in the sham rats (LCipsiP < 0.001 and LCcontraP < 0.001), although there was no difference between sides (Fig. 4G). Moreover, no c-Fos/Fluoro-Gold colabelled LC neurons projected to the spinal cord in CCI-LT rats. In CCI-ST and -LT rats, the expression of the noradrenaline synthesizing enzyme DBH was also studied in the superficial dorsal horn of LC projecting segments (Supplementary Fig. 7). A bilateral increase in the expression of DBH fibres was evident in CCI animals just after nerve injury that was significant in the L5 and L6 lumbar dorsal horn sections (ST, P < 0.05 versus sham; Supplementary Fig. 7A and B). By contrast, there was a loss of noradrenergic fibres after CCI-LT in the L6 segment (LT, P < 0.05 versus sham animals; Supplementary Fig. 7A). These data demonstrate lateralized changes in the activation of the LC→spinal cord pathway along neuropathy development.
DREADD-mediated inactivation of the noradrenergic-LCipsi or LCcontral→ spinal cord pathway was explored behaviourally and like the global LC blockade (Fig. 2B and D), intrathecal CNO administration following hM4D(Gi)-DREADD administration to the LCipsi enhanced thermal and mechanical hypersensitivity in the ipsilateral hindpaw of CCI-ST rats, yet not at later times (Fig. 5A). This was shown by the significant increase in the acetone score (P < 0.001), a decrease in the mechanical withdrawal threshold (P < 0.05) and an increase in the number of ipsilateral paw lifts in the cold plate test of CCI-ST-CNO as opposed to CCI-ST-saline animals (P < 0.001). When exploring the outcome of blocking the LCcontra→ spinal cord pathway, no significant changes in evoked pain responses were observed (Fig. 5B). Like after global LCipsi or LCcontra inhibition, no significant effect was found in sham animals or in the response of the contralateral hindpaw of CCI animals after blocking the LCipsi or LCcontra→ spinal cord pathway (Fig. 5A and B and Supplementary Fig. 8A and B).
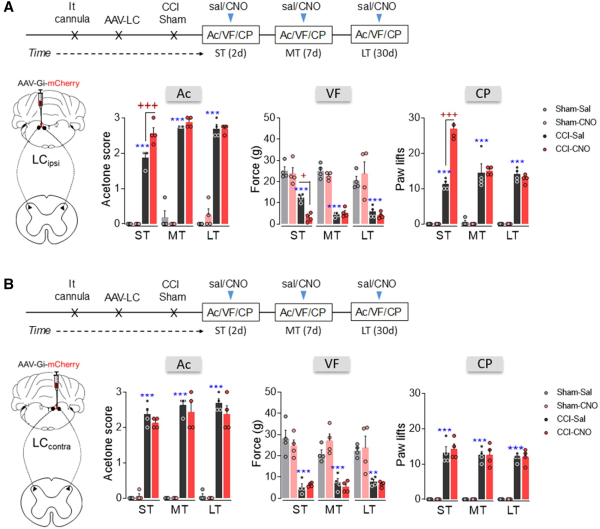
Figure 5
Effect of chemogenetic inhibition of the LCipsi/contra→spinal cord pathway on the time course of nociceptive behaviour after nerve injury. (A and B) Timeline and representative image of noradrenergic-LCipsi→spinal cord or LCcontra→spinal cord pathway inhibition using the AAV-Gi-mCherry strategy in TH:Cre rats. Response of the ipsilateral paw in the acetone, von Frey and cold plate tests after CNO (3 μM, i.t.) administration to rats in the short term (ST), mid-term (MT) and long term (LT) after CCI (n = 4 animals/group: **P < 0.01, ***P < 0.001 versus sham-sal; +P < 0.05, +++P < 0.001 versus CCI-sal, two-way ANOVA with repeated measures, Newman–Keuls post hoc test). Ac = acetone test; CP = cold plate test; d = days; i.t. = intrathecal; sal = saline; SC = spinal cord; VF = von Frey test.
The rM3D(Gs)-DREADD activation of the noradrenergic-LCipsi or LCcontra→ spinal cord pathway was also explored. Like global LC activation (Fig. 2E and F), DREADD-mediated activation of noradrenergic-LCipsi neurons projecting to the spinal cord reduced peripheral stimulus-evoked hypersensitivity in CCI-ST, -MT and -LT rats, as shown by the significant analgesic effects in the acetone, von Frey and cold plate tests (Fig. 6A). However, no change in thermal or mechanical allodynia was evident at any time point when the LCcontra to spinal cord pathway was activated (Fig. 6B). To verify that the local activation of α2-adrenoreceptors is still able to produce analgesia in these animals, the α2-adrenoreceptor agonist clonidine was intrathecally administered producing a clear analgesic effect in CCI-LT rats (acetone test P < 0.001, von Frey test P < 0.05 versus CCI-LT-saline; Fig. 6B). Finally, and like after global LCipsi or LCcontra activation, no change was found in sham animals or in the contralateral hindpaws of CCI animals (Fig. 6A and B and Supplementary Fig. 9A–C).
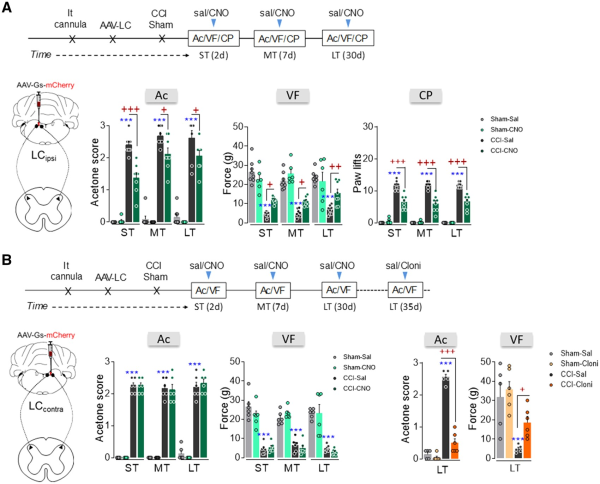
Figure 6
Effect of chemogenetic activation of the LCipsi/contra→spinal cord pathway on the time-course of nociceptive behaviour after nerve injury. (A and B) Timeline and representative image of noradrenergic-LCipsi→spinal cord or LCcontra→spinal cord pathway activation using the AAV-Gs-mCherry strategy in LE-TH:Cre rats. (A) Response of the ipsilateral paw in the acetone, von Frey and cold plate tests after CNO (3 μM, i.t) administration to ras in the short term (ST), mid-term (MT) and long term (LT) after CCI (n = 6–8 animals/group: ***P < 0.001 versus sham-sal; +P < 0.05, ++P < 0.01, +++P < 0.001 versus CCI-sal). (B) Response of the ipsilateral paw of CCI-ST, -MT and -LT rats in the acetone and von Frey tests after CNO (3 μM, i.t) administration (n = 6 animals/group: ***P < 0.001 versus sham-sal). In addition, the response of the ipsilateral paw of CCI-LT rats was evaluated in the acetone and von Frey tests after clonidine (20 μg, i.t) administration (n = 5–6 animals/group: ***P < 0.001 versus sham-sal; +P < 0.05, +++P < 0.001 versus CCI-sal). Two-way ANOVA with or without repeated measures, Newman–Keuls post hoc test. Ac = acetone test; Cloni = clondine; CP = cold plate test; d = days; i.t. = intrathecal; sal = saline; SC = spinal cord; VF = von Frey test.
Bilateral chemogenetic inhibition of the LC→rACC pathway relieves pain-induced depression
One of the main LC targets involved in mood and pain-related emotions is the rACC. Administration of Fluoro-Gold into the ipsilateral rACC produced abundant labelling of neurons in the LCipsi (≈80%), suggesting that the projection of the LC to the rACC is lateralized. Most marked neurons were located in the central LC along the rostro-caudal axis (Fig. 7A and B and Supplementary Fig. 6B). Bilateral administration of Fluoro-Gold to the rACC was also performed and as expected, there was a similar distribution of Fluoro-Gold-positive neurons bilaterally in the LC and preferentially in the dorsal pole (Fig. 7C–E and Supplementary Fig. 6 C). The expression of c-Fos in the LC neurons of LT animals was also explored (Fig. 7F–H). As found previously (Fig. 4G), there was a similar bilateral increase in neurons expressing c-Fos in the dorsal LC pole of CCI-LT rats (LCipsi and LCcontraP < 0.001 versus sham: Fig. 7G). Following bilateral administration of the Fluoro-Gold tracer to the rACC, >20% of c-Fos positive neurons projected specifically to the rACC and these projection neurons were located in the dorsal pole of the LC (Fig. 7H). No differences were found between the sides of the LC, demonstrating a bilateral overactivation of this pathway in CCI-LT animals in agreement with western blotting experiments about TH and phosphorylated CREB (Supplementary Fig. 5). Bilateral blockade of the LC-rACC pathway using hM4D(Gi)-DREADD had no significant effect on the evoked pain (acetone, von Frey and cold plate tests; Fig. 8A and Supplementary Fig. 10A and B). However, a reversion of the depressive phenotype was observed in CCI-LT rats (immobility and climbing P < 0.01 versus CCI-saline; Fig. 8A). No impairment of spontaneous locomotion was found (Supplementary Fig. 10C).
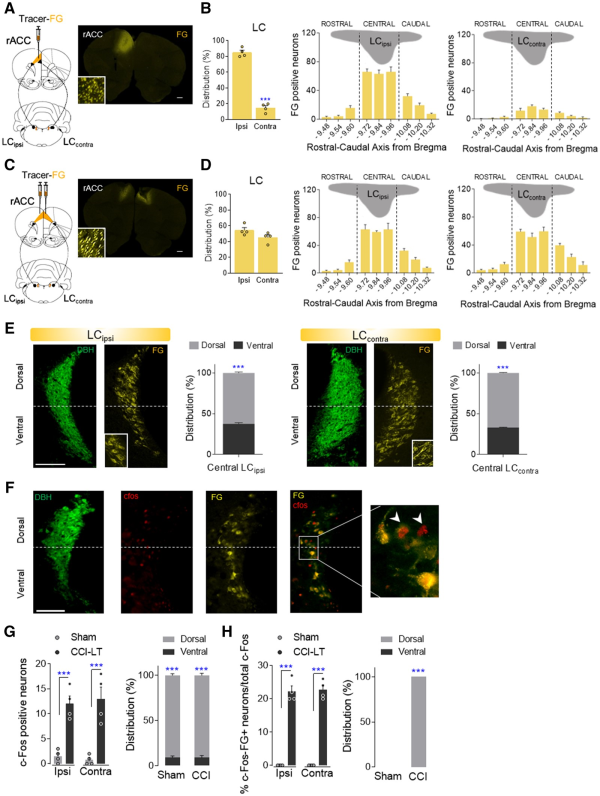
Figure 7
Neuroanatomical study of the LC projections to the rACC. (A and B) Retrograde Fluoro-Gold tracer strategy to target LC neurons that project to the ipsilateral rACC in wild-type rats and a representative image (scale bar = 500 μm). The ipsilateral and contralateral distribution of Fluoro-Gold-labelled LC neurons, and of Fluoro-Gold-positive neurons along the rostral-caudal axis of ipsilateral (LCipsi) and contralateral (LCcontra) LC (mean + SEM of the number of neurons per slice, n = 4 animals: ***P < 0.001 versus Ipsi, Student’s t-test; one-way ANOVA, Newman–Keuls post hoc test). (C and D) Retrograde Fluoro-Gold tracer strategy to target LC neurons that project to the bilaterally rACC and representative image (scale bar = 500 μm). Ipsilateral and contralateral distribution of Fluoro-Gold-labelled LC neurons and Fluoro-Gold-positive neurons along the rostral-caudal axis of LCipsi and LCcontra (mean + SEM of the number of neurons per slice, n = 4 animals: Student’s t-test and one-way ANOVA, Newman–Keuls post hoc test). (E) Representative immunofluorescence images of central LCipsi and LCcontra (−9.84 from bregma) showing the dorsoventral aspect (half height relative to DBH expression) (scale bar = 100 µm). The graphs show the dorsoventral distribution of the central LCipsi and LCcontra projection to the rACC (n = 4 animals: ***P < 0.001 versus ventral LC, Student’s t-test). (F) Representative immunofluorescence of the central LC showing the DBH, c-Fos, Fluoro-Gold expression and merged image (c-Fos+FG). The inset shows an example of c-Fos+FG- positive neurons (scale bar = 100 µm). (G and H) Numbers of c-Fos and c-Fos+FG-positive neurons of central LCipsi and LCcontra as well as the dorsoventral distribution in sham and CCI-LT rats (mean + SEM neurons per slice, n = 4 animals/group: ***P < 0.001 versus sham, two-way ANOVA, Newman–Keuls post hoc test; and ***P < 0.001 versus ventral LC, Student’s t-test). c-Fos (red); DBH = dopamine beta-hydroxylase (green); FG = Fluoro-Gold (yellow).
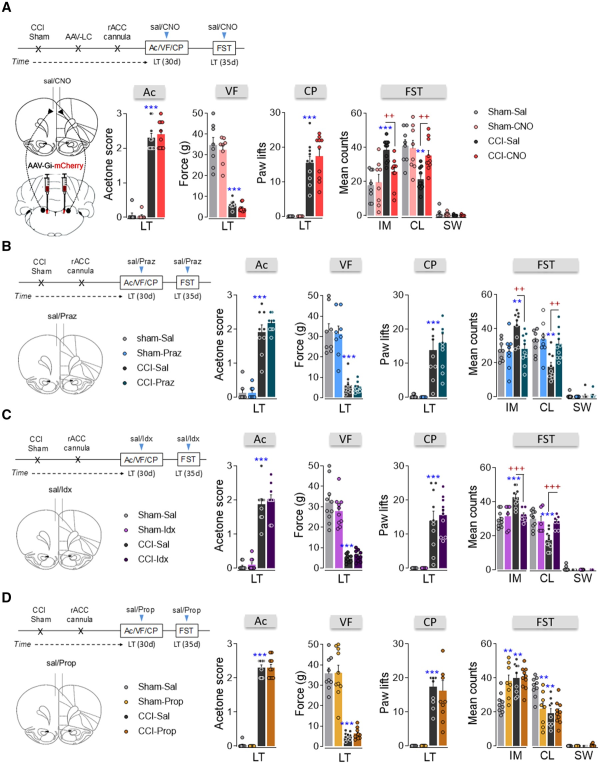
Figure 8
Effect of the chemogenetic inhibition of the LC-rACC pathway and the pharmacological blockade of adrenoreceptor activity in the rACC after long-term neuropathy. (A) Timeline and cartoon of the bilateral AAV-Gi-mCherry injection into the LC, and intra-rACC CNO (3 μM) administration to inhibit the noradrenergic-LC→rACC pathway of TH:Cre rats. Response of the ipsilateral hindpaw of CCI-LT rats in the acetone, von Frey and cold plate tests. The predominant behaviour in the FST (IM = immobility; CL = climbing; SW = swimming) was evaluated (n = 8–10 animals/group: **P < 0.01, ***P < 0.001 versus sham-sal; ++P < 0.01 versus CCI-sal, two-way ANOVA, Newman–Keuls post hoc test). (B–D) Timeline and cartoon of the pharmacological inhibition of α1-adrenoceptors (prazosin, 5 μg; n = 8–10 animals/group), α2-adrenoceptors (idazoxan, 9 μg; n = 6–10 animals/group) or β-adrenoceptors (propranolol, 1 μg; n = 7–10 animals/group) in the rACC of CCI-LT wild-type rats: **P < 0.01, ***P < 0.001 versus sham-sal; ++P < 0.01, +++P < 0.001 versus CCI-sal, two-way ANOVA, Newman–Keuls post hoc test. Ac = acetone test; CP = cold plate test; d = days; Idx = idazoxan; Praz = prazosin; Prop = propranolol; sal = saline; VF = von Frey test.
In addition, reduced c-Fos expression (LCipsi and LCcontraP < 0.001 versus CCI-sal: Supplementary Fig. 11A–C) and reduced c-Fos/Fluoro-Gold colabelling (LCipsiP < 0.01 and LCcontraP < 0.05 versus CCI-saline; Supplementary Fig. 11D) was observed in the LC after chemogenetic inhibition of the LC→rACC pathway in CCI-LT rats.
Blockade of α-adrenoreceptors in the rACC relieves pain-induced depression
To test whether blocking adrenoreceptor activity in the rACC might reverse pain-induced depression, we administered the α1-adrenoreceptor antagonist prazosin (5 µg/0.5 µl), the α2-adrenoreceptor antagonist idazoxan (9 µg/0.5 µl) or the β-adrenoreceptor antagonist propranolol (1 µg/0.5 µl) intra-rACC. Prazosin and idazoxan administration reversed depressive-like behaviour (prazosin: immobility and climbing P < 0.01, idazoxan: immobility and climbing P < 0.001 versus CCI-saline), although they did not change pain behaviours (acetone, von Frey and cold plate tests; Fig. 8B and C and Supplementary Fig. 12A–F). However, propranolol failed to modify pain- or depression-related behaviours in CCI-LT rats although it significantly increased depressive-like behaviour in sham (immobility and climbing P < 0.01 versus sham-saline; Fig. 8D and Supplementary Fig. 12G–I). Locomotor activity was not modified in any of the groups of rats evaluated (Supplementary Fig. 12B, E and H). These data indicate an involvement of α-adrenoreceptors in pain-induced depression at the rACC level.
Discussion
We demonstrate that there are time-dependent plastic changes in the noradrenergic-LC system as pain develops from acute to chronic, and that these changes are associated with behavioural despair.
Sensorial hypersensitivity appears immediately after nerve injury, whereas anxiodepressive and cognitive symptoms arise after several weeks (4–6 weeks).,,,,, To assess noradrenergic-LC changes when nociceptive signalling persists, LC activity in both hemispheres was explored at various times after unilateral nerve injury (from CCI-ST to CCI-LT). Immunohistochemistry revealed that there is increased c-Fos expression after nerve injury, both in the short and long term. However, the expression of c-Fos is higher in the ipsilateral side of the LC than on the contralateral side in the short term, while the increase is of equal magnitude bilaterally in the LC of CCI-LT animals. These results show that the nerve injury-induced LC changes vary on either side of the body, as well as with the duration of the injury. Chemogenetic suppression of the LC activity shows that activity of LCipsi neurons contributes to a milder pain phenotype, yet only in the early phase of nerve injury. However, LCcontra blockade did not modify pain perception at any time point. Contrary to sensorial sensitivity, the blockade of either the LCipsi or the LCcontra completely reversed the depressive phenotype developed at CCI-LT. Furthermore, bilateral stronger expression of TH, pCREB and c-Fos of the LC was found in the long term, in agreement with previous data in major depression disorder patients (TH) and in CCI-LT animals were both LCs were evaluated as a pool.,, Overall, the results indicate an early lateralized activation of the LC that reduces the pain phenotype but a later bilateral activation that leads to behavioural despair in nerve-injured animals. Interestingly, hM4D(Gi)-DREADD-mediated inhibition of the LC did not affect sensorial exploration or depressive-like phenotype in sham animals, suggesting a weak tonic drive of LC activity in the uninjured state. We also explored the consequences of activating the LC globally. In sham animals, global LC activation had no effect on the sensory threshold, although significant depressive behaviour was triggered when the LCipsi or LCcontra was activated chemogenetically, consistent with previous findings showing that the optogenetic/chemogenetic activation of LC neurons is itself anxiogenic., In terms of neuropathic pain, rM3D(Gs)-DREADD activation of LC neurons relieved pain at any time point, albeit with different contributions of the LCipsi and LCcontra. Indeed, global chemogenetic activation of the LCipsi produced a clear antineuropathic effect at all time points after nerve injury. However, global activation of LCcontra did not combat thermal and mechanical hypersensitivity at any time point. Furthermore, as depressive-like behaviour is associated with long-term pain, the effect of LC activation was explored in the FST. Chemogenetic LC activation did not modify the pain-induced depression-like state, perhaps reflecting that maximal activation had already been achieved by long-term nerve injury.
In the light of these findings, we explored the role of LC→spinal cord pathway along pain development. As occurred with global LC activity, hM4D(Gi)-DREADD blockade of the LCipsi→spinal cord projection rather than the LCcontra projection reduced pain in the short term. This was consistent with the c-Fos activation of the LCipsi neurons projecting to the spinal cord in the short term but not the long term (Fig. 2F). Accordingly, there are more DBH in the fibres of the spinal cord in the CCI-ST, and fewer in the CCI-LT. Alterations in spinal DBH at different time points from nerve injury have been shown before in several rodent models. We extend these previous findings evaluating changes in spinal DBH along with spontaneous activity of LC→spinal cord projecting neurons (c-Fos) and the behavioural effect of the chemogenetic LC blockade at two different time points from nerve injury. Overall, these changes would suggest an activation of the noradrenergic descending LC pathway to the spinal cord in the short term, reducing the pain phenotype soon after injury but later failing when the insult persists. Furthermore, chemogenetic activation of both LC to spinal cord neurons produces analgesia when neuropathy is well established (from 4 weeks after nerve injury), and we now show that this effect is mainly contributed by the LCipsi and can be triggered at any time point from injury. Our global evaluation of the LC and of the specific projection to the spinal cord seems to refute the idea that the LC serves to generate pain under conditions of chronic pain (review in ref. ) Indeed, intracerebroventricular administration of the neurotoxin anti-dopamine-β-hydroxylase saporin (anti-DβH-saporin) or intra-LC administration of lidocaine dampened the evoked pain in conditions of long-term nerve injury. However, intracerebroventricular injection of anti-DβH-saporin disrupts all noradrenergic nuclei (A1–A7), some of which contribute to sensorial hypersensitivity (A5),, although our experiments focused only on the LC. On the other hand, lidocaine administered at the level of the LC will block the activity of noradrenergic and non-noradrenergic cells, and our approach is to target only noradrenergic LC cells. Another important issue is the modular composition of the LC, whereby different projections may have opposing actions in a time-dependent manner. In this sense, the pain-inhibitory actions of noradrenaline in the spinal cord may be countered or even superseded by the activation of supraspinal facilitating pathways to the dorsal reticular nucleus (contributing to evoked pain) to the trigeminal spinal nucleus caudalis (evoked pain) or to the prefrontal cortex (PFC, spontaneous pain). Thus, further studies will be necessary to untangle the actions of the LC globally and in a modular fashion, also bearing in mind the strain differences that have been described in the noradrenergic pathways (see Llorca-Torralba et al. for a review).
One of the LC-related networks involved in affective dimensions and manifestations of pain-related expression of emotions is the rACC., Accordingly, hyperactivity in the ACC is seen in depressed patients and it has chronic neuropathic pain-induced anxiodepressive-like consequences. In this line, a bilateral increase of noradrenaline in the prefrontal cortex has been associated with long-term neuropathic pain (6 weeks after nerve injury), suggesting an overactivation of the noradrenergic system in long-term pain. Furthermore, optogenetic activation of the LC-ACC projection enhances excitatory neurotransmission in vitro. As such, the Fluoro-Gold and c-Fos co-labelling here reveals a significant bilateral increase in c-Fos expressing neurons that specifically project to the rACC in CCI-LT. The blockade of the LCipsi and LCcontra neurons projecting to the rACC completely reverses the depressive-like behaviour, yet it did not modify stimulus-evoked pain responses. Furthermore, site-specific pharmacological blockade indicates that α1- and α2-adrenoreceptors within the rACC are necessary for this behaviour. Mechanistically, electrophysiological studies in the medial PFC indicated that noradrenaline-persistent responses are mainly mediated by synergy between presynaptic α1-adrenoreceptor-mediated enhancement of glutamate release and postsynaptic α2-adrenoreceptor-mediated inhibition of hyperpolarization-activated cyclic nucleotide-gated cation channels. Thus, long-term pain overactivates the LC-ACC pathway (α-adrenoreceptors), leading to behavioural despair and overactivation of the LC-basolateral amygdala (BLA) pathway (β-adrenergic receptors) mediates anxiety and enhanced aversive learning. A similar phenomenon has been described when evaluating bilateral chemogenetic activation of the LC using resting-state functional MRI, which rapidly interrupts ongoing behaviour, dampens exploratory activity and augments anxiety, in conjunction with synchronized hyperconnectivity in the salient (that includes the ACC) and the amygdala networks. Zerbi’s findings, together with other previous studies showing that chemogenetic activation of the LC→PFC pathway in neuropathic pain animals (without anxiety) increases anxiogenic-like behaviour as well as spontaneous pain, raise important questions about the involvement of the LC→ACC pathway in anxiety and that of non-evoked pain in long-term neuropathic pain. Thus, experiments will be needed to determine whether anxiety is triggered by different LC projections (LC→BLA and LC→ACC). Indeed, from a translational point of view it will be particularly relevant to determine whether the blockade of β-adrenergic receptors, which has been shown to have an anxiolytic effect at BLA level, also has a similar beneficial effect at the ACC level.
Pain induces plasticity in the brain and it potentiates communication between brain nuclei by enhancing specific pathways, at the same time silencing other nuclei and pathways. Our study shows short-term asymmetrical endogenous LC activation after nerve injury, as witnessed by the c-Fos activity, and by the fact that global LC and LC-spinal cord pathway blockade attenuates injury-induced neuropathic pain ipsilaterally during the early post-injury period. However, this restorative LC analgesia fails over time, with the harmful bilateral activation of the LC and their projections to the rACC. These projections contribute to depression in the long term through α1- and α2-adrenoceptor activity. These data indicate that pain-induced depression is not a direct consequence of the failure of the descending pain inhibition at LC level (LC→spinal cord pathway). On the other hand, therapeutic approaches should focus on increasing noradrenaline exclusively at the spinal cord level to avoid any deleterious effects at supraspinal sites. These findings explain why drugs that systemically increase noradrenaline availability are rarely used (e.g. reboxetine, desipramine), while drugs with complementary serotoninergic or opioidergic actions [antidepressants (serotonin-noradrenaline reuptake inhibitors), tramadol, tapentadol] are more commonly chosen to treat pain or pain–depression comorbidity.
Acknowledgements
We are very grateful to Mr Santiago Muñoz, Ms Paula Reyes Perez and Ms Elena Marín Álvarez for their excellent technical assistance. The Central Services of Scientific and Technological Research, Health Sciences and Animal Research from the University of Cádiz.
Funding
This study was supported by grants cofinanced by the ‘Fondo Europeo de Desarrollo Regional’ (FEDER)-UE ‘A way to build Europe’ from the ‘Ministerio de Economía y Competitividad’ (MINECO: RTI2018-099778-B-I00) and by the ‘Ministerio de Salud-Instituto de Salud Carlos III’ (PI18/01691); the ‘Consejería de Salud de la Junta de Andalucía’ (PI-0134–2018); the ‘Programa Operativo de Andalucía FEDER, Iniciativa Territorial Integrada ITI 2014–2020 Consejería Salud, Junta de Andalucía’ (PI-0080–2017); the "Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía" (PEMP-0008-2020), Instituto de Investigación e Innovación en Ciencias Biomédicas de Cádiz (INiBICA LI19/06IN- CO22); the ‘Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía’ (CTS-510); the ‘Centro de Investigación Biomédica en Red de Salud Mental-CIBERSAM’ (CB/07/09/0033) and the Academy of Finland (315043).
Competing interests
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
CCI: chronic constriction injury
CNO: clozapine N-oxide
DREADD: designer receptor exclusively activated by designer drugs
FST: forced swimming test
LC: locus coeruleus
LT/MT/ST: long/mid/short term
PFC: prefrontal cortex
rACC: rostral anterior cingulate cortex
References
- 1. Bravo L, Llorca-Torralba M, Suarez-Pereira I, Berrocoso E. Pain in neuropsychiatry: Insights from animal models. Neurosci Biobehav Rev. 2020;115:96–115.
- 2. Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691.
- 3. Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474.
- 4. Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83.
- 5. Hirschberg S, Li Y, Randall A, Kremer EJ, Pickering AE. Functional dichotomy in spinal- versus prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife. 2017;6:e29808.
- 6. Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience. 2016;338:93–113.
- 7. Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator. J Neurosci Res. 2017;95(6):1336–1346.
- 8. Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319.
- 9. Isingrini E, Perret L, Rainer Q, et alResilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19(4):560–563.
- 10. Zhang H, Chaudhury D, Nectow AR, et alAlpha1- and beta3-Adrenergic receptor-mediated mesolimbic homeostatic plasticity confers resilience to social stress in susceptible mice. Biol Psychiatry. 2019;85(3):226–236.
- 11. Bernard R, Kerman IA, Thompson RC, et alAltered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16(6):634–646.
- 12. Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994;62(2):680–685.
- 13. Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55–68.
- 14. Perez-Caballero L, Torres-Sanchez S, Romero-López-Alberca C, González-Saiz F, Mico JA, Berrocoso E. Monoaminergic system and depression. Cell Tissue Res. 2019;377(1):107–113.
- 15. Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387.
- 16. Finnerup NB, Attal N, Haroutounian S, et alPharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173.
- 17. Chandler DJ, Jensen P, McCall JG, Pickering AE, Schwarz LA, Totah NK. Redefining noradrenergic neuromodulation of behavior: Impacts of a modular locus coeruleus architecture. J Neurosci. 2019;39(42):8239–8249.
- 18. Uematsu A, Tan BZ, Ycu EA, et alModular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci. 2017;20(11):1602–1611.
- 19. Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry. 2015;77(3):236–245.
- 20. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107.
- 21. Berrocoso E, De Benito MD, Mico JA. Role of serotonin 5-HT1A and opioid receptors in the antiallodynic effect of tramadol in the chronic constriction injury model of neuropathic pain in rats. Psychopharmacology (Berl)2007;193(1):97–105.
- 22. Llorca-Torralba M, Pilar-Cuellar F, Bravo L, et alOpioid activity in the locus coeruleus is modulated by chronic neuropathic pain. Mol Neurobiol. 2019;56(6):4135–4150.
- 23. Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–8082.
- 24. David-Pereira A, Sagalajev B, Wei H, Almeida A, Pertovaara A, Pinto-Ribeiro F. The medullary dorsal reticular nucleus as a relay for descending pronociception induced by the mGluR5 in the rat infralimbic cortex. Neuroscience. 2017;349:341–354.
- 25. Bravo L, Mico JA, Rey-Brea R, Pérez-Nievas B, Leza JC, Berrocoso E. Depressive-like states heighten the aversion to painful stimuli in a rat model of comorbid chronic pain and depression. Anesthesiology. 2012;117(3):613–625.
- 26. Berrocoso E, Mico J-A, Vitton O, et alEvaluation of milnacipran, in comparison with amitriptyline, on cold and mechanical allodynia in a rat model of neuropathic pain. Eur J Pharmacol. 2011;655(1-3):46–51.
- 27. Alba-Delgado C, Llorca-Torralba M, Horrillo I, et alChronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry. 2013;73(1):54–62.
- 28. Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl)1995;121(1):66–72.
- 29. Llorca-Torralba M, Suarez-Pereira I, Bravo L, et alChemogenetic silencing of the locus coeruleus-basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatry. 2019;85(12):1021–1035.
- 30. Alba-Delgado C, Llorca-Torralba M, Mico JA, Berrocoso E. The onset of treatment with the antidepressant desipramine is critical for the emotional consequences of neuropathic pain. Pain. 2018;159(12):2606–2619.
- 31. Bravo L, Torres-Sanchez S, Alba-Delgado C, Mico JA, Berrocoso E. Pain exacerbates chronic mild stress-induced changes in noradrenergic transmission in rats. Eur Neuropsychopharmacol. 2014;24(6):996–1003.
- 32. Tervo DGR, Proskurin M, Manakov M, et alBehavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell. 2014;159(1):21–32.
- 33. Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007;146(4):1785–1794.
- 34. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 2007.
- 35. McCall JG, Al-Hasani R, Siuda ER, et alCRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605–620.
- 36. Sciolino NR, Plummer NW, Chen YW, et alRecombinase-dependent mouse lines for chemogenetic activation of genetically defined cell types. Cell Rep. 2016;15(11):2563–2573.
- 37. Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136(3):348–355.
- 38. Hughes SW, Hickey L, Hulse RP, Lumb BM, Pickering AE. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neuropathic pain following tibial nerve injury. Pain. 2013;154(9):1680–1690.
- 39. Ma W, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970(1-2):110–118.
- 40. Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160(1):174–185.
- 41. Marques-Lopes J, Pinho D, Albino-Teixeira A, Tavares I. The hyperalgesic effects induced by the injection of angiotensin II into the caudal ventrolateral medulla are mediated by the pontine A5 noradrenergic cell group. Brain Res. 2010;1325:41–52.
- 42. Martins I, Carvalho P, de Vries MG, et alIncreased noradrenergic neurotransmission to a pain facilitatory area of the brain is implicated in facilitation of chronic pain. Anesthesiology. 2015;123(3):642–653.
- 43. Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127(2):160–164.
- 44. Kaushal R, Taylor BK, Jamal AB, et alGABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAalpha1 receptors in the medial prefrontal cortex. Neuroscience. 2016;334:148–159.
- 45. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167.
- 46. Mayberg HS, Liotti M, Brannan SK, et alReciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682.
- 47. Sellmeijer J, Mathis V, Hugel S, et alHyperactivity of anterior cingulate cortex areas 24a/24b drives chronic pain-induced anxiodepressive-like consequences. J Neurosci. 2018;38(12):3102–3115.
- 48. Suto T, Eisenach JC, Hayashida K. Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. Pain. 2014;155(10):1935–1942.
- 49. Koga K, Yamada A, Song Q, et alAscending noradrenergic excitation from the locus coeruleus to the anterior cingulate cortex. Mol Brain. 2020;13(1):49.
- 50. Zhang Z, Cordeiro Matos S, Jego S, Adamantidis A, Seguela P. Norepinephrine drives persistent activity in prefrontal cortex via synergistic alpha1 and alpha2 adrenoceptors. PLoS ONE. 2013;8(6):e66122.
- 51. Zerbi V, Floriou-Servou A, Markicevic M, et alRapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron. 2019;103(4):702–718.e705.
- 52. Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(3 Suppl):S49–S64.
- 53. Bravo L, Llorca-Torralba M, Berrocoso E, Mico JA. Monoamines as drug targets in chronic pain: Focusing on neuropathic pain. Front Neurosci. 2019;13:1268.
- 54. Hughes S, Hickey L, Donaldson LF, Lumb BM, Pickering AE. Intrathecal reboxetine suppresses evoked and ongoing neuropathic pain behaviours by restoring spinal noradrenergic inhibitory tone. Pain. 2015;156(2):328–334.
- 55. Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27(7):348–354.
