Introduction
Myelin, the multilayered lipid-rich-membrane sheath that wraps axons concentrically, is essential for transmitting electrical impulses in the nervous system. These impulses encode signals for executing numerous functions conserved in mammals, vertebrates and invertebrates. Myelinating Schwann cells integrate axonal signals to regulate myelin formation during development. For instance, axon-derived type III neuregulin-1 (Nrg1) binds to ErbB2/3 receptors on Schwann cells to activate small GTPases RAC1/CDC42 and promote myelination. Prion protein (PrPC) from axon interacts with G protein-coupled receptors (GPCRs) on Schwann cells, leading to trimeric G protein complex activation, RAC1/CDC42 activation, and cAMP-driven myelination., Thus, cyclic adenosine monophosphate (cAMP) in Schwann cells is an essential second messenger for promyelination. However, as an effector of RAC1/CDC42, how P21-activated kinase 2 (PAK2) plays a role in these promyelinating pathways is unclear.
PAKs, a family of serine/threonine protein kinases, are divided into two groups based on the homology of amino acid sequences: Group I (PAK1, PAK2 and PAK3); and Group II (PAK4, PAK5 and PAK6)., PAKs are directly downstream to small GTPases, RAC1 and CDC42. While Group I PAK members share considerable homology, each of them still exhibits distinct functions.Pak1 and Pak3 knockout mice present no or minimal phenotype, but Pak2 knockout is embryonically lethal in mice. Because ablation of Rac1 or Cdc42 arrests Schwann cells in their early stage of myelination, and PAKs are major druggable effectors of the small GTPases, it is important to clarify if and how PAK2 affects myelination.
We generated mice with a conditional deletion of Pak2 in Schwann cells. Our results revealed that PAK2 activity is indispensable for myelination. Moreover, this promyelinating effect can be regulated by myelin lipids, which may provide potential mechanisms for curbing or facilitating PAK2’s effect in myelination.
Materials and methods
Mice
Mice carrying floxed Pak2 alleles, (Pak2f/f, C57BL/6J background) were crossed with myelin protein zero-cre (Mpz-cre) mice (Strain #017927, Jackson Laboratory) to delete Pak2 in Schwann cells (scPak2−/−). In addition, Pak2f/f mice were bred with Synapsin I-cre (Syn-cre) mice (Strain #003966, Jackson Laboratory) to ablate Pak2 in neurons (nPak2−/−). Pak2f/+:Mpzcre+ and Pak2f/+:Syncre+ mice were backcrossed at least nine times onto the C57BL/6J background. Littermate Pak2f/f mice were used as controls. PCR analysis for genotyping Pak2 and Cre alleles used the following primers: for Pak2, forward was 5′-ATCTTCCCAGGCTCCTGACT-3′ and reverse 5′-TGAAGCTGCATCAATCTATTCTG-3′; for Mpz-cre and Syn-cre, forward was 5′-ACCCTGTTACGTATAGCCGA-3′ and reverse 5′-CTCCGGTATTGAAACTCCAG-3′. Controls were age- and sex-matched littermates. However, when evaluating body weight, male and female mice were separated for comparison. Mice were maintained on a standard 12-h light/12-h dark cycle, ad libitum access to food and water, and housed in plastic cages with standard rodent bedding.
Animal ethics statements
All animal experiments and procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Houston Methodist Research Institute and were performed in accordance with federal and institute guidelines and regulations for the care and use of experimental vertebrate animals. The authors complied with the ARRIVE guidelines for reporting.
Rotarod test
This method was previously described. Mice were placed on an accelerating (0.1 rpm/s) rotarod (Columbus Instruments) that progressed from 16 to 28 rpm over 120 s. After positioning, the mice walked until they fell or finished the 120 s run. Each mouse received 2 days of training before the test. All mice participated in daily trials for 3 days, with a 30-min break separating each trial. The latency to fall was averaged for each mouse.
Hindlimb clasping test
Mice were held by the tail 30 cm above a tabletop and video-recorded for 20 s. Abnormal postures, such as hindlimb clasping and sustained straining of paws, were documented. A researcher unaware of the genotype examined the videos and recorded the duration of clasping and paw straining.
Nerve conduction studies
Mouse nerve conduction studies (NCS) were previously described. In brief, mice were anaesthetized with isoflurane and compound muscle action potential (CMAP) measurements were taken from the intrinsic foot muscles using needle electrodes. Stimulation electrodes were placed at the mouse’s sciatic notch and ankle, with recordings made on the paw. Supramaximal stimulations were used to elicit CMAP and the conduction velocity (CV) was calculated between distal and proximal stimulation sites.
Morphometric analysis and electron microscopy
The morphometric analysis has been detailed previously. Semi-thin sections from Epon-embedded sciatic nerves were stained with toluidine blue and imaged using a Leica THUNDER microscope. We segmented nerve images based on a U-net architecture in the convoluted neural network (CNN) model. After learning from manually segmented nerve images, this model automatically segmented myelin sheaths from axons. Using the inner and outer areas of each nerve fibre, the program calculated both inner and outer radii through a designated formula. The radii led to computing the myelin thickness by subtracting the inner radius from the outer radius and dividing the result by two, as well as the g-ratio, representing the ratio of the inner radius to the outer radius. Axon density was assessed by dividing the number of axons by the area containing all captured nerve fibres.
Electron microscopic analysis of mouse sciatic nerves was performed as previously described. The ultrathin sections were stained with uranyl acetate and lead citrate. Imaging was carried out using a Zeiss Gemini 300 Scanning Electron Microscope.
Animal surgery and intraneural injection
Green fluorescent protein (GFP)-only, GFP-WT-PAK2 and GFP-K278R-PAK2 lentivirus were purchased from Applied Biological Materials and injected into sciatic nerves using a published protocol. Pups at P3 were anaesthetized with isoflurane inhalation and positioned under a Leica stereomicroscope. The sciatic nerve was exposed surgically. A fine glass needle (Fivephoton Biochemicals) containing 2 μl of 1010 cfu/ml lentivirus particles mixed with 0.01% Fast Green was injected into the sciatic nerve using a micromanipulator until the nerves appeared green. The contralateral sciatic nerve, as a control, received a 2 μl PBS with 0.01% Fast Green injection. Once the injection was completed, the wound was closed with Histoacryl glue. At Day 30 post-injection, sciatic nerves were dissected for immunofluorescence staining.
Immunofluorescence staining
This method was previously described. In brief, for teased nerve fibre staining, sciatic nerves were fixed in 4% paraformaldehyde (PFA) overnight and teased into individual fibres on glass slides. For paraffin nerve staining, sciatic nerves were fixed, embedded in paraffin and sectioned into 5 μm-thick slices. The slides were incubated with primary antibodies at 4°C overnight. Following a wash, the slides were incubated with secondary antibodies for 1 h. The stained slides were observed under a Leica THUNDER fluorescent microscope.
Cell culture and cAMP measurement
Schwann cells were obtained from the sciatic nerves of 3-day-old Pak2f/f and scPak2−/− mice. Schwann cells were immortalized using an SV40-T lentiviral vector (Applied Biological Materials Inc). Human wild-type PAK2 and kinase-dead PAK2-K278R plasmids were transfected into Pak2−/− Schwann cells to generate overexpressing wild-type PAK2 (Pak2-OE) and kinase-dead PAK2 (Pak2-K278R) cell clones.
The four Schwann cell lines, Pak2+/+, Pak2−/−, Pak2-OE and Pak2-K278R, were treated with PBS, Nrg1 (50 ng/ml, Cat. No. 377HB, R&D Systems) or synthetic flexible tail (residues 23–50) of PrPC peptides (2 μM, GenScript) for 30 min. The cells were lysed using 0.1 M HCl lysis buffer. The following steps were carried out according to the manufacturer’s protocol (Enzo Life Sciences, Cat. No. ADI-900-066), which involved competition between the sample cAMP and a cAMP-alkaline phosphatase conjugate. cAMP levels were determined using a cAMP standard curve in the context of an ELISA-based assay.
Nrg1 and PrPC stimulation and GTPase pull-down assay
Pak2+/+ and Pak2−/− Schwann cells were plated onto poly-L-lysine (PLL)-coated 100 mm plates and grown in Dulbecco’s modified Eagle medium (DMEM)/F12 supplemented with 10% fetal bovine serum (FBS) until they reached 80% confluency. To induce serum and mitogen starvation, cells were washed twice with pre-warmed Hanks’ Balanced Salt Solution (HBSS) and incubated in DMEM/F12 containing 0.5% FBS (starvation medium) for 20 h. After the incubation, three replicates of each plate were treated for 1 h at 37°C and 5% CO2 using either starvation medium with a vehicle (PBS plus 0.02% FBS; mock medium), 50 ng/ml Nrg1, or 2 μM PrPC peptide.
Following the stimulation period, cells were washed twice with PBS containing phosphatase inhibitors (Cat. No. 5872, Cell Signaling) and lysed with 1× lysis buffer. Total protein concentration was determined using the BCA assay (Prod. No. 23225, Thermo Scientific). The active GTPase pull-down assay was performed according to the manufacturer’s instructions for the active RAC1 detection kit (Cat. No. 8815, Cell Signaling) and active CDC42 detection kit (Cat. No. 8819, Cell Signaling), with samples treated with GTPγS (positive control) and GDP (negative control). The immunoprecipitated materials and total protein samples were then prepared for immunoblot analysis.
Immunoblotting
This method has been described previously. Whole cells or chopped sciatic nerves were lysed in RIPA buffer (Cat. No. R0278, Sigma) with a proteinase/phosphatase inhibiter cocktail. Protein concentration was measured using a BCA assay. Equal volumes of proteins were separated using SDS-PAGE gels and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking, primary and secondary antibodies were used to detect target proteins. Chemiluminescence (Cat. No. NEL103001, Perkin Elmer) was used for detection and ImageJ software quantified the protein bands.
Antibodies and materials
PAK1 (Cat. No. 2602), PAK2 (Cat. No. 2608), PAK3 (Cat. No. 2609), PAK4 (Cat. No. 52694), Phospho-PAK2 (Ser20) (Cat. No. 2607), Phospho-PAK1 (Ser144)/PAK2 (Ser141) (Cat. No. 2606), Phospho-PAK1 (Thr423)/PAK2 (Thr402) (Cat. No. 2601), β-Tubulin (Cat. No. 2128) and β-actin (Cat. No. 4970) antibodies were from Cell Signaling Technology. PAK5 (Cat. No. ab110069), PAK6 (Cat. No. ab154752) and GAPDH (Cat. No. ab9485) antibodies were from Abcam. MBP (Cat. No. MAB386) and Neurofilament 200 (Cat. No. MAB5266) antibodies were from Millipore. Sphingomyelin (SM, #860062), L-α-phosphatidylinositol (PI, #840042), Ganglioside GM3 (GM3, #860058), cholesterol (CHOL, #700100), 18:1 cholesteryl oleate (CHOL-E, #700269), L-α-phosphatidylserine (PS, #840032) and C18:1 Galactosyl (β) Ceramide (G-Cer, #860596) were from Avanti Polar Lipids. N-hexanoyl-D-sphingosine (C6-Cer, #H6524) and D-sphingosine (SPH, #S7049) were from Sigma Aldrich.
Protein structure analysis
AlphaFold-Multimer v3 (https://doi.org/10.1101/2021.10.04.463034) was run locally with default parameters with Nvidia 24 Gbyte GPUs. Uniprot90 was used as the sequence database for the generation of multiple sequence alignments (MSAs), which are input features to AlphaFold-Multimer. No template structures from the Protein Data Bank (PDB) were used. Twenty-five models of each complex were made (5 seeds × 5 AlphaFold-Multimer parameter sets). Structures were visualized with PyMOL.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism software version 9.0 and data were represented as the mean ± standard deviation (SD). P-values were derived from either a two-tailed Student’s t-test or repeated-measures ANOVA. A P-value < 0.05 was deemed statistically significant.
Results
PAK2 is highly expressed during the development of the peripheral nerves
We evaluated PAKs’ expression in the mouse sciatic nerve. PAK1 and PAK2 were detectable by western blot in the wild-type mouse sciatic nerves at postnatal Day (P) 7, while PAK3, 4, 5 and 6 proteins were weakly expressed or not detectable (Fig. 1A). PAK2 had the highest expression among all PAKs. PAK1 expressed higher at embryonic Day (E) 18.5 and P1 but maintained at a moderate level from P7 to adulthood. PAK2 expression was higher from E18.5 to P14, but downregulated in adulthood (Fig. 1B). Immunostaining revealed that PAK2 was present in the myelin of the mouse sciatic nerve and cytoplasm of neurons (Fig. 1C and D). While mice with Pak1 knockout were asymptomatic with normal morphology in peripheral nerves, it does raise a question about the Pak2 function in the peripheral nerve.
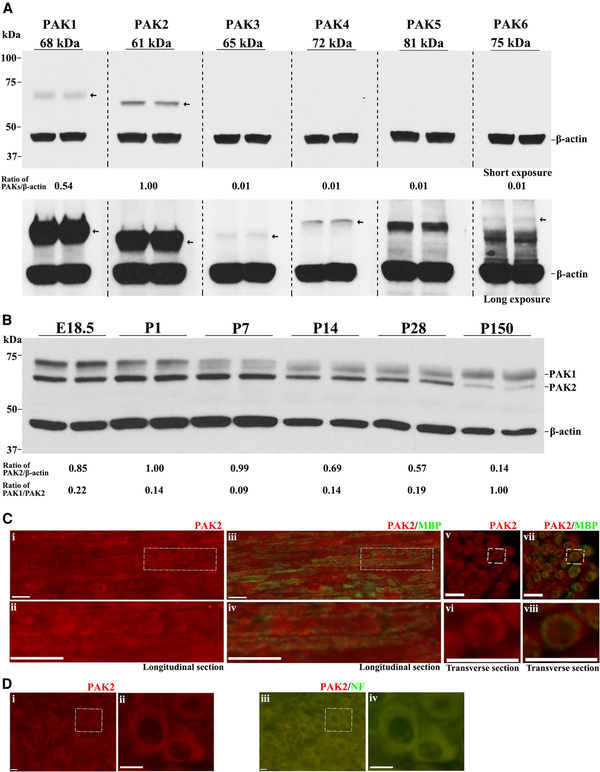
Figure 1
PAK2 is highly expressed during peripheral nerve development. (A) The expression levels of P21-activated kinases (PAKs) were analysed by western blot in the sciatic nerves of P7 wild-type (WT) mice. Each antibody was tested in duplicates of nerve lysate. PAK1 and PAK2 were detectable in mouse sciatic nerves, while PAK3, PAK4, PAK5 and PAK6 were barely detectable or undetectable. β-Actin served as a loading control. The relative protein level of the PAKs was determined by normalizing to β-actin. The top panel was from a short exposure. The bottom panel was developed by a long exposure. This experiment was performed three times using different samples. (B) Total PAK1 and PAK2 levels were measured by western blot in E18.5, P1, P7, P14, P28 and P150 wild-type mouse sciatic nerves. PAK1 and PAK2 levels were normalized against β-actin levels. PAK2 levels were significantly higher in the developing peripheral nerves (E18.5 to P14) than those in mature ones (P150). PAK1 is highly expressed in peripheral nerves during the embryonic period (E18.5) and gradually decreases during the maturation of the peripheral nerves. For each developmental stage, duplicates of nerve samples were used in each experiment. The experiment was replicated with different samples. (C) Longitudinal (i–iv) and transverse (v–viii) sections of 3-week-old wild-type sciatic nerves were stained with antibodies against PAK2 (red) and myelin basic protein (MBP) (green, stain myelin). The staining showed PAK2 localization predominantly in myelin. The top panels [C(i, iii, v and vii)] show the original images, while the bottom panels [C(ii, iv, vi and viii)] are the magnified view of the white boxes in the top panels. The specificity of the PAK2 antibody was confirmed through knockout mouse tissue (Supplementary Fig. 1). (D) A section of the dorsal root ganglia (DRG) from a 3-week-old wild-type mouse was reacted with PAK2 (red) and neurofilament-200 (NF, green) antibodies. PAK2 was localized in the cytoplasm of the DRG neurons. [D(ii and iv)] Magnified view of the white box. Scale bars = 10 μm.
Conditional knockout of Pak2 in Schwann cells
We crossed Pak2f/f mice with Schwann cell-specific Mpzcre mice (expresses Cre at ∼E14.5) to generate Schwann cell-specific knockout mice. Exon 2 of the Pak2 alleles was excised during Mpz-cre recombination (Fig. 2A). PCR confirmed efficient Cre-mediated recombination (Fig. 2B). Western blot of scPak2−/− sciatic nerve confirmed a reduction in PAK2 levels (Fig. 2C), with minimal PAK2 likely from axons and connective tissues. A subtle decrease in PAK1 level was also detected in scPak2−/− nerves (Fig. 2C). ScPak2−/− pups were born in the expected Mendelian ratio and were similar in size to their wild-type (WT) and heterozygous siblings at birth. However, by P4 weeks, scPak2−/− mice had impaired growth (Fig. 2D and E), with death occurring between P6 and 10 weeks.
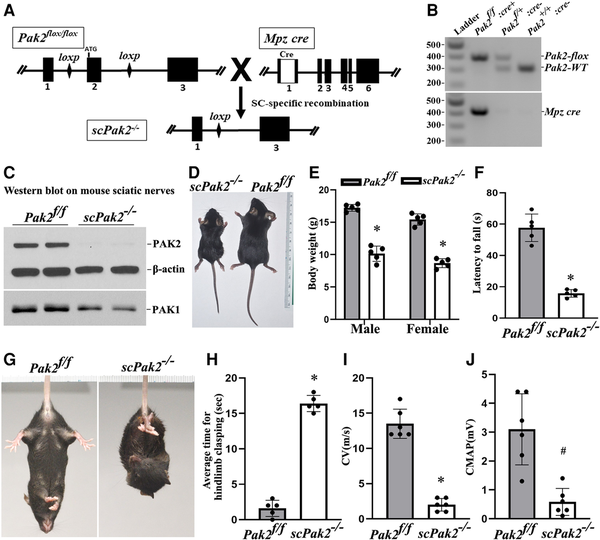
Figure 2
Schwann cell-specific Pak2 knockout mouse. (A) Exon 2 in Pak2 gene was flanked by LoxP. Mpz activates Cre recombinase expression in Schwann cells at embryonic Day 14.5. Upon Cre recombination, the exon 2 of Pak2 between loxP sites was removed to produce Schwann cell-specific knockout mice (scPak2−/−). (B) Mice were genotyped by PCR. The amplified wild-type (WT) Pak2 allele is 391 bp, and the amplified Pak2 flox allele is 306 bp. Lane 1 = DNA ladder (M); Lane 2 = Pak2flox/flox:Mpz-Cre+ (scPak2−/−); Lane 3 = Pak2flox/+:Mpz-Cre− (Pak2f/+); Lane 4 = Pak2wt/wt:Mpz-Cre− (Pak2+/+). (C) At P21, sciatic nerves with stripped epineurium were analysed by western blot using an antibody against PAK1 and PAK2. In Pak2f/f nerves, both PAK2 and PAK1 were detectable. However, in scPak2−/− nerves, PAK2 expression was hardly detectable, while PAK1 level also exhibited a minor reduction. β-Actin was used as a loading control. The nerves of each genotype were from two different samples. (D). One-month-old Pak2f/f and scPak2−/− littermate were placed next to a centimetre measure. (E) The body size of the 1-month-old scPak2−/− mice was significantly smaller than that of their Pak2f/f littermates (P < 0.001, n = 5 in each group). (F) Rotarod analysis of motor function at 1 month of age revealed a significant reduction in scPak2−/− mice, compared to that in Pak2f/f mice (P < 0.001, n = 5 in each group). (G) Tail-suspension test showed hind paw clasping in 1-month-old scPak2−/− mice, but not in control littermates. (H) Quantification of hindlimb clasping test. Data are presented as the average time for hindlimb splaying. One-month-old scPak2−/− mice exhibited significantly increased hindlimb clasp compared to that in their Pak2f/f littermates (P < 0.001, n = 5 for each genotyping group). (I and J) Nerve conduction studies showed that conduction velocity (CV) and compound muscle action potential (CMAP) were significantly decreased in 1-month-old scPak2−/− mice compared to Pak2f/f mice (P < 0.05, n = 6 in each genotype). Mean ± SD. *P < 0.001, #P < 0.01.
scPak2−/− mice manifest behaviour dysfunction
The scPak2−/− mice developed tremors by 2 weeks of age and progressive paralysis. At 4 weeks of age, these mice exhibited hindlimb clasping when suspended by the tail (Fig. 2G and H). Rotarod analyses showed significant impairment of motor performance in 4-week-old scPak2−/− mice (Fig. 2F). NCS revealed significantly reduced nerve CV (Fig. 2I) and CMAP (Fig. 2J), consistent with abnormal development of myelin.
Ablation of Pak2 in Schwann cells arrests myelination
We examined nerve morphology on the cross-sections of mouse sciatic nerve under light microscopy and electron microscopy. In Pak2f/f sciatic nerves, large-diameter axons were well myelinated. However, in scPak2−/− mice, we observed axons with thin myelin sheaths and numerous abnormal aggregates of unsorted large-calibre axons that formed axon bundles (Fig. 3A and E, arrowheads). We quantified myelinated nerve fibres using a machine learning-based segmentation. Our results showed a significant decrease in myelinated nerve fibres and the thickness of the myelin sheath in scPak2−/− nerves, compared to those in Pak2f/f nerves (Fig. 3B and C). Moreover, an increased g-ratio in the scPak2−/− nerves confirmed a reduction in myelin thickness (Fig. 3D). These findings demonstrate that the absence of PAK2 in Schwann cells leads to the failure of myelin development.
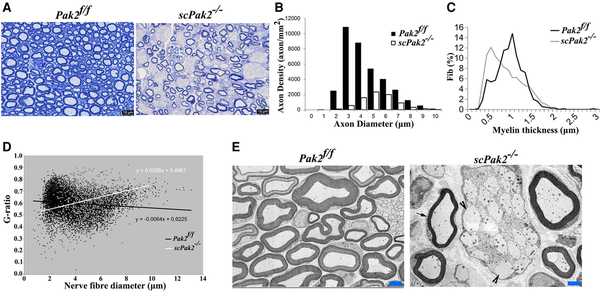
Figure 3
Morphometric analysis on mouse sciatic nerve. (A) The semithin sections on 1-month-old scPak2−/− sciatic nerves showed a reduction in the number of myelinated nerve fibres, which appeared smaller in diameter compared to that in the Pak2f/f nerves. There were no signs of demyelination, such as myelin debris, broken myelin or excessive macrophages. (B) The morphometric analysis confirmed a significant decrease in myelinated nerve fibres of scPak2−/− nerves, compared with that in the Pak2f/f nerves (P < 0.001, n = 3 in each genotype). (C) Myelin sheath thickness was reduced in scPak2−/− sciatic nerves. (D) A plot of g-ratio against nerve fibre diameter supports the observed reduction of myelin thickness in scPak2−/− mice. (E) Electronic microscopy images revealed scPak2−/− axons either failed to be myelinated (arrowhead) or thinly myelinated (arrow). Scale bars = 2 μm.
Restoration of PAK2 kinase activation, not the protein per se, in scPak2−/− sciatic nerves rescues dysmyelination
To verify whether the loss of Pak2 directly leads to dysmyelination, we locally injected 2 μl of 1010 cfu/ml lentiviral particles expressing WT-PAK2 or kinase-dead PAK2 into the sciatic nerves of P3 scPak2−/− pups. We also injected 2 μl GFP-lentivirus or PBS into the contralateral sciatic nerve as a control. At P30, we teased the sciatic nerves into individual nerve fibres, which were stained with myelin basic protein (MBP) antibodies. We imaged the internodes to confirm the transfection of PAK2 in the lentivirus-injected nerves (Fig. 4A). Quantification of MBP fluorescence intensity (a well-known myelin protein as an indicator of myelination) showed a significant increase of MBP in scPak2−/− nerves injected with GFP-WT-Pak2 lentivirus, compared with the internodes in scPak2−/− nerve fibres transfected with GFP-only lentivirus (Fig. 4B). Furthermore, internodal lengths increased by 40% in scPak2−/− nerves transfected with GFP-WT-Pak2 lentivirus (Fig. 4C). In contrast, Schwann cells expressing a kinase-dead mutant of Pak2 failed to restore MBP expression and internodal lengths in scPak2−/− sciatic nerves (Fig. 4B and C). The GFP-only and PBS-injected scPak2−/− nerves showed similar pathological changes to those in non-injected scPak2−/− nerves. Additionally, PAK2 overexpression did not increase segmental demyelination in wild-type sciatic nerves when compared to nerves with normal PAK2 levels (Supplementary Fig. 2) (0.80 ± 0.2% for nerves with PAK2 overexpression versus 0.73 ± 0.2% for nerves with normal PAK2 levels; n = 3, P > 0.05). Together, these findings supported the essential role of PAK2 kinase activity in myelination.
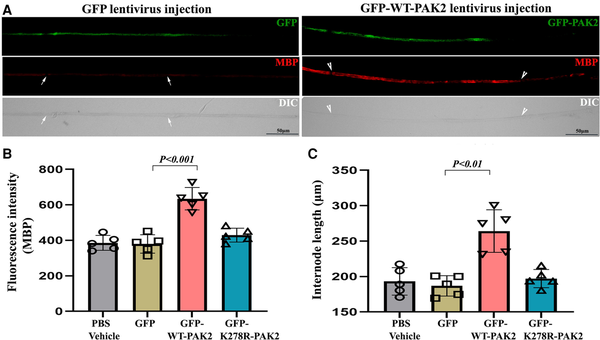
Figure 4
Intraneural lentivirus delivery restores PAK2 expression in Schwann cells of scPak2−/− sciatic nerves in vivo. (A) A sciatic nerve from a 3-day-old scPak2−/− mouse was surgically exposed and locally injected with 2 μl lentivirus particles at 1010 cfu/ml of GFP-only, GFP-WT-PAK2 (wild-type PAK2) or GFP-K278R-PAK2 (kinase-dead PAK2 mutation). The contralateral sciatic nerve was injected with 2 μl PBS as a control. At P30, the sciatic nerves were dissected. After fixation, teased nerve fibres were stained with myelin basic protein (MBP) antibody and imaged. (B) The MBP fluorescence intensity of each internode within nerve fibres was quantified and the average intensity for each mouse was calculated. The intensity of MBP was significantly increased in GFP-positive fibres (between arrowheads) from scPak2−/− nerves transfected with GFP-WT-Pak2 lentivirus, but not GFP-positive fibres from scPak2−/− nerves transfected with GFP-K278R-Pak2 lentivirus, compared with fibres (between arrows) in scPak2−/− nerve fibres transfected with GFP-only lentivirus (mean ± SD, P < 0.001, n = 10–20 fibres per mouse, n = 5 mice per group). (C) Internodal lengths of each nerve fibre were quantified. The average internodal length increased by 40% in scPak2−/− nerves treated with GFP-WT-Pak2 lentivirus (264.2 ± 30 μm for internodes in scPak2−/− nerves injected with GFP-WT-Pak2 lentivirus versus 186.9 ± 14 μm for internodes in scPak2−/− nerve fibres injected with GFP-only lentivirus, mean ± SD, P < 0.01, 10–20 fibres per mouse, n = 5 mice per group). This rescue was not observed in nerve fibres injected with kinase-dead Pak2 mutation. DIC = differential interference contrast; GFP = green fluorescent protein; SD = standard deviation.
PAK2 is a part of Nrg1- or PrPC-driven RAC1/CDC42 signalling in Schwann cells
Nrg1 or PrPC from axons promotes myelination through CDC42, RAC1 and cAMP., Because PAK2 is the effector of the small GTPases RAC1 and CDC42, we tested whether Nrg1 and PrPC signalling are mediated through PAK2 activity using affinity precipitation and immunoblot assays.
Schwann cell culture was stimulated by PrPC or Nrg1. The results showed that either PrPC or Nrg1 significantly enhanced the activation of CDC42 and RAC1 in Pak2+/+, but not in Pak2−/− Schwann cells (Fig. 5A and B). This blockage of RAC1 and CDC42 activation by the absence of PAK2 in Schwann cells was not expected and has not been reported. Notice the basal level of RAC1 and CDC42 activation and the total level of RAC1 or CDC42 were not changed in Pak2−/− Schwann cells (Fig. 5A, PBS lanes). Furthermore, PrPC or Nrg1 significantly increased the phosphorylation of PAK2 at Thr402 (Fig. 5A and C), which is a known indicator of PAK2 activation by RAC1/CDC42. These findings provide strong evidence that PAK2 activity is required in RAC1/CDC42 signalling activated by promyelinating factors PrPC or Nrg1.
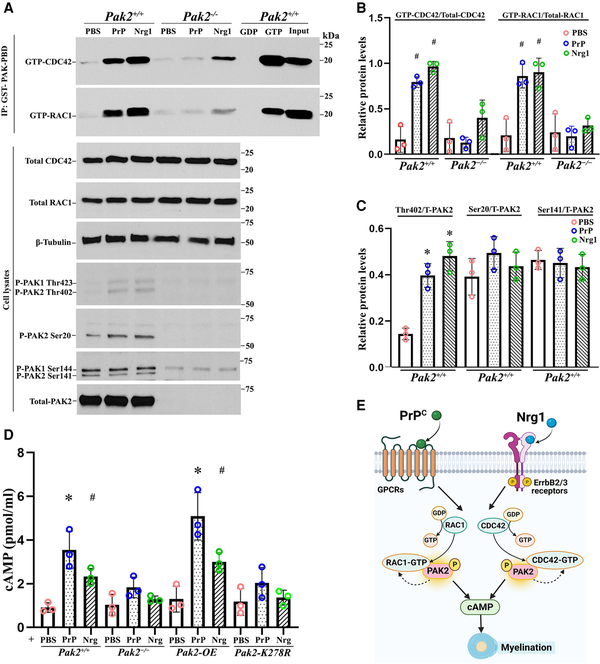
Figure 5
PAK2 is essential for Nrg1- or PrPC-driven CDC42/RAC1 activation and cAMP levels. (A) Endogenous RAC1 and CDC42 activity were measured using an affinity-precipitation assay with recombinant GST-PAK-PBD. Western blots were performed with antibodies for total RAC1, total CDC42, total PAK2 and phosphorylated-PAK2 (Thr402, Ser20 and Ser141) in Pak2+/+ and Pak2−/− Schwann cells stimulated with prion protein (PrPC) and Nrg1. (B) The levels of GTP-CDC42 and GTP-RAC1 were normalized against total CDC42 and total RAC1, respectively. After 30 min of exposure to PrPC or Nrg1, GTP-CDC42, and GTP-RAC1 levels significantly increased in Pak2+/+ Schwann cells, compared with PBS-treated Schwann cells (#P < 0.05). Pak2−/− Schwann cells had no or minimal response to the stimulation. (C) The level of Thr402, but not Ser20 and Ser 141, significantly increased in PrPC or Nrg1-induced Pak2+/+ Schwann cells, compared with those in the vehicle (*P < 0.01). Total and phosphorylated-PAK2 proteins were undetectable in Pak2−/− Schwann cells. (D) Human WT-PAK2 and kinase-dead PAK2-K278R plasmids were transfected into Pak2−/− Schwann cells to obtain Pak2-OE and Pak2-K278R cell clones. Pak2+/+, Pak2−/−, Pak2-OE and Pak2-K278R Schwann cells were treated with PBS, PrPC or Nrg1 for 30 min. The intracellular cyclic adenosine monophosphate (cAMP) concentrations were measured by direct cAMP ELISA assay in cell lysates. PrPC and Nrg1 significantly increased cAMP levels in Pak2+/+ and Pak2-OE Schwann cells, but not Pak2−/− and Pak2-K278R Schwann cells, compared with that in PBS (mean ± SD, *P < 0.01, #P < 0.05). (E) A proposed model highlights PAK2 serves as a crucial convergence point for axon-derived Nrg1 and PrPC promyelination pathways, subsequently facilitating the activation of RAC1/CDC42 and cAMP level within Schwann cells. The curved arrows indicate the proposed feedback stimulation from PAK2 to CDC42/RAC1. SD = standard deviation; WT = wild-type.
Nrg1 or PrPC increases cAMP level through PAK2 activity
cAMP is a well-known second messenger to promote myelination in Schwann cells. Nrg1 or PrPC may elevate cAMP levels in Schwann cells to promote myelination., We performed an ELISA assay to measure cAMP levels. Upon stimulation with Nrg1 or PrPC, Schwann cells displayed significantly increased cAMP levels in both wild-type and PAK2-overexpression conditions, but not in Pak2−/− Schwann cells (Fig. 5D). Moreover, Nrg1 or PrPC failed to increase cAMP levels in Schwann cells expressing a kinase-dead Pak2 mutant (Fig. 5D), underscoring an enabling role of PAK2 kinase activity in cAMP signalling. These findings, together with observations in scPak2−/− mice above, further support that PAK2 activity is necessary for myelination. PAK2 also appears to be a convergence point for multiple promyelination signalling pathways, as both Nrg1 and PrPC exert their promyelinating effects through PAK2 kinase activity (Fig. 5E).
Ablation of Pak2 in neurons does not cause dysmyelination or axon degeneration
PAK2 is expressed in neurons (Fig. 1D). To determine PAK2 function in neurons, we generated a neuron-specific conditional knockout (nPak2−/−) by crossing Pak2f/f with Syn-cre mice (Fig. 6A and B). Morphometric analysis of the nPak2−/− sciatic nerves revealed no significant difference in axon diameter, axon density, myelin thickness and g-ratio when compared to Pak2f/f nerves (Fig. 6C–G). In line with these findings, both CMAP and CV were not significantly different between Pak2f/f and nPak2−/− mice (Fig. 6H and I). This indicates that promyelination by PAK2 is independent of neurons.
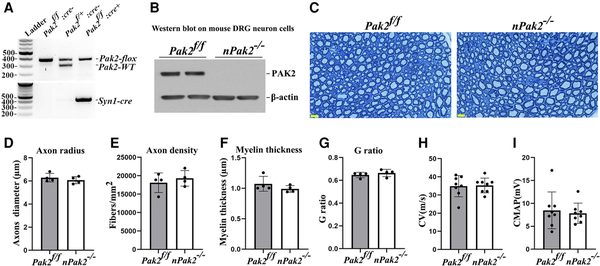
Figure 6
Neuronal-specific Pak2 conditional knockout mice. (A) Pak2f/f mice were crossed with Syncre mice to generate a conditional neuronal knockout (nPak2−/−). The genotype of conditional knockouts was identified by PCR. (B) A western blot analysis indicated that PAK2 level was undetectable in neuron cells from nPak2−/− dorsal root ganglia (DRGs). The neuron cells of each genotype were derived from two separate samples. (C) Semi-thin sections of sciatic nerves showed myelinated nerve fibres were similar between 6-month-old Pak2f/f and nPak2−/− mice. Scale bars = 10 µm. (D–G) Morphometric evaluations revealed no difference in axon radius, axon density, myelin thickness and the g-ratio between the sciatic nerves of 6-month-old Pak2f/f and nPak2−/− mice (P > 0.05; n = 4 for both groups). (H) Nerve conduction studies showed no significant difference in conduction velocity (CV) between 6-month-old Pak2f/f and nPak2−/− mice (P > 0.05, n = 8 in each genotype). (I) Compound muscle action potential (CMAP) revealed normal values in mutants. Mean ± SD. SD = standard deviation.
Promyelination by small GTPases is independent of PAK1
RAC1/CDC42 activation depends on PAK2 activity in PrPC or Nrg1 signalling (Fig. 5), which raises a question on PAK1’s roles in the molecular cascade as PAK1 and PAK2 share a high homology in their amino acid sequences. We performed an affinity precipitation and immunoblot assay for PAK1 (Fig. 7) similar to those for PAK2 (Fig. 5). It showed that RAC1/CDC42 activation was not blocked by the ablation of Pak1 in Schwann cells (Fig. 7A and B).
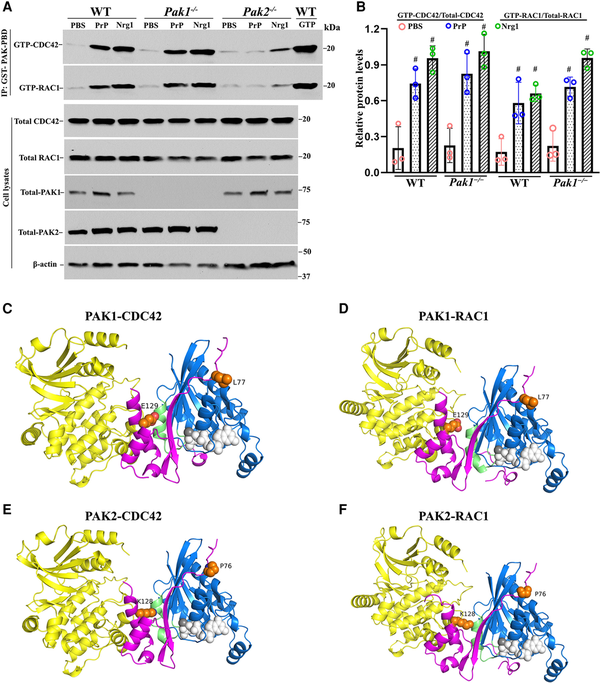
Figure 7
Comparative analysis of PAK1 and PAK2 in Nrg1- or PrPC-driven CDC42/RAC1 activation and protein structure analysis. (A and B) The activity of CDC42 and RAC1 were evaluated through an affinity-precipitation assay using recombinant GST-PAK-PBD. Western blot analyses were conducted using antibodies against RAC1, CDC42, PAK2 and PAK1 in Schwann cells of wild-type (WT), Pak1−/− and Pak2−/−, which were stimulated with prion protein (PrPC) or Nrg1. Upon stimulation with PrPC or Nrg1, GTP-CDC42 and GTP-RAC1 levels significantly increased in wild-type Schwann cells when compared to the Schwann cells exposed to PBS (#P < 0.05). On the contrary, Pak2−/− Schwann cells showed minimal or no response to the stimulation. (C–F) AlphaFold-Multimer models of full-length PAK1 and PAK2 with CDC42 and RAC1. (C) PAK1/CDC42. (D) PAK1/RAC1. (E) PAK2/CDC42. (F) PAK2/RAC1. Regions with pLDDT (a confidence measure produced by AlphaFold-Multimer per residue) <50 are not shown. These regions are intrinsically disordered regions. The domains are coloured as follows: PAK kinase domain (yellow); PAK PBD regions (magenta); CDC42/RAC1 (blue); CDC42/RAC1 Switch 2 loop/helix (green); GTP from PDB: 5CJP (white spheres); Mg2+ ion (grey sphere); residue differences between PAK1 and PAK2 in the PBD region (orange spheres).
To clarify the difference between PAK2 and PAK1, we used AlphaFold-Multimer (v3) to model complexes of the full-length proteins (Fig. 7C–F). In each image, the PAK kinase domains are in yellow, the PAK PBD domain (which binds CDC42/RAC1) is in magenta, CDC42/RAC1 is in blue, and the switch two domain of the CDC42/RAC1 is in green. The RAS domain of CDC42/RAC1 is in an active conformation in all four complexes, like that of GTP-bound CDC42 in PDB entry 5CJP (0.3 Å RMSD over backbone atoms). The complex structures are very similar to the crystallographic structures of the PAK1 PBD domain with CDC42 (PDB: 1e0a) and the PAK1 PBD domain with RAC3.
As expected, the structures of the four complexes are very similar. The CDC42 structures are identical in the PAK1 and PAK2 complexes, as are the RAC1 structures with PAK1 and PAK2. Thus, AlphaFold-Multimer does not predict any difference between PAK1 and PAK2 that might affect conformational changes in the switch two, the domain determining the activation of RAS domains. There are only two residues in the PBD regions that are different between PAK1 and PAK2 and might affect how they interact with CDC42 and RAC1. These are L77 (PAK1) versus P76 (PAK2) and E129 (PAK1) versus K128 (PAK2). Close inspection of the interactions of these residues does not indicate any obvious changes in affinity. E129/K128 does interact with the switch two domains of CDC42 and RAC1, which could be responsible for the differential effects on CDC42 and RAC1 following PAK binding. However, the mechanism for these differences remains to be investigated.
Taken together, these promyelination pathways are dependent on PAK2, but not PAK1, despite high amino acid homology between PAK1 and PAK2.
Myelin lipids regulate PAK2 activity
Myelin contains a high content of lipids (71%). Sphingosine has been shown to bind to and activate PAK., We stimulated Schwann cells with 10 different myelin lipids and measured PAK2 activity. Four of the 10 lipids [cholesteryl ester (CHOL-E), phosphatidyl (PI), N-hexanoyl-D-sphingosine (C6Cer) and GM3 ganglioside (GM3)] induced phosphorylation at Thr402 of PAK2 in the soluble fraction of Schwann cells (Fig. 8A and C). Interestingly, Sphingosine (SPH) inhibited PAK2 in the soluble fraction but enhanced PAK2 activity in the insoluble fraction (Fig. 8A–D). The two different effects of sphingosine on PAK2 activation have been shown between two different cell fractions from 3T3-L1 mouse pre-adipocytes.
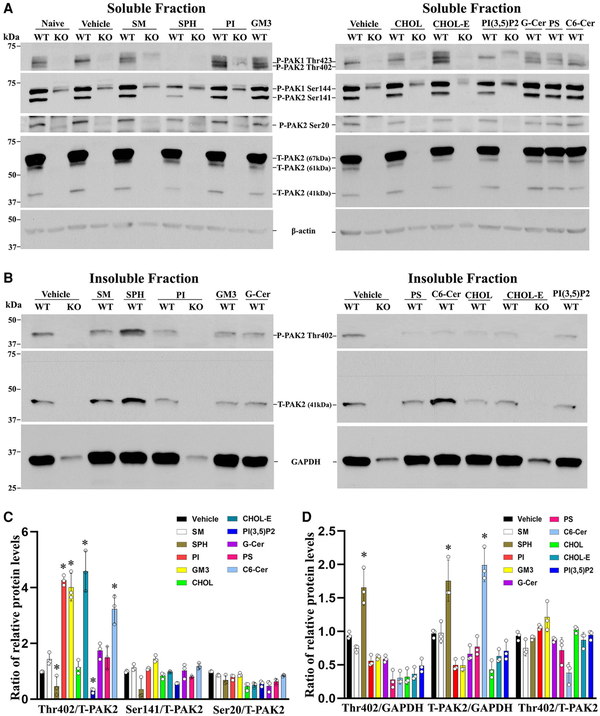
Figure 8
PAK2 activity is regulated by myelin lipids in Schwann cells. (A) Subconfluent Pak2+/+ and Pak2−/− Schwann cell cultures grown on poly-L-lysine (PLL)-coated wells were starved and stimulated with the indicated lipids (100 μM) or vehicle for 30 min. Cells were lysed in RIPA buffer and the supernatants (soluble fraction) were collected. Total PAK2 and its phosphorylation levels were determined by western blots using the indicated antibodies in Pak2+/+ Schwann cells, but not in Pak2−/− Schwann cells. β-Tubulin was used as loading control. (B) The pellets were solubilized by sonication in RIPA lysis buffer containing 1% NP40 and the supernatants (insoluble fraction) were obtained. Western blot of phosphorylated and total PAK2 was performed in the insoluble fraction. The phosphorylated PAK2 proteins (Ser20 and Ser141) were not detectable in the insoluble fraction. GAPDH was used as loading control. (C) Densitometric analysis: the Thr402 level, but not Ser20, Ser141 and total PAK2, significantly increased in the soluble fraction of Pak2+/+ Schwann cells after treatment with cholesteryl oleate, L-α-phosphatidylinositol (PI), C6 ceramide or ganglioside GM3, compared to that in vehicles. Conversely, Thr402 level was decreased by sphingosine and PI(3,5)P2. *P < 0.01. (D) In the insoluble fraction of Pak2+/+ Schwann cells, the Thr402 PAK2 level showed a significant increase following sphingosine treatment compared to that by vehicle. Total PAK2 levels were elevated when treated with sphingosine and N-Hexanoyl-D-sphingosine. *P < 0.01. C6 Ceramide = N-Hexanoyl-D-sphingosine; Chol = cholesterol; CholE = cholesteryl oleate; Gal Cer = Galactosyl(β) Ceramide; GM3 = Ganglioside GM3; KO = Pak2−/− Schwann cells; PS = L-α-phosphatidylserine; SM = sphingomyelin; SPH = sphingosine; WT = Pak2+/+ Schwann cells.
Discussion
Our study produces several important discoveries that are original. First, it demonstrates PAK2 activity as a novel promyelinating factor. Myelination is essential for the normal functions of the peripheral nerves, including action potential propagation. Disruption of myelination has been found in various peripheral neuropathies, such as those congenital hypomyelination neuropathies, Charcot-Marie-Tooth (CMT) 1A, B, C, D, E, CMT4B, C, D, F and J. Interestingly, we did observe a decrease of PAK2 activity in a CMT1E (Trembler-J) mouse nerves (Supplementary Fig. 3). Thus, PAK2 could be a potential molecular target to be manipulated in repairing abnormal myelination in these diseases.
Second, the depletion of PAK2 activity blocks signals driven by either Nrg1 or PrPC. Thus, PAK2 acts as a converging point indispensable to multiple promyelination pathways. This finding is not only novel but also reveals a broad regulatory effect of PAK2 on myelination.
Abnormal axonal radial sorting in Pak2-deficient nerves suggests that the PAK2 effect on myelination takes place at the early stage of myelin development. This is also in line with the highest expression of PAK2 found in developing mouse sciatic nerves. Because deletion of PAK2 blocks RAC1/CDC42 activation (Fig. 5), this finding is also consistent with previous studies that Cdc42 or Rac1 ablation in Schwann cells causes severe dysmyelination with abnormal radial sorting., Nrg1 or PrPC functions as key axonal signals that interact with distinct Schwann cell receptors to initiate a cascade of molecular events promoting myelination., Therefore, PAK2 activity is critical for Schwann cell-axon interactions.
While ablation of PAK2 suppressed CDC42 activity, there were still residual activities of CDC42 noticed (Fig. 5, lane 6). How the residual activity occurs is still unclear. However, when Nrg1 binds to the ErbB2/3, it activates not only the CDC42 pathway but also triggers other signallings like focal adhesion kinase (FAK) that could, in turn, affect CDC42 activity.
The exact mechanisms for PAK2 to drive Schwann cell’s wrapping of axon remain to be determined. Promyelinating factors (Nrg1, PrPC) from axons interact with their receptors on the adaxonal membrane (inner mesaxons) of Schwann cells that are anchored on axolemma via various junctions (adherence junctions, tight junctions, gap junctions). These junctions are usually stabilized on the actin network. The actin subject to the regulation of PAK activity, which could be a mechanism of PAK2 to advance the wrapping of Schwann cell inner membrane. In this context, myelin lipids in the vicinity of PAK2 may directly affect PAK2 activation.
Furthermore, a previous study has shown that CDC42 predominantly affects Schwann cell proliferation while RAC1 regulates Schwann cell process extension. The latter by RAC1, not CDC42, is mainly driven by the interaction between laminin in the extracellular matrix and β1-integrin on Schwann cells. PAK2 can also be cleaved by caspase-3 at D212 to release its c-terminal to promote apoptosis via nuclear translocation. Each of these specific mechanisms could contribute to the phenotypes in scPak2−/− mice and remains to be determined. However, this does not alter the conclusion in the current study.
Third, protein-protein interactions are crucial for numerous biological functions, either positively (activation) or negatively (inhibition). For example, PAK2 activity is known to be stimulated by the binding of small GTPases CDC42 or RAC1. Our study has uncovered a fascinating reciprocal activation between PAK2 and CDC42/RAC1 in Schwann cells, occurring under the drive of axonal Nrg1 or PrPC. Small GTPases activate PAK2, but PAK2 activity, in turn, promotes the activation of CDC42 and RAC1 upon the protein complex formation. This reciprocal effect is specific to PAK2, but not PAK1, as ablation of PAK1 made no difference in small GTPase activity (Fig. 7).
This reciprocal effect is also promyelination-specific. The basal activity of CDC42/RAC1 was unchanged in the absence of Pak2 (Fig. 5), while CDC42/RAC1 activation was blocked in scPak2−/− Schwann cells upon the stimulation of Nrg1 or PrPC. Our structural analysis did reveal differences of two specific amino acids between PAK2-CDC42/RAC1 and PAK1-CDC42/RAC1 interfaces. It remains to be determined whether the difference is sufficient to explain the reciprocal effect of PAK2-CDC42/RAC1. Nevertheless, these findings do explain the normal peripheral nerve in Pak1 knockout mice and the severe dysmyelination in scPak2−/− mice. Thus, the PAK2-dependence in small GTPase activation underscores the significance of understanding the promyelinating effect conferred by the specificity of the PAK2-GTPase reciprocal effect. This significance is further highlighted by the mutations in the FRABIN/FGD4 gene causing an inherited peripheral neuropathy in humans as FGD4 encodes the small RhoGTPase CDC42-guanine nucleotide exchange factor Frabin and activates CDC42.
Fourth, specific myelin lipid species may enhance or inhibit PAK2 activity. Lipids account for ∼71% of the dry weight of myelin membranes. Myelin has its unique lipid composition with high levels of cholesterol (∼26% by weight) and glycolipids (31%; for instance, cerebroside and sulphatide account for 14%–26% and 2%–7% of myelin lipids, respectively). During development, most myelin is formed during the first 2 years of postnatal life. In rodents, myelination occurs predominantly during the first month of life. It has been estimated that the myelin membrane surface area of one glial cell expands ∼6500-fold during its maturation. The composition of various myelin lipids also dynamically evolves over the course of early development. How the nervous system senses the myelin maturation to prevent over-myelination is still unclear. Our findings of myelin lipid effect on PAK2 activation provide a potential mechanism allowing myelin lipids to directly regulate PAK2 activity, thereby promoting or curbing myelination.
PAKs have been a ‘hot’ target in therapeutic development through searching small molecule inhibitors of PAKs., Less attention has been given to PAK activators. Our study suggests that PAK2 activity may be manipulated via the delivery of specific myelin lipids or even lipid diet modification.,
Our finding is likely applicable to the CNS as there is an increase of PAK2 expression in the developing brain. In fact, Pak2 haploinsufficiency has been linked to brain development abnormalities and autism aetiology.
Of great interest, mice depleted of PAK1 do not display any evident peripheral nerve phenotype or pathology, despite a high homology of amino acid sequence between PAK1 and PAK2. Instead, PAK1 hyperactivity has been found to regulate myelin junctions in adult mice with peripheral nerve diseases. In contrast, the present study demonstrates a prominent role of PAK2 during the early development of the peripheral nerve. We uncovered a novel molecular mechanism differentiating PAK2 from PAK1 through a reciprocal effect between small GTPase and PAK2, not PAK1. Together, each PAK family member exerts distinct biological functions through unique molecular mechanisms.
In summary, this study uncovers a novel promyelinating factor, PAK2. It converges multiple well established promyelinating signalling pathways during glia-axon interactions, such as Nrg1 and PrPC. PAK2 exerts its promyelinating effect through small GTPases, which distinct its function from PAK1. Because myelin lipids can enhance or inhibit PAK2 activation, the lipid effect could function as a feedback mechanism to promote or curb myelination, a potential therapeutic approach for future exploration.
Acknowledgements
The authors thank Zafar Wazir for his assistance in the Rotarod test and mouse genotyping.
References
- 1. Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. 2015;7:a020529.
- 2. Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–928.
- 3. Mogha A, D'Rozario M, Monk KR. G protein-coupled receptors in myelinating Glia. Trends Pharmacol Sci. 2016;37:977–987.
- 4. Küffer A, Lakkaraju AK, Mogha A, et al The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464–468.
- 5. Bacallao K, Monje PV. Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS One. 2015;10:e0116948.
- 6. Rane CK, Minden A. P21 activated kinases: Structure, regulation, and functions. Small GTPases. 2014:5:e28003.
- 7. Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117(Pt 19):4343–4354.
- 8. Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255.
- 9. Hu B, Arpag S, Zhang X, et al Tuning PAK activity to rescue abnormal myelin permeability in HNPP. PLoS Genet. 2016;12:e1006290.
- 10. Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene pak3. J Neurosci. 2005;25:6641–6650.
- 11. Kelly ML, Chernoff J. Mouse models of PAK function. Cell Logist. 2012;2:84–88.
- 12. Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32:17251–17261.
- 13. Guo L, Moon C, Zheng Y, Ratner N. Cdc42 regulates Schwann cell radial sorting and myelin sheath folding through NF2/merlin-dependent and independent signaling. Glia. 2013;61:1906–1921.
- 14. Benninger Y, Thurnherr T, Pereira JA, et al Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061.
- 15. Phee H, Au-Yeung BB, Pryshchep O, et al Pak2 is required for actin cytoskeleton remodeling, TCR signaling, and normal thymocyte development and maturation. Elife. 2014;3:e02270.
- 16. Radu M, Lyle K, Hoeflich KP, Villamar-Cruz O, Koeppen H, Chernoff J. p21-Activated kinase 2 regulates endothelial development and function through the Bmk1/Erk5 pathway. Mol Cell Biol. 2015;35:3990–4005.
- 17. Moiseev D, Wazir Z, Liu D, Li J, Hu B. C698r mutation in Lrsam1 gene impairs nerve regeneration in a CMT2P mouse model. Sci Rep. 2022;12:12160.
- 18. Moiseev D, Hu B, Li J. Morphometric analysis of peripheral myelinated nerve fibers through deep learning. J Peripher Nerv Syst. 2019;24:87–93.
- 19. Katona I, Zhang X, Bai Y, et al Distinct pathogenic processes between Fig4-deficient motor and sensory neurons. Eur J Neurosci. 2011;33:1401–1410.
- 20. Gonzalez S, Fernando RN, Perrin-Tricaud C, Tricaud N. In vivo introduction of transgenes into mouse sciatic nerve cells in situ using viral vectors. Nat Protoc. 2014;9:1160–1169.
- 21. Renkema GH, Pulkkinen K, Saksela K. Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol Cell Biol. 2002;22:6719–6725.
- 22. Arthur-Farraj P, Wanek K, Hantke J, et al Mouse Schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733.
- 23. Parker MI, Meyer JE, Golemis EA, Dunbrack RL. Delineating the RAS conformational landscape. Cancer Res. 2022;82:2485–2498.
- 24. Poitelon Y, Kopec AM, Belin S. Myelin fat facts: An overview of lipids and fatty acid metabolism. Cells. 2020;9:812.
- 25. Bokoch GM, Reilly AM, Daniels RH, et al A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–8144.
- 26. Roig J, Tuazon PT, Traugh JA. Cdc42-independent activation and translocation of the cytostatic p21-activated protein kinase gamma-PAK by sphingosine. FEBS Lett. 2001;507:195–199.
- 27. Li J. Inherited neuropathies. Semin Neurol. 2012;32:204–214.
- 28. Myers JP, Robles E, Ducharme-Smith A, Gomez TM. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J Cell Sci. 2012;125(Pt 12):2918–2929.
- 29. Nodari A, Zambroni D, Quattrini A, et al Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075.
- 30. Jakobi R, McCarthy CC, Koeppel MA, Stringer DK. Caspase-activated PAK-2 is regulated by subcellular targeting and proteasomal degradation. J Biol Chem. 2003;278:38675–38685.
- 31. Varusai TM, Kolch W, Kholodenko BN, Nguyen LK. Protein-protein interactions generate hidden feedback and feed-forward loops to trigger bistable switches, oscillations and biphasic dose-responses. Mol Biosyst. 2015;11:2750–2762.
- 32. Horn M, Baumann R, Pereira JA, et al Myelin is dependent on the Charcot-Marie-Tooth type 4H disease culprit protein FRABIN/FGD4 in Schwann cells. Brain. 2012;135(Pt 12):3567–3583.
- 33. Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52:419–434.
- 34. Webster HD. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 1971;48:348–367.
- 35. Barnes-Velez JA, Aksoy Yasar FB, Hu J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innovation (Camb). 2023;4:100360.
- 36. Rudolph J, Crawford JJ, Hoeflich KP, Chernoff J. p21-activated kinase inhibitors. Enzymes. 2013;34(Pt B):157–180.
- 37. Senapedis W, Crochiere M, Baloglu E, Landesman Y. Therapeutic potential of targeting PAK signaling. Anticancer Agents Med Chem. 2016;16:75–88.
- 38. Wang Y, Zeng C, Li J, et al PAK2 haploinsufficiency results in synaptic cytoskeleton impairment and autism-related behavior. Cell Rep. 2018;24:2029–2041.
