Established Facts
• Aortopulmonary window (APW) is a rare congenital heart disease.
• Although surgical closure is the primary option, percutaneous therapy may also be a suitable alternative.
Novel Insights
• Cardiac 64-slice computed tomography and 3D reconstruction are more sensitive, specific, and accurate for the correct diagnosis of APW.
• This is the first study to report an APW patient treated successfully using a transcatheter closure with a symmetrical membranous ventricular septal occluder.
Introduction
Aortopulmonary window (APW), a birth defect in which there is a hole between the ascending aorta and pulmonary artery, results from incomplete development of septa between the aorta and pulmonary artery []. APW is a rare lesion and is usually accompanied by other cardiovascular anomalies such as atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), and an interrupted aortic arch []. Due to the left to right blood shunting, APW may lead to pulmonary hypertension and congestive heart failure []. Surgery is the standard treatment for the vast majority of patients with APW. However, recently several reports have shown that transcatheter closure may represent another option. For example, several APW cases were treated with Rashkind umbrella devices (Bard, New Providence, NJ, USA) and various Amplatzer Occluders (AGA Medical Corporation, Plymouth, MN, USA) [,]. Herein, we report closure of an APW using a symmetrical membranous ventricular septal occluder device (SVSDO, Beijing Starway Medical Technology, China), originally designed for closure of membranous VSD.
Case Report
A 42-year-old woman without a significant medical history presented with progressive exertional dyspnea for 2 years. Physical exam showed cardiac dullness, as well as a grade 3/6 continuous murmur in the left second intercostal space. Electrocardiogram showed features of left ventricular hypertrophy and chest X-ray revealed cardiomegaly. Ultrasonography showed a PDA with a diameter of approximately 10 mm, a prominent left superior vena cava, and an increased left ventricular end-diastole with a diameter of 56 mm. Left ventricular ejection fraction was normal.
The patient was scheduled for transcatheter closure of the PDA after informed consent was obtained. Right heart catheterization was then performed, which showed mild pulmonary hypertension of approximately 47/16 (26) mm Hg. However, an aortogram revealed a right-sided aortic arch but no PDA. The operation was discontinued because of the patient's complaint of discomfort. Thereafter, we performed cardiac computed tomography and 3D reconstruction, which displayed an APW with an axial diameter of 5 mm and 2 mm in longitudinal length. The APW was associated with the right-sided aortic arch, a descending aorta malformation, and persistent left superior vena cava (Fig. 1a, b). Subsequently, we carried out an ascending aortogram and confirmed the presence of an APW of 5.5 mm in diameter. We proceeded to correct the malformation with a SVSDO with a 10-mm symmetrical disc (Beijing Starway Medical Technology, China) to close the APW, after obtaining informed consent from the patient. We established a tract by first crossing a Terumo guide wire (Terumo Company, Japan) from the ascending aorta into the pulmonary artery through the defect and then trapping and pulling the guide wire out through the right femoral vein. After positioning an 8-Fr sheath across the APW from the venous access site, we loaded the SVSDO and deployed it across the APW under angiographic and transthoracic echocardiographic guidance. Angiography in the shallow right anterior oblique projection showed correct positioning of the device. Repeated angiography and echocardiography demonstrated no residual leak or obstruction of the coronary arteries (Fig. 1c, d). Electrocardiogram showed no ST-segment or T-wave changes. No clinical symptoms or heart murmurs were detected in the following days. The patient was then started on aspirin (100 mg per day) for 6 months and has been followed up for over 1 year. During the follow-up period, ultrasonography showed no residual leak and a reduction of the left ventricular end-diastolic diameter to 45 mm.
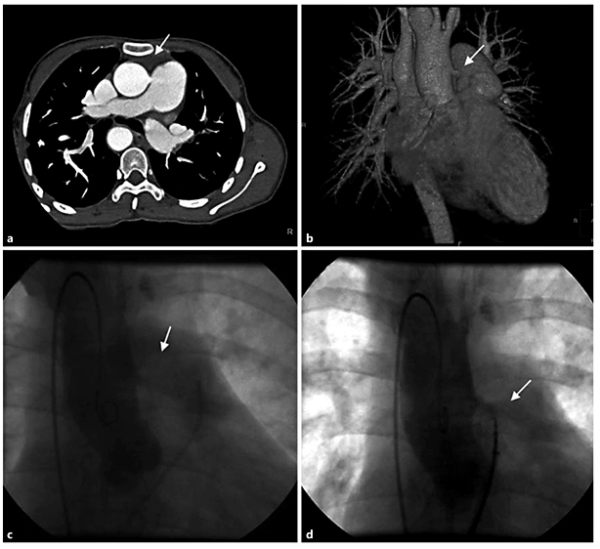
Fig. 1
Images showing the presence and closure of APW. a, b Cross-sectional and three-dimensional cardiac computed tomography imaging. The communication between the aorta and the pulmonary artery was visualized (arrow). c, d Ascending aortogram in AP projection before and after closure of APW using a SVSDO with a 10-mm symmetrical disc showing the good device position and no significant shunt.
Discussion
APW is a rare congenital septal defect that allows communication between the ascending aorta and the pulmonary trunk and/or right pulmonary artery. Based on the anatomical characteristics, APW has been classified into 4 types, namely a proximal defect, distal defect, total defect, and intermediate defect []. Approximately 90% of APW are of the proximal category []. The majority of APW cases present with congestive heart failure and pulmonary hypertension due to massive pulmonary blood flow [,].
Although angiography is considered the gold standard for the judgment of the defect location and the possible coexistence of other cardiac structural malformations, APW may be detected using echocardiography due to its association with both heart failure and pulmonary hypertension. However, the distal and total types of APW are often misdiagnosed as PDA, VSD, or ASD, with misdiagnosis rates as high as 30.9% []. More advanced techniques, including cardiac 64-slice computed tomography and 3D reconstruction, are more sensitive, specific, and accurate for evaluating the structural and spatial relationship between the heart and large blood vessels in complex congenital cardiac malformations. Indeed, the value of cardiac 64-slice computed tomography in APW diagnosis of this case was remarkable. In our case, the key to the correct diagnosis of the APW was to find the simultaneous development of the aorta and pulmonary trunk through an ascending aortogram in the right anterior oblique position [].
The early diagnosis of APW, followed by a timely repair, is crucial to significantly decrease mortality, reduce patient anxiety, and improve quality of life []. Although surgical closure is the primary option, percutaneous therapy may also be a suitable alternative. In several selected cases, transcatheter closure using certain devices has been described []. For example, Stamato et al. [ ]described the transcatheter closure of an APW in a 3-year-old child using a modified double umbrella occluder system. Furthermore, Naik et al. [ ]and Peer et al. [ ]used an Amplatzer ASD device to close a 6.0-mm APW and an Amplatzer duct occluder to close a 3.0-mm APW. In addition to these studies, Li et al. [] reported the successful treatment of a residual shunt after surgical repair of an APW with a muscular ventricular septal occluder. In our experience, one critical requirement in choosing an appropriate closure device is that the candidate device should match the shape and margin of the defect. In this case, we chose the SVSDO device for closure, and this is the first report of an APW closure case with this device. The SVSDO device, whose connecting waist was 3 mm long and both ventricular disks were 10 mm long, has been commonly used for treating membranous VSD. In our case, the APW was 2 mm in longitudinal length and 5 mm in diameter, as measured by computed tomography, making the SVSDO device an ideal candidate as the waist length was 3 mm. On the contrary, the waist length of the muscle VSD occluder was 5 mm and that of the PDA occluder was 7 mm, both of which were too long for this case. More importantly, the breadth of the edge of the SVSDO device was an optimal distance between the coronary ostia and aortic valve, which lowered the risk of embolization postoperatively.
In conclusion, a correct diagnosis of an APW can be challenging and may require multiple imaging examinations, utilizing different techniques []. For treatment, in addition to traditional surgical correction, transcatheter closure of APW with a muscular VSD occluder or a membranous VSD occluder is a feasible choice, depending on the type, location, and size of the APW.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1. Naik GD, Chandra VS, Shenoy A, Isaac BC, Shetty GG, Padmakumar P, Jayranganath M: Transcatheter closure of aortopulmonary window using Amplatzer device. Catheter Cardiovasc Interv 2003;59:402-405.
- 2. Demir IH, Erdem A, Saritas T, Demir F, Erol N, Yucel IK, Aydemir NA, Celebi A: Diagnosis, treatment and outcomes of patients with aortopulmonary window. Balkan Med J 2013;30:191-196.
- 3. Peer SM, Donofrio MT, Gaur L, Sinha P: Tricuspid atresia with aortopulmonary window: challenges in achieving a balanced circulation. Interact Cardiovasc Thorac Surg 2013;17:441-443.
- 4. Noonan PM, Desai T, Degiovanni JV: Closure of an aortopulmonary window using the Amplatzer Duct Occluder II. Pediatr Cardiol 2013;34:712-714.
- 5. Stamato T, Benson LN, Smallhorn JF, Freedom RM: Transcatheter closure of an aortopulmonary window with a modified double umbrella occluder system. Cathet Cardiovasc Diagn 1995;35:165-167.
- 6. Kumar A, Singh DK, Gupta VK: Aortopulmonary window: a rare congenital heart defect. J Clin Diagn Res 2016;10:Pj01-Pj02.
- 7. Bagtharia R, Trivedi KR, Burkhart HM, Williams WG, Freedom RM, Van Arsdell GS, McCrindle BW: Outcomes for patients with an aortopulmonary window, and the impact of associated cardiovascular lesions. Cardiol Young 2004;14:473-480.
- 8. Li X, Zhu D, Feng Y: Transcatheter closure of late-onset residual aortopulmonary septal defect using a muscular ventricular septal occluder. Int Heart J 2014;55:89-91.
- 9. Chattranukulchai P, Satitthummanid S, Puwanant S, Srimahachota S, Singhatanadgige S, Boonyaratavej S: Undetected large aortopulmonary window in an adult: a confluence of great vessels. J Am Coll Cardiol 2013;62:e439.
- 10. Wong J, Mathur S, Giese D, Pushparajah K, Schaeffter T, Razavi R, Greil GF: Analysis of aortopulmonary window using cardiac magnetic resonance imaging. Circulation 2012;126:e228-e229.
