Introduction
Myocardial infarction (MI) is universally managed according to the ST segment changes in electrocardiography (ECG). Rapid revascularization should be performed when ST elevation in precordial derivations is detected in an acute anterior MI. Concomitant ST segment changes in inferior derivations are detected in almost half of the patients with acute anterior MI []. Classic ECG theory is based on the idea that the amplitude of remote ST segment depression is proportional to the amplitude of ST segment elevation from the facing leads [,], while the discovery of ST segment changes in reciprocal derivations is considered a milestone in research literature [,,]. Some researchers, for instance, have posited that ST segment elevation in inferior derivations is secondary to transmural infarctions caused by occlusion of the wrapped left anterior descending artery (LAD), whereas many others have put forward hypotheses to explain the pathophysiology of reciprocal ST segment depression in inferior derivations.
Little et al. [] reported that reciprocal alterations are the underlying cause of ST segment depression in acute MI. However, in another study, Schuster and Bulkley [] asserted that ST segment depression is secondary to ischemia of those adjacent areas due to a lack of collateral blood flow from the occluded artery. The most well-accepted theory is explained by Sasaki et al. [], who defended the claim that ST segment depression in inferior derivations in acute anterior MI is secondary to the concomitant effect of benign electrical alterations and adjacent myocardial ischemia. It seems clear, therefore, that ST segment changes occur as a result of factors that cause ST elevation or depression.
Since admission ST segment changes in inferior derivations in acute anterior MI occur secondary to a combination of benign electrical phenomena and myocardial ischemia, we attempted to investigate the prognostic effect of these ST segment changes. The aim of this prospective study was to compare the outcomes among first acute anterior MI patients who have ST segment depression, no ST segment change, or ST segment elevation in inferior derivations.
Materials and Methods
Patients and Study Design
Between January 2015 and February 2016, three hundred eighty-six consecutive patients of all ages with a first acute anterior MI with a symptom duration of <12 h and who underwent primary percutaneous coronary intervention (PCI) were prospectively enrolled into this study. Patients admitted to the emergency department of a tertiary heart center in Istanbul, Turkey, were included in this study if they had persistent ischemic chest pain with ST segment elevation in precordial derivations. ST segment elevation MI was diagnosed according to guidelines, in which it is defined as elevation in 2 contiguous leads, and it is ≥0.25 mV in men below the age of 40 years, ≥0.2 mV in men over the age of 40 years, and ≥0.15 mV in women in leads V2-V3 and/or ≥0.1 mV in other leads []. Excluded patients were those who underwent urgent angiography for ST segment elevation MI other than acute anterior MI, non-ST segment elevation MI, or unstable angina pectoris. Patients with confounding features on their ECG such as a paced rhythm and poor quality and those who underwent urgent coronary artery bypass surgery (CABG) in their hospitalization period or received thrombolysis before primary PCI were also excluded to minimize bias. Among the 386 prospectively enrolled patients, those with a previous MI or PCI (n = 23), those who underwent urgent CABG (n = 6), and those with a paced rhythm or poor-quality ECG (n = 3) were excluded.
The 354 patients who were enrolled into this study had no known coronary artery disease, no previous MI, and no PCI admission to an emergency department with acute anterior MI treated with primary PCI. Among the 354 patients, 206 had ST segment depression in inferior derivations, 108 had no ST change, and 40 had ST elevation in inferior derivations. Our cardiology team informed patients about this study, and their informed consent was obtained. The Local Ethical Committee of our hospital approved the study protocol.
Baseline demographic characteristics and related clinical information were obtained from each patient at the time of their emergency department admission. Before the primary PCI, transthoracic echocardiography was administered to the participants using a Vivid 7 system (GE Vingmed Ultrasound AS, Horten, Norway). An expert on cardiovascular imaging conducted the transthoracic echocardiography. For each patient, the left ventricular ejection fraction (LVEF) was calculated using the Simpson method [], and the pulmonary arterial peak systolic pressure was calculated using the simplified Bernoulli equation [].
Blood values obtained from venous blood samples at hospital admission were recorded. White blood cells, hemoglobin levels, and neutrophil counts were measured as part of the automated complete blood count using a Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland Inc., Galway, Ireland). Biochemical measurements were taken using Siemens Healthcare Diagnostic Products kits and calibrators (Marburg, Germany). Creatinine kinase isoenzyme-MB levels were measured using an immune-inhibition method (Architect C 8000; Abbott Inc.).
A standard 12-lead ECG (Cardiovit AT-10 plus; Schiller; filter: 150 Hz, 25 mm/s, 10 mm/mV) was obtained from all patients prior to primary PCI. ECG were scanned at 300 DPI, and images were amplified (×10). Precordial (V1-V6) R and Q wave amplitudes were measured manually using a caliper on an individual basis. The first negative deflection of a QRS complex was accepted as a Q wave, regardless of its pathologic formation. QRS duration was calculated using the same program. ST segment elevation and depression were measured at the J point using the same caliper. An angiography was performed using nonionic (Omnipaque 300 [ioheksol]) contrast dye in all patients. The type of antiplatelet agent added to the asetylsalicylic acid was left to the interventional cardiologist; clopidogrel, prasugrel, and ticagrelor were the available agents at the hospital. The duration and pressure of balloon inflation, the number of inflations, and the choice of interventional equipment, including balloons and stents, were left to the discretion of the interventional cardiologist performing the procedure. A trained study coordinator evaluated in-hospital events.
The study population was divided into 3 groups according to their admission ST segment change in inferior derivations: ST depression (group 1), no ST change (group 2), and ST elevation (group 3).
Definitions
In-hospital mortality was the primary end point. Cardiogenic shock, in-hospital target vessel revascularization, stent thrombosis, recurrent MI, and major adverse cardiac events (MACE) were also noted. In-hospital mortality was defined as death from any cause during hospitalization. Cardiogenic shock was defined as a systolic pressure less than 90 mm Hg or a systolic pressure drop greater than or equal to 40 mm Hg for more than 15 min without new-onset arrhythmia, hypovolemia, or sepsis. MACE included death, recurrent MI, stent thrombosis, and target vessel revascularization. Hypertension (HTN) was defined as a systolic pressure greater than 140 mm Hg or a diastolic pressure greater than 90 mm Hg or previously diagnosed HTN. Diabetes mellitus (DM) was defined as use of insulin or antidiabetic agents in the patient's medical history or a fasting glucose level greater than 126 mg/dL. Hyperlipidemia was defined as serum total cholesterol ≥240 mg/dL, serum triglycerides ≥200 mg/dL, low-density lipoprotein cholesterol ≥130 mg/dL, or previously diagnosed hyperlipidemia. A wrapped LAD denoted am LAD observed on a postperfusion coronary angiogram that perfused at least one fourth of the inferior wall of the left ventricle in the right anterior oblique projection.
Follow-Up
All follow-up data were obtained during the patients' hospitalization period. The primary end points were in-hospital and long-term (18 months) mortality. In-hospital cardiogenic shock, recurrent MI, stent thrombosis, target lesion revascularization, and MACE were also separately evaluated. Mortality evaluation was obtained from the hospital's database or by follow-up interviews (directly or by telephone).
Statistical Analysis
In the first step, the study population was divided into 3 groups according to their admission ST segment change in inferior derivations as follows: group 1 (ST depression, n = 206), group 2 (no ST change, n = 108), and group 3 (ST elevation, n = 40). In the second step, baseline characteristics were compared among the 3 groups. Quantitative variables are expressed as means ± SD. The Kolmogorov-Smirnov test was used for normality testing. All continuous variables showed skewed distributions and were compared using the Kruskal-Wallis test. Categorical variables are expressed as numbers and percentages, and Pearson's χ2 or Fisher's exact test was used to evaluate the differences. Logistic regression models were used for mortality and MACE by total ST segment change in inferior derivations. Two logistic regression multivariable models were used: model 1 (unadjusted) and model 2 (adjusted). The variables' covariates in model 2 were: demographics (age and sex); BMI; HTN; DM; hyperlipidemia; current smoking; chronic renal failure; onset-to-door time; door-to-balloon time; the first measurement of systolic blood pressure and heart rate; the first measurement during hospitalization of the following laboratory values: admission blood urea nitrogen, white blood cell count, hemoglobin, CRP, and glucose; creatine kinase-MB; troponin I; medication (type of antiplatelet agent); ejection fraction; and RV S' velocity. A 2-tailed p value <0.05 was considered statistically significant, and 95% CI were presented for all OR and HR. Analyses were performed using Statistical Package for Social Sciences software, version 20.0 (SPSS; IBM, Armonk, NY, USA). After a follow-up period of 19.6 ± 4.0 months, the median survival times of the 3 groups were compared using the Kaplan-Meier survival method. Overall survival was calculated from the day of diagnosis to the day of death or last follow-up. Patients lost to follow-up were censored at the time of the last follow-up. Differences between the groups were analyzed using the log-rank test.
Results
Baseline Characteristics and Angiography, Laboratory, and Echocardiography Findings
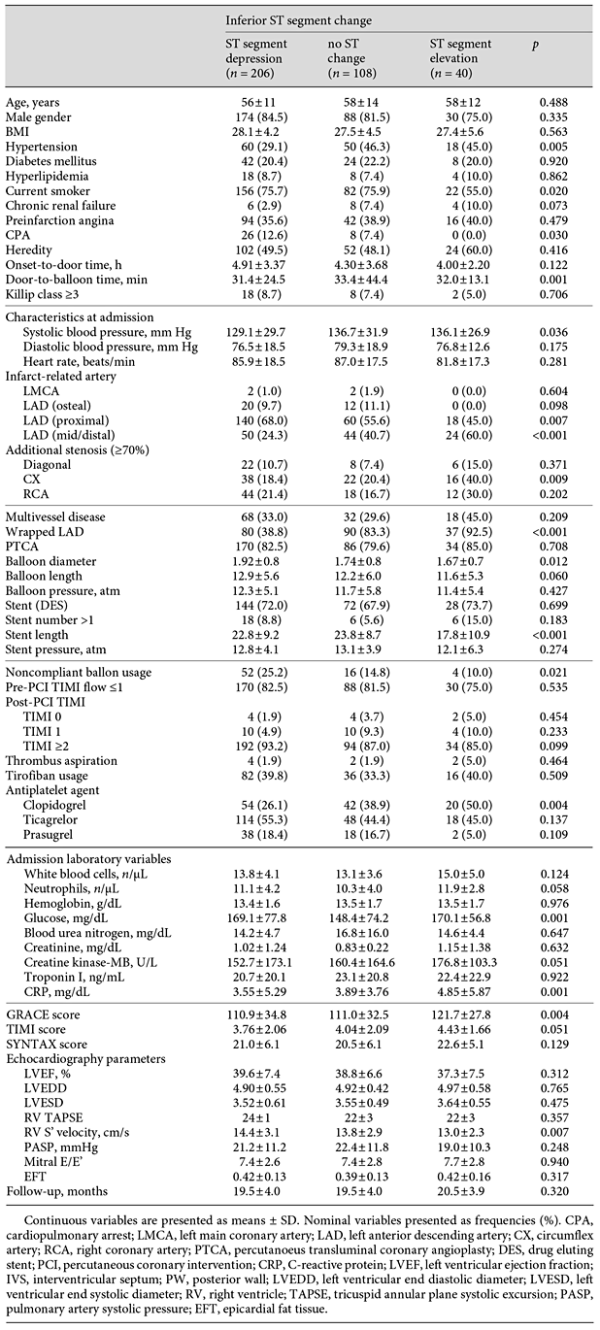
Baseline characteristics, categorized by admission totals of ST segment change in inferior derivations, are listed in Table 1. A total of 354 patients (mean age 57.6 ± 12.9 years, males: 82.5%) with a first acute anterior MI participated in this study. The patients in group 1 had notably lower incidences of HTN than those in the other groups (p = 0.005). The 3 groups were similar in age range, gender, BMI, and the presence of DM, hyperlipidemia, preinfarction angina, and chronic renal failure. The door-to-balloon time was remarkably higher in group 2 (p = 0.001), and the systolic blood pressure was notably lower in group 1 (p = 0.036). Diastolic blood pressure and heart rate did not differ between groups. In considering the culprit artery, LAD proximal occlusion was more frequent in group 1 (p = 0.007), and LAD mid/distal occlusion was found to be significantly higher in group 3 (p < 0.001). Additional CX stenosis was detected more frequently in group 3 (p = 0.009), but multivessel disease did not differ among the 3 groups. Moreover, Pre-PCI TIMI ≤1 did not differ between groups, although post-PCI TIMI 1 flow was detected more frequently in group 1 (p = 0.025). Admission white blood cell and neutrophil counts did not differ among the 3 groups (p = 0.124 and p = 0.058, respectively); however, CKMB, glucose, and CRP serum levels were statistically higher in group 3 (p = 0.051, p = 0.001, and p = 0.001, respectively). LVEF was statistically similar among the 3 groups (p = 0.312), and GRACE, TIMI, and SYNTAX scores were all higher in group 3 (p = 0.004, p = 0.051, and p = 0.129, respectively).
ECG Findings
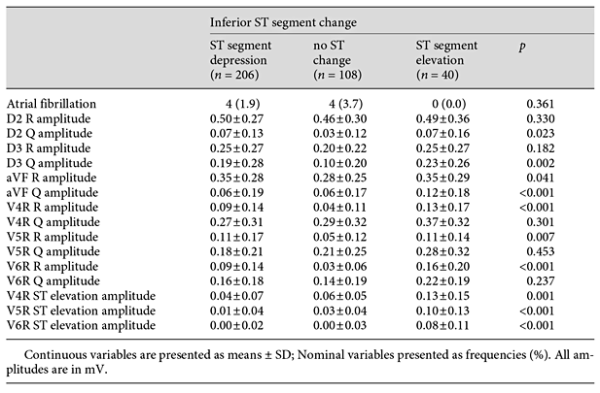
Table 2 summarizes the ECG findings. Atrial fibrillation at admission did not differ between groups, although D2, D3, and aVF Q amplitudes in ECG were remarkably higher in group 3 (p = 0.023, p < 0.002, and p < 0.001, respectively). V4R, V5R, and V6R R amplitudes in ECG were also remarkably higher in group 3 (p < 0.001, p = 0.007, and p < 0.001, respectively), as were the V4R, V5R, and V6R ST elevations in ECG (p = 0.001, p < 0.001, and p < 0.001, respectively).
Outcomes
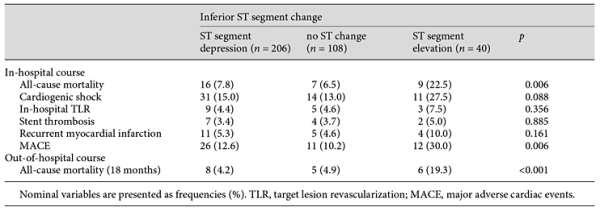
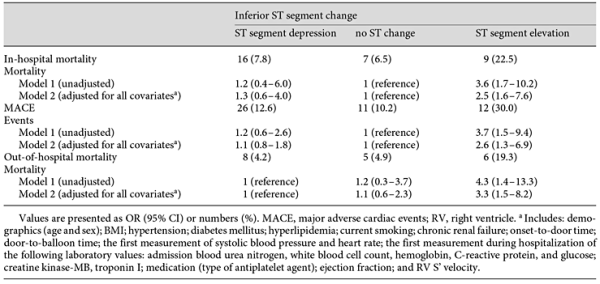
Table 3 presents the patient outcomes according to their admission ST segment change in inferior derivations. All-cause mortality and MACE were found to be higher in group 3 (p = 0.006 and p = 0.006, respectively). Table 4 lists both unadjusted and adjusted logistic regressions for in-hospital events (mortality and MACE) by group. The in-hospital mortality rates were 3.6 times higher in group 3 (95% CI 1.7-10.2) than in group 1, and MACE was 3.7 times higher in group 3 (95% CI 1.5-9.4) than in group 1, which was used as the reference for both outcomes. The patients were followed up for a mean period of 19.6 ± 4.0 months. The 18-month Kaplan-Meier overall survival rates were 88.3, 88, and 62.5%, respectively. The Kaplan-Meier cumulative survival curve is shown in Figure 1.
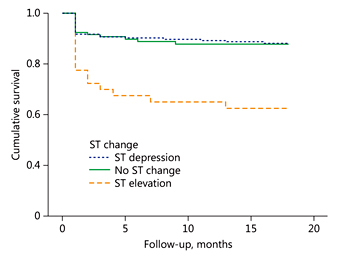
Fig. 1
Kaplan-Meier cumulative survival curve of the ST elevation, no ST change, and ST depression groups.
Discussion
Our study presents outcome comparisons of ST segment depression, no ST change, and ST segment elevation in inferior derivations that were detected in the admission ECG of patients with a first acute anterior MI and who were treated with primary PCI. As a result of these comparisons, ST segment elevation in inferior derivations was found to be an independent prognostic factor for in-hospital mortality, MACE, and out-of-hospital mortality.
Reciprocal ST segment changes in inferior derivations have been at the center of investigations since Ferguson et al. [] presented ST segment depression as a benign electrical phenomenon. Despite studies defending only electrical phenomena, a recent study using magnetic resonance imaging (MRI) demonstrated that patients with ST segment depression have a larger mass of salvaged myocardium and a larger mass of ischemic myocardium []. Sasaki et al. [] combined the 2 well-accepted theories, which include benign electrical phenomenon and ischemia at a distance. These 2 separate mechanisms are responsible not only for ST segment depression in inferior derivations but also for all ST segment changes in inferior derivations.
However, a benign electrical phenomenon is insufficient, by itself, to explain inferior ST segment elevation in acute anterior MI. Interruption of the collateral flow from an occluded LAD or a direct occlusion wrapped LAD is the underlying reason for ST segment elevation. Because the wrapped LAD is directly responsible for critical ischemia of larger myocardial tissue, it has also been reported to be a useful predictor of adverse clinical outcomes in patients with acute anterior MI []. Thus, by combining these considerations, the prognostic effect of inferior ST segment changes in acute anterior MI, which has not been tested before, is now an object of curiosity.
ST segment elevation in inferior derivations was found to be secondary to transmural infarct in some parts of the inferior wall of the heart, which clarified the higher MACE and mortality in this group. The ST segment depression group was expected to have worse outcomes than the ST segment elevation group. However, the groups with ST segment depression and no ST change in inferior derivations displayed similar outcomes, which were both better than that of the ST elevation group. Even though a high incidence of proximal LAD occlusions was identified as the culprit artery in the ST depression group and the no ST change group, inflammation markers such as CRP and neutrophils were found to be higher in the ST elevation group. CRP and neutrophils were associated with adverse clinical outcomes in patients with MI, which clarifies the lower LVEF and worse clinical outcomes in the ST segment elevation group []. Despite statistical insignificance, the LVEF was lower in group 3 than in the other groups, which explains the larger mass of ischemic myocardium.
ST segment depression was evaluated in the literature for its association with multivessel disease. Piérard et al. [] found no notable relation between reciprocal ST depression and multivessel disease. On the other hand, Kouvaras et al. [] and Kürüm et al. [] demonstrated an association between reciprocal ST segment depression and multivessel disease. In our study, there was not a notable relation between ST segment changes and multivessel disease or additional RCA stenosis. Despite statistical insignificance, multivessel disease and RCA stenosis were more frequent in the ST segment elevation group. Q waves are strongly connected with myocardial contractile function [], and their amplitudes in inferior derivations were all higher in the ST segment elevation group, which had the lowest LVEF. The Q wave amplitudes in V4-6R derivations were also higher in group 3, as were the additional RCA stenosis and wrapped LAD. The higher amplitudes of Q waves were considered to be secondary to a larger mass of ischemic myocardium in the ST elevation group. Additionally, V4-6R ST elevation amplitudes were also higher in group 3, which was also considered to be the reason for the statistically lower RV S' velocity in group 3. The impaired right ventricular contractile function was thought to be one of the underlying reasons for the highest observed mortality rate in the ST elevation group.
GRACE, TIMI, and SYNTAX scores are well-accepted clinical and anatomical scores whose predictive value for in-hospital and long-term outcomes for patients with coronary artery disease has been demonstrated in numerous studies [,,]. In our study, those valuable scores were all higher, respectively, in the patients who had ST segment elevation in inferior derivations. The worst prognostic effect of ST segment elevation in inferior derivations was also proven by its close association with those risk scores.
Study Limitations
The current study has several limitations. First, this was a single-center, observational study; however, it was conducted in a high-volume, interventional center for primary PCI, and all consecutive patients who met the criteria were included, thus limiting the selection bias. Some of the patients who had previous MI, PCI, or CABG were excluded because their ECG may have been affected before the reference event.
Conclusion
Our study indicates that ST segment elevation in inferior derivations is an independent prognostic factor for in-hospital mortality, MACE, and out-of-hospital mortality in patients with first acute anterior MI, compared to ST depression and no ST change in inferior derivations. This result confirms that, in acute anterior MI, ST segment elevation in inferior derivations has the worst outcome.
Statement of Ethics
This article does not contain any studies in human participants or animals performed by any of the authors.
Conflict of Interest
The authors have no conflicts of interests to disclose.
References
- 1. Sasaki K, Yotsukura M, Sakata K, Yoshino H, Ishikawa K: Relation of ST-segment changes in inferior leads during anterior wall acute myocardial infarction to length and occlusion site of the left anterior descending coronary artery. Am J Cardiol 2001;87:1340-1345.
- 2. Marriott JHL: Myocardial infarction; in Marriott JHL (ed): Practical Electrocardiography. Baltimore, Williams & Wilkins, 1972, pp 226-253.
- 3. Goldberger AL: S-T segment depressions: ischemic causes; in Goldberger AL (ed): Myocardial Infarction: Electrocardiographic Differential Diagnosis. St Louis, Mosby, 1979, pp 158-167.
- 4. Little WC, Rogers EW, Sodums MT: Mechanism of anterior ST-segment depression during acute inferior myocardial infarction: observations during coronary thrombolysis. Ann Intern Med 1984;100:226-229.
- 5. Schuster EH, Bulkley BH: Ischemia at a distance after acute myocardial infarction: a cause of early postinfarction angina. Circulation 1980;62:509-515.
- 6. Thygesen K, Alpert JS, White HD: Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-2538.
- 7. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358-367.
- 8. Oh JK, Hagler DJ, Cabalka A, Reeder GS, Cetta F Jr, Seward JB: Transesophageal and intracardiac echocardiography; in Oh JK, Seward JB, Tajik AJ (eds): The Echomanual, ed 3. Philadelphia, Lippincott Williams & Wilkins; 2006, pp 29-58.
- 9. Ferguson DW, Pandian N, Kioschos JM, Marcus ML, White CW: Angiographic evidence that reciprocal ST-segment depression during acute myocardial infarction does not indicate remote ischemia (analysis of 23 patients). Am J Cardiol 1984;53:55-62.
- 10. Kidambi A, Mather AN, Uddin A, Motwani M, Ripley DP, Herzog BA, McDiarmid A, Gunn J, Plein S, Greenwood JP: Reciprocal ECG change in reperfused ST-elevation myocardial infarction is associated with myocardial salvage and area at risk assessed by cardiovascular magnetic resonance. Heart 2013;99:1658-1662.
- 11. Kobayashi N, Maehara A, Brener SJ, Généreux P, Witzenbichler B, Guagliumi G, Peruga JZ, Mehran R, Mintz GS, Stone GW: Usefulness of the left anterior descending coronary artery wrapping around the left ventricular apex to predict adverse clinical outcomes in patients with anterior wall ST-segment elevation myocardial infarction (from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol 2015;116:1658-1665.
- 12. Lim P, Moutereau S, Simon T, Gallet R, Probst V, Ferrieres J, Gueret P, Danchin N: Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am J Cardiol 2013;111:31-37.
- 13. Piérard LA, Sprynger M, Gilis F, Carlier J: Significance of precordial ST-segment depression in inferior acute myocardial infarction as determined by echocardiography. Am J Cardiol 1986;57:82-85.
- 14. Kouvaras G, Spyropoulou M, Bacoulas G: The significance of a persistent precordial ST segment greater than or equal to 0.1 mV depression in acute inferior myocardial infarction (coronary angiographic and ventriculographic findings). Angiology 1986;37:57-62.
- 15. Kürüm T, Oztekin E, Ozçelik F, Eker H, Türe M, Ozbay G: Predictive value of admission electrocardiogram for multivessel disease in acute anterior and anterior-inferior myocardial infarction. Ann Noninvasive Electrocardiol 2002;7:369-373.
- 16. Phibbs B, Marcus F, Marriott HJ, Moss A, Spodick DH: Q-wave versus non-Q wave myocardial infarction: a meaningless distinction. J Am Coll Cardiol 1999;33:576-582.
- 17. GRACE Investigators: Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J 2001;141:190-199.
- 18. Morrow DA, Antman EM, Parsons L, de Lemos JA, Cannon CP, Giugliano RP, McCabe CH, Barron HV, Braunwald E: Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA 2001;286:1356-1359.
- 19. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW: The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219-227.