Introduction
The left atrium (LA) functions as a conduit for pulmonary venous return during early ventricular diastole and plays an important role in left ventricular filling []. LA size is known to be a marker for cardiovascular risk: large LA have been linked to mortality in association with left heart diseases [,]. The differential diagnosis for the etiologies of a small LA, however, is limited and includes acute and chronic pulmonary embolism (PE) [,] and chronic obstructive pulmonary disease (COPD) []. We have previously adopted a technology of 4-chamber volumetric analysis (4CVA) [,] in order to explore the cardiac chambers' size as assessed by computed tomography pulmonary angiography (CTPA) in patients with acute PE. Utilizing this software we found that among patients with acute PE a decreased LA volume was associated with an increased 30-day mortality []. Since the LA is the conduit to the left ventricle (LV), and underfilling of the LV may result in hypotensive shock, we hypothesized that a small LA in patients without PE may also be a predictor of mortality.
Methods
Study Design and Setting
We conducted a historical cohort study of all patients admitted between 2011 and 2015 to a large tertiary academic hospital serving an urban population of approximately 500,000. This study was reviewed and approved by the Institutional Review Board, with a waiver of informed consent.
Participants
This study included patients aged 18 years and older who underwent nongated CT pulmonary angiography (CTPA) as part of an investigation for suspected PE and had an echocardiogram available within 24 h of the CTPA. Patients diagnosed with PE based on their CTPA scan were excluded from this study.
CT Acquisition
All patients were scanned by a multidetector CT scanner (Mx8000 IDT or Brilliance-64, or iCT-256; Philips Medical Systems) with 16, 64, or 128 detector rows. The reconstructed slice thickness was 1-2 mm with an increment of 0.5-1 mm. Scans were acquired according to our routine nonelectrocardiographic-gated protocol with contrast injections of 70-100 mL of iodinated contrast material at a concentration of 300 mg iodine/mL (Ultravist; Schering) and at rates of 3-4 mL/s. To optimize visualization of the pulmonary arteries, an automated bolus-tracking technique was used with a region of interest placed within the main pulmonary artery. Five seconds after reaching a threshold of 100 Hounsfield units at the region of interest, scanning began covering the chest from the lung bases to the thoracic inlet. All scans were obtained in a cranio-caudal direction during a single breath hold.
Volumetric Analysis of the Cardiac Chambers
An automated volumetric measurement of the LA was obtained using fully automatic software (Comprehensive Cardiac Analysis, Extended Brilliance Workspace, Version 4.5, or Pulmonary Arterial Analysis, IntelliSpace, Portal Version 6; Philips Healthcare, Best, The Netherlands). The software adapts an anatomical model of the heart chambers to the CT image volume [,,]. The output consists of a 3-dimensional (3D) graphic display of the heart segmented into its main structures. The volume of the LA was automatically calculated as the product of a single voxel volume and the sum of all of the voxels included in it. The software allows the relevant segmentation structure to be color coded and viewed simultaneously in both 3D and 2D superimposed on the reference image in axial, coronal, sagittal, or cardiac views (short axis, vertical long axis, horizontal long axis). Each structure was inspected visually on the reference images for conformity to the imaged cardiac anatomy in order to validate the correctness of the segmentation. Figure 1 demonstrates cases of the volumetric model of the 4 cardiac chambers based on CT pulmonary angiography. In the event that the automatic segmentation was visually assessed as incorrect, the patient's data were excluded from this study. Patients included were only those with a fully available automatic segmentation (all cardiac chambers).
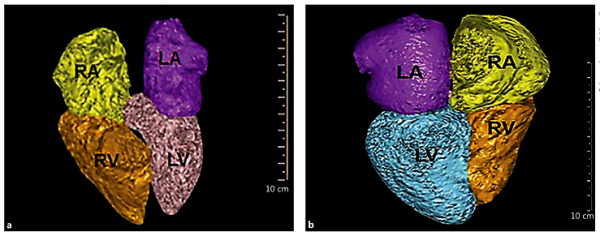
Fig. 1
Representative cases of the volumetric model of the 4 cardiac chambers based on CT pulmonary angiography. a Fifty-nine-year-old female with a very small LA (20.1 mL/m2) who died within 11 days of the CT. The patient was admitted due to sepsis of an undetermined source after a recent removal of a brain tumor. b Fifty-nine-year-old woman with a normal LA volume (LA volume/body surface area 57.04 mL/m2). The patient was admitted due to COPD exacerbation and discharged 24 h after her CT ruled out pulmonary emboli. LA, left atrium (purple); LV, left ventricle (light blue); RA, right atrium (yellow); RV, right ventricle (orange).
Echocardiography
Echocardiography was performed within 1 day of the CTPA in all patients. Echocardiography was performed in a standard manner using the same equipment (iE33; Philips Medical Systems, Bothell, WA, USA). LA volume was calculated using the biplane area length method at end systole []. All volumetric measurements were divided by the body surface area (BSA) and reported as milliliter/square meter. We defined patients with a very small LA (VSLA) as those with an LA volume/BSA value in the lowest 5th percentile.
The CTPA studies of the 11 patients with a VSLA were reviewed again separately by a fellowship-trained cardiothoracic radiologist (D.C.) in order to qualitatively assess the possible etiology of the small LA. The review was done blinded to the clinical outcome and clinical background of the patients. In addition, their echocardiographic studies were reviewed and analyzed for the etiology of the small LA by a cardiologist with a specialization in advanced echocardiography and heart failure (Y.T.). This review was done blinded to the clinical outcome and clinical background of the patients as well. The echocardiographic evaluation for the etiology of the VSLA of the 11 patients with the VSLA included transmitral E/e for LA pressure [] and inferior vena cava size and collapsibility for right atrial pressure [,]. LV and right ventricle (RV) chamber sizes, areas, and volumes were evaluated for intravascular volume status and function [], and the LV output tract was observed for stroke distance in order to assess the intravascular volume status []. Stroke volume was calculated as: cross-sectional area × velocity-time integral []. In order to evaluate right-sided hemodynamics pulmonary artery systolic pressure, TAPSE, and RV fractional area change were assessed [,]. In addition, a general overview was taken into consideration when screening for space-occupying lesions.
Variables, Data Source, and Measurement
Age, gender, major comorbidities (atrial fibrillation, ischemic heart disease, a prior permanent pacemaker, valvulopathies, cerebrovascular accident/transient ischemic attack, lung diseases, chronic kidney disease, malignancy, and mechanical ventilation), laboratory measurements (hemoglobin, creatinine, and estimated glomerular filtration rate [eGFR]), and all-cause mortality were extracted from the electronic health record.
Statistical Methods
Categorical variables are reported as numbers and percentages, and continuous variables are reported as means ± SD or medians (IQR). Continuous variables were tested for a normal distribution using histograms and Q-Q plots. Patients were divided into 2 groups according to their LA volume on the CTPA (lower 5th percentile vs. higher volumes). Continuous variables were compared between groups using an independent samples t test or a Mann-Whitney test and categorical variables were compared using Fisher's exact test. Correlation between continuous variables was evaluated using Spearman's rank correlation coefficient. Univariate cox regression was used to evaluate the crude association between each predictor and mortality. Age, gender, and variables with a significance level of less than 0.2 were included in the multivariate analysis. Multivariate cox regression was used to evaluate the association between the group and mortality after controlling for potential confounders. The survival plot was adjusted to the variables included in the multivariate analysis. The follow-up time was obtained using the reverse censoring method. Two-tailed p values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS (IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
A total of 241 patients were included in this study, and 63.1% of them were female. The median age was 72 years (IQR 54-81), and the median follow-up time for this cohort was 22.7 months (range 0.03-54.3). The correlation between LA volume according to the CTPA and echocardiographic measurements of the LA was 0.634 (p < 0.001).
The median LA volume/BSA according to 4CVA was 43.6 mL/m2 (IQR 33.3-57.2). An LA volume/BSA of 24 mL/m2 was found to be the threshold value for the lower 5th percentile (n = 11), and thus patients with an LA volume/BSA below 24 mL/m2 were regarded as having a VSLA. Table 1 presents the comparison of the characteristics of patients with VSLA (lowest 5th percentile) and all other patients. There was no statistical difference in baseline characteristics.
Expert Analysis of the Imaging Modalities
The expert interpretation of the main etiology of VSLA is shown in Table 2. According to analysis of patients' echocardiograms, 6 individuals had findings compatible with severe dehydration causing a low stroke volume, 2 had a right heart failure, and 3 had a space-occupying lesion which compressed the LA; of them, 1 also had a cardiac tamponade. Similar to the echocardiogram, the expert radiologist analysis of the CTPA revealed space-occupying lesions in 3 patients, 1 of whom had a pericardial effusion as well. No definite etiology for the small LA was demonstrated in the CTPA of the other 8 patients with a VSLA.
VSLA and Mortality
Among the 11 patients with a VSLA (volume/BSA <24 mL/m2) according to 4CVA, 6 (54.5%) patients died during the median follow-up of 22.5 months (IQR 0.03-31.3), while 53/230 (23.0%) patients with a higher LA volume died (median follow-up: 22.7 months, IQR 0.08-54.3). Patients with VSLA had an HR of 3.208 (95% CI 1.375-7.484; p = 0.007) for mortality in a univariate analysis. Other parameters associated with a higher mortality in the univariate analysis were: older age, valvulopathy, malignancy, lower hemoglobin concentrations, and a decreased eGFR (Table 3). In a multivariate analysis (adjusted for age, hypertension, valvulopathy, malignancy, hemoglobin, and eGFR), VSLA (HRadj = 3.6; 95% CI 1.46-8.87; p = 0.005), malignancy (HRadj = 2.28; 95% CI 1.32-3.93; p = 0.003), and lower hemoglobin concentrations (HRadj = 0.86; 95% CI 0.76-0.99; p = 0.032) were associated with a higher mortality (Table 3). Figure 2 demonstrates the comparison of the adjusted survival curve over the follow-up period between patients with an LA volume/BSA <24 mL/m2 versus higher LA volumes.
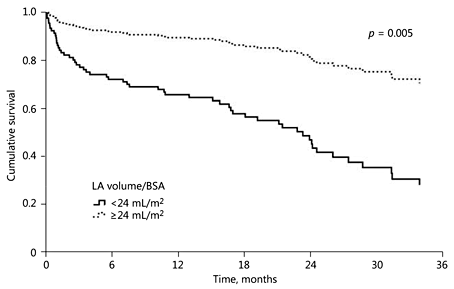
Fig. 2
Survival curve comparing very low LA volumes (LA/BSA <24 mL/m2) to higher LA volumes (LA/BSA ≥24 mL/m2) after adjustment for baseline characteristics. BSA, body surface area; LA, left atrium.
Discussion
Our data indicate that a VSLA volume on nongated CTPA is a significant predictor of mortality in patients without PE. Automated volumetric measurements, which are easily obtained in patients undergoing nongated CTPA, may thus serve as an important cautionary sign among patients with VSLA. This is the first study to explore the additional differential diagnoses of a small LA other than PE and COPD.
Filling of the LA is dependent on the pulmonary circulation. In instances such as PE, the pulmonary circulation is compromised and the LA volume may be decreased [,]. Underfilling of the LA has also been shown in patients with COPD []. Moreover, it was previously demonstrated via magnetic resonance angiography that the pulmonic vein area of patients with COPD is smaller, suggesting underfilling of their LA []. Proposed mechanisms for decreased left side filling in patients with COPD include an altered intrathoracic pressure, increased pulmonary veins resistance, compression of alveolar vessels [], increased heart rate [], systemic vasodilation [], and bulging of the septum due to right heart dilatation [,]. Investigating the etiologies of small LA size on CT, Cassagnes et al. [] reported that patients with emphysema had an inverse correlation between LA volume and the severity of emphysema. In a different study, patients with COPD were shown to have decreased LA filling as assessed by echocardiography, although absolute LA volumes were not significantly different [].
Previous available data concerning LA size as a prognostic marker focused mostly on large atrial volumes. In a recent review regarding LA size and function, only left heart side pathologies were regarded []. In our cohort individuals with a VSLA, that is to say LA indexed to BSA below 24 mL/m2, had a 3.6 times higher chance of mortality compared to patients with higher LA volumes. Moreover, almost a fifth of the patients with a VSLA died within 30 days. A VSLA, therefore, may serve as an important marker for deteriorated health.
Oncologic patients are known to suffer from dehydration []. Indeed, most of the individuals included in this group were either dehydrated or had a compressed LA due to a tumor. Nevertheless, in our cohort there was no statistical difference in the prevalence of malignancy between the groups. Thus, the finding of a VSLA does not represent a single etiology.
Although it has been suggested that the LA should be primarily assessed echocardiographically [], echocardiography might be limited by errors that can arise from foreshortening and the use of geometric assumptions of biplane volume calculations. In addition, technical difficulties such as achieving a convenient acoustic window and the timing of various atrial events are known limitations, which CT and MRI are devoid of [].
The individual analysis of the imaging modalities of patients with a VSLA in our cohort demonstrated some of the underlying pathologies responsible for the decreased LA size. This pathophysiological investigation may have crucial implications if performed in real time. The underlying pathologies - severe dehydration, right heart failure, compression of the LA due to a space-occupying lesion, and cardiac tamponade - may be treatable in certain cases. A rapidly obtained clue to underfilling, blocked pulmonary circulation, or LA compression could affect patient management. It should be noted that assessment of the LA volume using 4CVA required no additional imaging tests or radiation exposure, and it was not time consuming due to the automaticity of the software which provides quantitative results. However, the use of nongated CT is likely associated with some blurring of myocardial borders due to volume averaging from cardiac motion between systole and diastole.
Limitations
The limitations of our study should be regarded. First, this is a small-scale study. Only 11 patients were included in the lower 5th percentile. Indeed, larger scale cohorts are indicated. Second, this study was performed retrospectively. The association of the VSLA with mortality was observational. The real-time hemodynamic status of the patients, as well as data regarding treatments, such as fluid balance and oncological therapy, were not available. A prospective study may better demonstrate the clinical implications of our findings. An advantage of this study is, however, the use of echocardiography which was obtained within 24 h of the CTPA. This tool enabled us to generate a more comprehensive imaging assessment, for these 2 modalities are complementary to one another: CTPA with its large field of view and echocardiography with its detailed real time cardiac anatomic, functional, and hemodynamic evaluation.
To conclude, a new marker for mortality has been regarded for the first time. VSLA, assessed using 4CVA technology, may be suggestive of an increased risk of mortality among patients who undergo nongated CTPA in whom PE was excluded. Volumetric analysis may provide viable and easily obtained information, which may aid in risk stratification. Large-scale trials including real-time clinical data collection are required to further investigate our findings.
Conflict of Interest
Dr. Galit Aviram declares financial activity related to this paper: the author's institution received a research grant from Philips Medical Systems.
None of the other coauthors has conflicts of interests to declare.
References
- 1. Hoit BD: Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493-505.
- 2. Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA: Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or = 65 years of age (the Cardiovascular Health Study). Am J Cardiol 2006;97:83-89.
- 3. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D: Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation 1995;92:835-841.
- 4. Marston NA, Auger WR, Madani MM, Kimura BJ, Strachan GM, Raisinghani AB, DeMaria AN, Blanchard DG: Assessment of left atrial volume before and after pulmonary thromboendarterectomy in chronic thromboembolic pulmonary hypertension. Cardiovasc Ultrasound 2014;12:32.
- 5. Aviram G, Soikher E, Bendet A, Shmueli H, Ziv-Baran T, Amitai Y, Friedensohn L, Berliner S, Meilik A, Topilsky Y: Prediction of mortality in pulmonary embolism based on left atrial volume measured on CT pulmonary angiography. Chest 2016;149:667-675.
- 6. Cassagnes L, Pontana F, Molinari F, Faivre JB, Santangelo T, Algeri E, Duhamel A, Remy J, Remy-Jardin M: Left atrial volume in chronic obstructive pulmonary disease. J Thorac Imaging 2014;29:233-239.
- 7. Aviram G, Shmueli H, Adam SZ, Bendet A, Ziv-Baran T, Steinvil A, Berliner AS, Nesher N, Ben-Gal Y, Topilsky Y: Pulmonary hypertension: a nomogram based on CT pulmonary angiographic data for prediction in patients without pulmonary embolism. Radiology 2015;277:236-246.
- 8. Abadi S, Roguin A, Engel A, Lessick J: Feasibility of automatic assessment of four-chamber cardiac function with MDCT: initial clinical application and validation. Eur J Radiol 2010;74:175-181.
- 9. Ecabert O, Peters J, Schramm H, Lorenz C, von Berg J, Walker MJ, Vembar M, Olszewski ME, Subramanyan K, Lavi G, et al: Automatic model-based segmentation of the heart in CT images. IEEE Trans Med Imaging 2008;27:1189-1201.
- 10. Lorenz C, von Berg J: A comprehensive shape model of the heart. Med Image Anal 2006;10:657-670.
- 11. To AC, Flamm SD, Marwick TH, Klein AL: Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging 2011;4:788-798.
- 12. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-133.
- 13. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB: Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713, quiz 786-788.
- 14. Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT: Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol 2006;1:749-753.
- 15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al: Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14.
- 16. Ristow B, Na B, Ali S, Whooley MA, Schiller NB: Left ventricular outflow tract and pulmonary artery stroke distances independently predict heart failure hospitalization and mortality: the Heart and Soul Study. J Am Soc Echocardiogr 2011;24:565-572.
- 17. Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography: Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167-184.
- 18. Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, Schwab K, Meldrum DR: Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol 2010;56:1435-1446.
- 19. Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M, Sainty JM, Orehek J: Left atrial and ventricular filling in chronic obstructive pulmonary disease: an echocardiographic and Doppler study. Am J Respir Crit Care Med 2000;162:670-675.
- 20. Smith BM, Prince MR, Hoffman EA, Bluemke DA, Liu CY, Rabinowitz D, Hueper K, Parikh MA, Gomes AS, Michos ED, et al: Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest 2013;144:1143-1151.
- 21. Casiglia E, Pavan L, Marcato L, Leopardi M, Pizziol A, Salvador P, Zuin R, Pessina AC: Subjects with obstructive pulmonary disease tend to be chronically vasodilated. Clin Sci (Lond) 1998;95:287-294.
- 22. Jardin F, Gueret P, Prost JF, Farcot JC, Ozier Y, Bourdarias JP: Two-dimensional echocardiographic assessment of left ventricular function in chronic obstructive pulmonary disease. Am Rev Respir Dis 1984;129:135-142.
- 23. Settle HP, Engel PJ, Fowler NO, Allen JM, Vassallo CL, Hackworth JN, Adolph RJ, Eppert DC: Echocardiographic study of the paradoxical arterial pulse in chronic obstructive lung disease. Circulation 1980;62:1297-1307.
- 24. Steiner N, Bruera E: Methods of hydration in palliative care patients. J Palliat Care 1998;14:6-13.