Introduction
The Global Registry of Acute Coronary Events (GRACE) risk score (GRS) predicts the risk of death and myocardial infarction (MI) in patients with acute coronary syndrome (ACS) [-]. The final GRACE model includes only 8 of the most prognostically significant baseline variables to make the score clinically usable. The discriminatory performance of the GRACE model leaves room for improvement. Attempts have been made to improve the model by adding multiple blood biomarkers [-]. These are mostly emerging biomarkers that are not routinely measured. It is mostly a composite of different rather than a single biomarker that has provided incremental value over and above the GRACE score. Therefore, identifying a single biomarker that can be routinely measured not only to predict prognosis but also to improve the GRS would be useful.
A glycaemic matrix is notably absent in the GRACE score even though post-ACS prognosis is worse in patients with known diabetes mellitus (DM). Addition of fasting plasma glucose (FPG) [, ], admission plasma glucose (APG) [-], and glycosylated haemoglobin (HbA1c) [, ] has been variably successful in improving the GRACE model. The GRACE model predicts post-discharge prognosis up 4 years []. We investigate whether FPG and/or 2-h post-load plasma glucose (2h-PG) improves the performance of the GRS in predicting short- to intermediate-term major adverse cardiac events (MACE) in patients with MI but without known DM.
Materials and Methods
The study methods have been described previously []. This analysis includes consecutive post-MI survivors without known DM who underwent a routine pre-discharge oral glucose tolerance test (OGTT) with standard follow-up after discharge. Information on demographics, risk factors for coronary artery disease, past medical history including history of previous MI and revascularisation, prescribed medications, haemodynamic parameters, troponin I, renal function test, Killip class, and presence of ST-segment depression GRS for risk of death or MI from discharge to 6 months were collected from the Myocardial Infarction National Audit Project (MINAP) database. All participants underwent pre-discharge OGTT on/after the third day of admission. Venous plasma glucose was measured after ≥8 h of overnight fast and 2 h after administration of 75 g glucose in 200 mL water. Clinically unstable patients were tested later. We excluded patients who were transferred out of our centre before the OGTT or did not tolerate it. Glucose was measured using the glucose oxidase method. The patients diagnosed with impaired glucose tolerance (IGT) and new DM were referred to the endocrinologist for appropriate management.
MI was defined as per the universal definition []. Patients were labelled “known DM” if they were aware of the diagnosis before admission, had it documented in their medical records, or were on anti-DM treatment. Admission HbA1c was not measured as it was not recommended in the guidelines at the time of data collection [-]. Patients with pre-existing diabetes were excluded. The glucometabolic states were defined as normal glucose tolerance: FPG <6.1 mmol/L and a 2-h PG <7.8 mmol/L; impaired fasting glucose: FPG 6.1–6.9 mmol/L and 2-h PG <7.8 mmol/L; IGT: FPG <7 mmol/L and 2-h PG 7.8–11 mmol/L, and new DM: FPG ≥7.0 and/or 2-h PG ≥11.1 mmol/L.
All participants were followed up for a median of 36 months for outcomes. Hospital and general practice records were reviewed ensuring complete follow-up. The first occurrence of MACE defined as death or non-fatal re-infarction (the only events predicted by GRS) was obtained from the hospital and general practice databases and confirmed by the office of public health intelligence. As routinely collected anonymised data on standard clinical practice were being retrospectively analysed, the East Yorkshire and North Lincolnshire Research Ethics Committee waived the need for formal ethical approval and patient consent [].
Statistics
Continuous variables are presented as medians (interquartile range, IQR) and categorical variables as counts and proportions. The baseline characteristics of the patients grouped as above and below the median 2h-PG (8.1 mmol/L) were compared using the Mann-Whitney test for non-parametric continuous variables and the χ2 test for categorical variables. Event-free survival was compared between groups using the Kaplan-Meier method. The dataset was censored at 6 months, and 1, 2, and 3 years, and analysed using multivariate Cox proportional hazard regression (MedCalc Statistical Software version 17.0.4, Ostend, Belgium). Gender, smoking status, hypercholesterolaemia, hypertension, history of MI and revascularisation, discharge diagnosis and medications, GRS, FPG, and 2h-PG were “entered” into the model. Multicollinearity was tested (MedCalc Statistical Software version 17.0.4), and variables with variance inflation factor <4 were included in the same model. Hazard ratios (HRs) and 95% CIs are reported.
The χ2 likelihood ratio test was used to compare nested models to determine if the logistic regression models including GRS and FPG or 2h-PG provided a significantly better fit than those with GRS alone. Akaike’s information criterion (AIC) was used to estimate the probability that a given nested or non-nested model including GRS and FPG and/or 2h-PG was the “best”-fitting model of those studied.
FPG and 2h-PG were added, individually and in combination, into logistic regression models containing GRS along with other covariates to calculate the predicted probabilities of MACE at each time point. The incremental predictive value of adding 2h-PG to models with FPG was analysed from these predicted probabilities by comparing the area under the receiver-operating characteristic (ROC) curve (AUC) (MedCalc Statistical Software version 17.0.4), using the category-free continuous net reclassification index (NRI>0) and integrated discrimination improvement (IDI). The event and non-event NRI were defined as net percentage of persons with and without the event of interest correctly assigned a higher and lower predicted risk, respectively. The overall NRI is the sum of the event and non-event NRI reported as a number. The IDI was defined as the mean difference in predicted risks between those with and without events.
Results
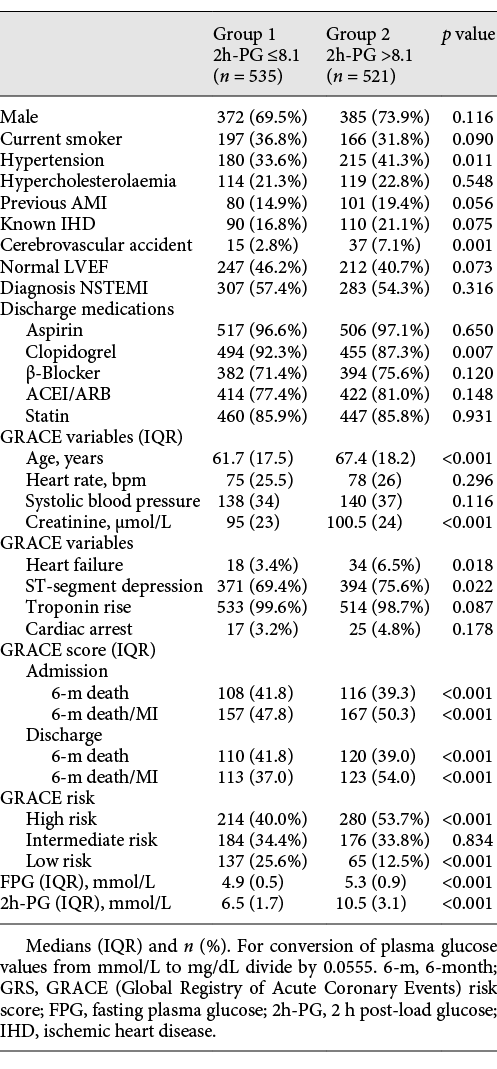
Baseline characteristics of the patients grouped as below or equal to (group 1) and above (group 2) the median 2h-PG (8.1 mmol/L) are shown in Table 1. OGTT was done on day 3 or later in 97.6% of patients; 61.6% of ST-elevationmyocardial infarction (STEMI) and 60.3% of non-STEMI (NSTEMI) patients had glucose measured on day 4 or later after admission. The timing of OGTT was similar in the NSTEMI (median 4.0; IQR 6–3) and STEMI patients (median 4.0; IQR 5–3, p = 0.420). Patients who had their OGTT on or before day 3 had lower FPG (median 5.0; IQR 5.4–4.7 vs. median 5.1; IQR 5.6–4.8, p = 0.002) but similar 2h-PG (median 8.1; IQR 10.4–6.4 vs. median 8.0; IQR 10.5–6.5, p = 0.762) compared to those done later. The patients with STEMI had higher FPG (median 5.1; IQR 5.6–4.8 vs. median 5.05; IQR 5.5–4.7, p = 0.020) but similar 2h-PG (median 8.2; IQR 10.4–6.6 vs. median 8.0; IQR 10.5–6.4, p = 0.323) than the NSTEMI patients.
Outcomes
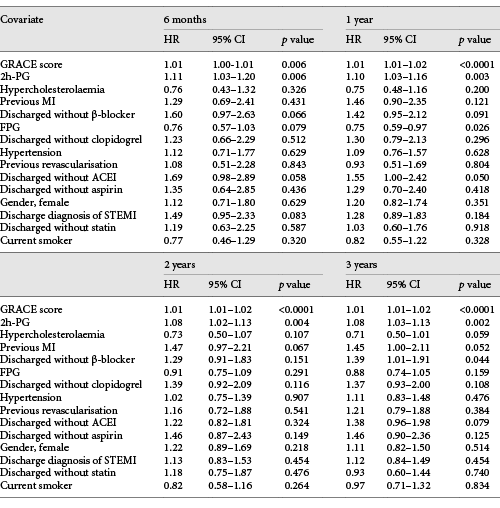
During the median follow-up of 36.5 months, there were 211 MACE (20.0%), 96 deaths (9.1%), and 115 non-fatal re-infarctions (10.9%). Group 2 had higher MACE than group 1 (HR 1.53, 95% CI 1.17–2.01, p = 0.002). The 2h-PG and GRS, but not FPG, independently predicted MACE at all time points (Table 2). The risk of MACE increased by 8–11% at various time points for each 1 mmol/L rise in 2h-PG.
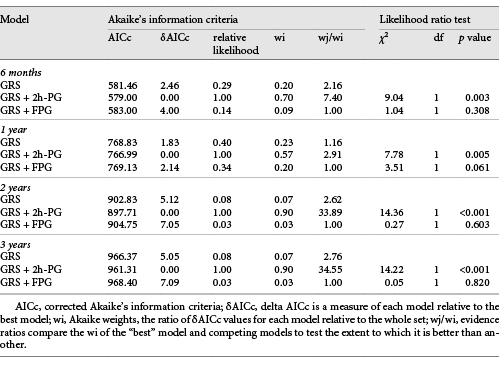
Addition of the 2h-PG, but not FPG, significantly improved the ability of a model including GRS to predict MACE at all time points during follow-up (Table 3). Models containing GRS and 2h-PG yielded the lowest corrected AIC and highest Akaike weight and evidence ratio compared to those with GRS alone and GRS with FPG (Table 3) suggesting that the model with GRS and 2h-PG is the “best”-fitting model compared to the other models tested.
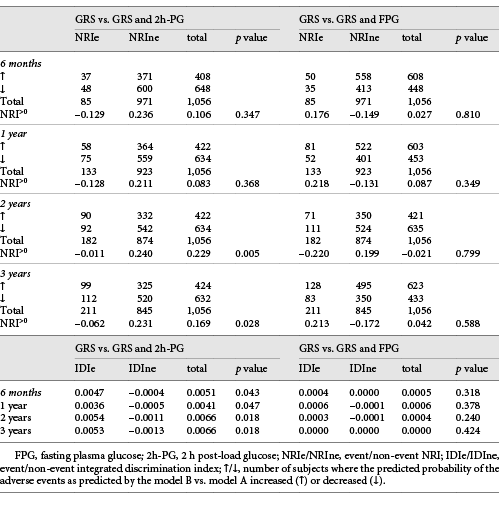
2h-PG, but not FPG, significantly improved the net reclassification of the GRS-containing model alone in predicting events during follow-up (Table 4). 2h-PG significantly improved NRI>0 by 17–23% at 2 and 3 years but not at 6 months and 1 year. Addition of FPG did not change reclassification. Integrated discrimination at all time points was similarly improved by 2h-PG (Table 4). The IDI was 0.41–0.66% at the various time points.
The c-statistic for the model containing GRS only, 2h-PG only, and GRS and 2h-PG were 0.73 (95% CI 0.71–0.76, p < 0.0001), 0.70 (95% CI 0.67–0.73, p < 0.0001), and 0.74 (95% CI 0.71–0.77, p <0.0001) respectively. The AUC for the GRS-only model was better than the 2h-PG-only model (δAUC 0.0320, p = 0.028). Addition of 2h-PG to the GRS-only model did not improve the c-static (δAUC 0.0053, p = 0.295) but did so when GRS was added to the 2h-PG-only model (δAUC 0.0373, p = 0.003).
Discussion/Conclusion
We show that 2h-PG, measured ≥3 days after the event, would improve performance of a model including GRS in predicting post-MI prognosis in the short- to intermediate-term in patients without known DM. GRS accurately predicts post-MI events in the short and long term [, ]. Multiple blood biomarkers have been added to the model to improve its performance [-]. Its performance improves when a composite score created mostly from multiple novel biomarkers that are not routinely measured is added. These approaches are too cumbersome to be used clinically and possibly costly with marginal gains. This study suggests that 2h-PG as single glycaemic matrix added to the GRS would improve its performance.
As the post-MI prognosis in patients with DM is inferior to those without, a glycaemic matrix would be a natural choice as an additional biomarker that could improve predictive performance of models containing GRS. The GRS model does not include DM history or a glycaemic matrix as a variable [, ]. In the GRACE registry DM, as a dichotomous categorical variable, did not independently predict the 6-month post-discharge events []. However, increasing FPG increased the risk of in-hospital mortality irrespective of a history of DM []. In the absence of OGTT, it is uncertain whether the raised FPG or undetected raised 2h-PG either to the IGT or DM range affected the outcomes in these patients. The 6-month post-ACS survival was affected by FPG only if it was above the diagnostic threshold for diabetes [].
FPG, APG, HbA1c, and AGT individually predicts adverse post-ACS prognosis in patients without known DM. The 2h-PG is a better predictor than both APG and FPG []. FPG, APG, and HbA1c, after adjusting for GRS, predict post-MI outcomes in some [, , , , ] but not other [, , , , , ] studies. Attempts at using APG [-], FPG [, ], and HbA1c [] to improve the predictive ability of models containing GRS have yielded conflicting results. This study suggests that in patients without known DM, 2h-PG but not FPG improves the performance of GRS-containing models in predicting post-MI prognosis in the short to medium term. Aronson et al. [] shows that FPG predicts mortality after MI and improves the prognostic models containing GRS. This study is somewhat different from ours. Most (73%) patients in their study had STEMI, FPG was measured in the first 24 h of admission, and post-load glucose was not measured []. In contrast, STEMI was diagnosed in 44% of our patients, and glucose was measured a lot later. As a binary dichotomous variable, troponin in the GRS model does not account for the effect of the degree of myonecrosis on prognosis. FPG is higher when measured within 24 h of MI than later and following STEMI compared to NSTEMI [-]. The effect of FPG on the predictive performance of the model in this study [] may have been exaggerated due to the higher levels of FPG measured early after STEMI combined with GRS unaffected by the higher volume of myonecrosis in STEMI. It is also unclear whether this effect would persist if 2h-PG was included in the models. The morbidity associated with 2h-PG rather than FPG in our study could be due to the progression of atherosclerosis seen after challenge but not with fasting hyperglycaemia [].
In the absence of HbA1c, we are unable to compare the effect of all the glycaemic matrices on prognosis. None of the patients had HbA1c measured as routine screening for undiagnosed diabetes as HbA1c was not recommended in the EASD Guidelines 2007 []. On the contrary, screening for diabetes using a non-invasive risk score and OGTT was recommended. Whether HbA1c would be a useful biomarker to predict post-MI prognosis is debated. HbA1c has been shown to predict post-ACS prognosis in some [, -] but not all studies [-]. The effect of APG, FPG, 2h-PG, and HbA1c on post-MI prognosis in patients without known diabetes has been compared in a few studies [, , , ]. The 2h-PG, but neither FPG nor HcA1c, predicted outcome in EUROASPIRE IV []. HbA1c ≥6.5%, when included in the same model as known DM, did not increase mortality, and new DM diagnosed by OGTT did, even when the HbA1c was <6.5% in another study []. Kowalczyk et al. [] suggests the usefulness of HbA1c in patients with IGT and new DM, but they do not report the effect of HbA1c on prognosis of patients without. The Emerging Risk Factors Collaboration [] published a large epidemiological dataset including individuals without DM or cardiovascular disease at baseline suggesting that additional assessment of HbA1c provided little incremental benefit for predicting the risk of cardiovascular disease over and above other glycaemic matrices.
The 2h-PG did not increase the c-statistic of the model containing GRS. Improving c-statistics of models containing powerful variables as GRS may be difficult as δAUC heavily depends on the strength of performance of the underlying clinical model. NRI>0 and IDI, tests devised to deal with this anomaly, improved when 2h-PG is added to GRS. The NRI>0 did not change for the 6-month and 1-year time points, but the IDI did. The NRI>0 counts the individuals with and without events whose calculated risk changes on addition of a variable into a model. IDI measures the amount of change in calculated risk for each individual with and without events incorporating both the direction and the extent of change in calculated risk, making it more meaningful than NRI>0.
The study is limited by its retrospective observational nature. Deaths were recorded from the general practice database linked to the national death register. Although local records are regularly updated, some re-infarctions admitted to other hospitals may have been missed. Information not available had to be excluded from statistical models. Inclusion of a mainly Caucasian population could affect its generalizability. Stress hyperglycaemia is less likely to have affected our result as the OGTT was done ≥3 days after the event. The stress response to an acute event subsides within 2–5 days with no further decrease thereafter []. The effect of random fluctuation in glycaemia, however, cannot be excluded. Although reproducibility of the OGTT results and its relation to a long-term glucometabolic state would be important for the diagnosis of DM, its relevance to assessing post-MI prognostic risk is less.
This study concludes that in patients without known diabetes, 2h-PG but not FPG, improves the performance of GRS-containing models in predicting post-MI prognosis in the short to medium term. Thus 2h-PG can be used as an additional prognostic biomarker in addition to GRS in these patients. There is an ongoing debate as to the choice of a glucose matrix for the detection of hyperglycaemia in this high-risk population. It may be reasonable to choose 2h-PG, the one that minimises the risk of missing the diagnosis and is additionally capable of providing prognostic information despite it being cumbersome to measure, in favour of HbA1c that is simpler and feasible to measure, and deemed sufficient for use in the low-risk general population for epidemiological purposes especially since there is no evidence of superiority of HbA1c over 2h-PG in predicting prognosis. Thus, an appropriately timed pre-discharge OGTT may be recommended for all patients without known diabetes admitted with MI.
Acknowledgments
We thank all the nurses that helped with the collection of data. We also thank the patients whose data were utilised for this study.
Statement of Ethics
As routinely collected anonymised data on standard clinical practice were being retrospectively analysed, the East Yorkshire and North Lincolnshire Research Ethics Committee waived the need for formal ethical approval and patient consent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no funding sources to declare.
Author Contribution
J.J. and S.C. conceived and designed the study. A.G. and S.C. contributed to acquisition of data and drafted the manuscript. S.C. analysed and interpreted the data. J.J., S.C., and T.S. critically revised the manuscript. A.G., J.J., S.C., and T.S. gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
References
- 1. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et alGlobal Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53.
- 2. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et alGRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33.
- 3. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153(1):29–35.
- 4. van Toorenburg M, van den Berg VJ, van der Ploeg T, Heestermans AA, Dirksen MT, Hautvast RW, et al Addition of routinely measured blood biomarkers significantly improves GRACE risk stratification in patients with myocardial infarction. Int J Cardiol. 2018;273:237–42.
- 5. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–76.
- 6. Klingenberg R, Aghlmandi S, Räber L, Gencer B, Nanchen D, Heg D, et al Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the GRACE score. Eur Heart J Acute Cardiovasc Care. 2018;7(2):129–38.
- 7. Eggers KM, Kempf T, Venge P, Wallentin L, Wollert KC, Lindahl B. Improving long-term risk prediction in patients with acute chest pain: the Global Registry of Acute Coronary Events (GRACE) risk score is enhanced by selected nonnecrosis biomarkers. Am Heart J. 2010;160(1):88–94.
- 8. Widera C, Pencina MJ, Meisner A, Kempf T, Bethmann K, Marquardt I, et al Adjustment of the GRACE score by growth differentiation factor 15 enables a more accurate appreciation of risk in non-ST-elevation acute coronary syndrome. Eur Heart J. 2012;33(9):1095–104.
- 9. Widera C, Pencina MJ, Bobadilla M, Reimann I, Guba-Quint A, Marquardt I, et al Incremental prognostic value of biomarkers beyond the GRACE (Global Registry of Acute Coronary Events) score and high-sensitivity cardiac troponin T in non-ST-elevation acute coronary syndrome. Clin Chem. 2013;59(10):1497–505.
- 10. O'Donoghue ML, Morrow DA, Cannon CP, et al: Multimarker Risk Stratification in Patients With Acute Myocardial Infarction. J Am Heart Assoc 2016;%20;5:JAHA.
- 11. Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, et alGRACE Investigators. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):402–9.
- 12. Aronson D, Hammerman H, Kapeliovich MR, Suleiman A, Agmon Y, Beyar R, et al Fasting glucose in acute myocardial infarction: incremental value for long-term mortality and relationship with left ventricular systolic function. Diabetes Care. 2007;30(4):960–6.
- 13. Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, et al Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111(23):3078–86.
- 14. Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, et al A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart. 2003;89(5):512–6.
- 15. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, et al Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36(4):1026–32.
- 16. Timóteo AT, Papoila AL, Rio P, Miranda F, Ferreira ML, Ferreira RC. Prognostic impact of admission blood glucose for all-cause mortality in patients with acute coronary syndromes: added value on top of GRACE risk score. Eur Heart J Acute Cardiovasc Care. 2014;3(3):257–63.
- 17. de Mulder M, van der Ploeg T, de Waard GA, Boersma E, Umans VA. Admission glucose does not improve GRACE score at 6 months and 5 years after myocardial infarction. Cardiology. 2011;120(4):227–34.
- 18. Correia LC, Rocha MS, Bittencourt AP, Freitas R, Souza AC, Almeida MC, et al Does acute hyperglycemia add prognostic value to the GRACE score in individuals with non-ST elevation acute coronary syndromes? Clin Chim Acta. 2009;410(1-2):74–8.
- 19. David RB, Almeida ED, Cruz LV, Sebben JC, Feijó IP, Schmidt KE, et al Diabetes mellitus and glucose as predictors of mortality in primary coronary percutaneous intervention. Arq Bras Cardiol. 2014;103(4):323–30.
- 20. Liu XJ, Wan ZF, Zhao N, Zhang YP, Mi L, Wang XH, et al Adjustment of the GRACE score by HemoglobinA1c enables a more accurate prediction of long-term major adverse cardiac events in acute coronary syndrome without diabetes undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2015;14(1):110.
- 21. She J, Deng Y, Wu Y, Xia Y, Li H, Liang X, et al Hemoglobin A1c is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiovasc Diabetol. 2017;16(1):97–0578.
- 22. George A, Bhatia RT, Buchanan GL, Whiteside A, Moisey RS, Beer SF, et al Impaired Glucose Tolerance or Newly Diagnosed Diabetes Mellitus Diagnosed during Admission Adversely Affects Prognosis after Myocardial Infarction: An Observational Study. PLoS One. 2015;10(11):e0142045.
- 23. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et alJoint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–38.
- 24. Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et alTask Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC)European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28(1):88–136.
- 25. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97.
- 26. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et alExpert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7.
- 27. Chattopadhyay S, George A, John J, Sathyapalan T. Two-hour post-challenge glucose is a better predictor of adverse outcome after myocardial infarction than fasting or admission glucose in patients without diabetes. Acta Diabetol. 2018;55(5):449–58.
- 28. Baeza-Román A, de Miguel-Balsa E, Latour-Pérez J, Carrillo-López A. Predictive power of the grace score in population with diabetes. Int J Cardiol. 2017;248:73–6.
- 29. Hage C, Malmberg K, Rydén L, Wallander M. The impact of infarct type on the reliability of early oral glucose tolerance testing in patients with myocardial infarction. Int J Cardiol. 2010;145(2):259–60.
- 30. Knudsen EC, Seljeflot I, Abdelnoor M, Eritsland J, Mangschau A, Arnesen H, et al Abnormal glucose regulation in patients with acute ST- elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol. 2009;8(1):6.
- 31. Tenerz A, Norhammar A, Silveira A, Hamsten A, Nilsson G, Rydén L, et al Diabetes, insulin resistance, and the metabolic syndrome in patients with acute myocardial infarction without previously known diabetes. Diabetes Care. 2003;26(10):2770–6.
- 32. Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004;147(5):803–7.
- 33. Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, et al Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124(6):704–11.
- 34. Moura FA, Figueiredo VN, Teles BS, Barbosa MA, Pereira LR, Costa AP, et alBrasilia Heart Study. Glycosylated hemoglobin is associated with decreased endothelial function, high inflammatory response, and adverse clinical outcome in non-diabetic STEMI patients. Atherosclerosis. 2015;243(1):124–30.
- 35. Geng J, Zhang Y, Wang B, Xie J, Xu B, Li J. Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease: A meta-analysis. Medicine (Baltimore). 2017;96(17):e6784.
- 36. Pararajasingam G, Høfsten DE, Løgstrup BB, Egstrup M, Henriksen FL, Hangaard J, et al Newly detected abnormal glucose regulation and long-term prognosis after acute myocardial infarction: comparison of an oral glucose tolerance test and glycosylated haemoglobin A1c. Int J Cardiol. 2016;214:310–5.
- 37. Shin D, Ahn J, Cha KS, Park JS, Oh JH, Lee HW, et alKorea Working Group on Myocardial Infarction Investigators. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis. 2016;27(1):40–6.
- 38. Lazzeri C, Valente S, Chiostri M, Attanà P, Mattesini A, Nesti M, et al Glycated haemoglobin and long-term mortality in patients with ST Elevation Myocardial Infarction. J Cardiovasc Med (Hagerstown). 2015;16(6):404–8.
- 39. Shahim B, De Bacquer D, De Backer G, Gyberg V, Kotseva K, Mellbin L, et al The Prognostic Value of Fasting Plasma Glucose, Two-Hour Postload Glucose, and HbA1c in Patients With Coronary Artery Disease: A Report From EUROASPIRE IV: A Survey From the European Society of Cardiology. Diabetes Care. 2017;40(9):1233–40.
- 40. Tailakh MA, Friger M, Zahger D, Sidi A, Mazor-Dray E, Novack V. Prospective study of the impact of diabetes mellitus newly diagnosed by glycated hemoglobin on outcomes in patients undergoing percutaneous coronary intervention. Eur J Intern Med. 2017;37:69–74.
- 41. Kowalczyk J, Mazurek M, Zielinska T, Lenarczyk R, Sedkowska A, Swiatkowski A, et al Prognostic significance of HbA1c in patients with AMI treated invasively and newly detected glucose abnormalities. Eur J Prev Cardiol. 2015;22(6):798–806.
- 42. Gyberg V, De Bacquer D, Kotseva K, De Backer G, Schnell O, Sundvall J, et alEUROASPIRE IV Investigators. Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: a report from EUROASPIRE IV—a survey from the European Society of Cardiology. Eur Heart J. 2015;36(19):1171–7.
- 43. Di Angelantonio E, Gao P, Khan H, Butterworth AS, Wormser D, Kaptoge S, et alEmerging Risk Factors Collaboration. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311(12):1225–33.