Introduction
The American Cancer Society estimates that, by 2030, over 22.1 million cancer survivors will live in the United States. Despite treatment advances, cancer remained the second leading cause of death in the United States in 2020, behind only cardiovascular disease. Along with the physical symptoms associated with tumor burden and toxicities of therapies, patients with cancer also suffer from comorbid psychological conditions at a higher rate than the general population. The prevalence of depression among patients with cancer is estimated to be four times higher than that in the general population, with up to 50% of patients experiencing clinical depression., However, only about 25% receive a formal diagnosis. Notably, depression has been linked to increased mortality and an overall poorer prognosis in patients with cancer., , Mindfulness‐based interventions (MBIs) have emerged as promising interventions for cancer‐related distress and affective disorders. Although the literature is abundant with research supporting MBIs in improving depressive symptoms, fatigue, and quality of life in patients with cancer, no studies have directly correlated mindfulness with survival. If depression reduces survival in this population, interventions effective in treating depression may also be predicted to improve survival (Figure 1).
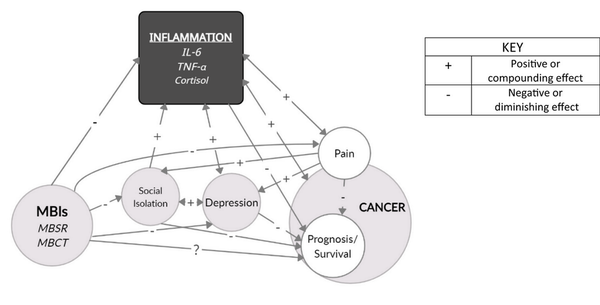
FIGURE 1
Mindfulness, inflammation and cancer prognosis. This conceptual framework illustrates the many relationships that suggest a possible correlation between mindfulness and cancer survival. IL‐6 indicates interleukin‐6; MBCT, mindfulness‐based cognitive therapy; MBI, mindfulness‐based intervention; MBSR, mindfulness‐based stress reduction; TNF‐α, tumor necrosis factor α.
History and overview of mindfulness
Derived from centuries‐old Buddhist traditions, mindfulness is defined as the nonjudgmental awareness of the present moment, with attention focused on the body and breath, allowing the mind to rest from rumination and worry. The mindfulness practitioner acknowledges distressing feelings and emotions with less reactivity and judgment, leading to greater acceptance and an overall sense of well‐being. There is a wide breadth of literature about mindfulness meditation as an effective modality in treating depression, anxiety, pain, and posttraumatic stress disorder., There is also evidence for its efficacy in improving symptom severity, quality of life, and clinical outcomes in several chronic illnesses, such as hypertension, diabetes, rheumatoid arthritis, Parkinson disease, urge incontinence, and pain.
Molecular biologist Jon Kabat‐Zinn first placed mindfulness into a scientific context when he established his Stress Reduction Clinic at the University of Massachusetts Memorial Medical Center in 1979., His formal mindfulness program evolved into an 8‐week program that greater than 25,000 people have since completed. The program, known as mindfulness‐based stress reduction (MBSR), consists of weekly group classes and independent study using various meditative practices, such as sitting, body scan, yoga, and walking meditations. MBSR has been integrated into many community and hospital‐based health systems.
Similar programs have been developed, such as mindfulness‐based cognitive therapy (MBCT), an adaptation for patients at high risk of depression relapse. MBSR and MBCT are similar in structure and format, but the latter includes principles of cognitive therapy in which individuals learn to break patterns of rumination and negative thinking. These two programs are the MBIs with the strongest evidence base in peer‐reviewed journals. Formal MBI programs share essential characteristics, following established protocols that allow for homogeneity in teacher training, program implementation, and research. Electronic adaptations, such as internet and app‐based MBIs, are increasingly popular, but these vary in length and are mostly self‐guided, with little formal structure.Loving‐kindness meditation (LKM) is a meditation strategy aimed at cultivating compassion toward oneself, others, and the world. Similar to mindfulness, it strengthens concentration and awareness; the two are often combined in therapeutic interventions and research.
Mindfulness in the general population
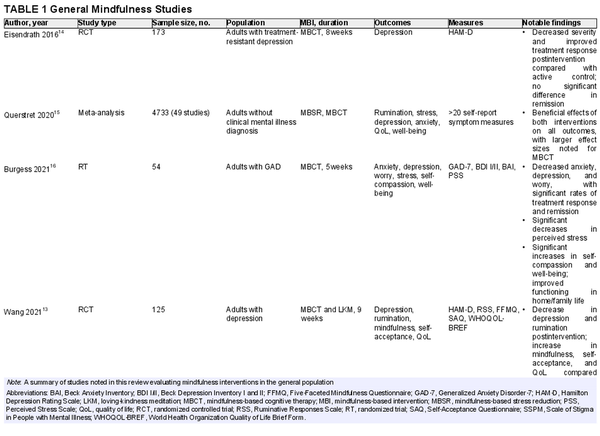
The evidence supporting MBIs for psychological well‐being is robust (Table 1)., , , A recent meta‐analysis by Querstret et al. compared MBSR and MBCT versus passive controls and found that MBIs caused a significant reduction in rumination, stress, depression, and anxiety and a significant improvement in quality of life and well‐being. Burgess and colleagues conducted a randomized trial looking at a 5‐week MBCT intervention for a community sample and found significant reductions in anxiety, depression, and worry, with large effect sizes noted for worry and depression. Esiendrath et al. evaluated the effects of an 8‐week MBCT program compared with a local health‐enhancement program as adjuncts to pharmacotherapy in patients with treatment‐resistant depression. The MBCT group had a significant reduction in depression severity after the intervention and a higher number of treatment responders than the health‐enhancement program. Another randomized controlled trial (RCT) by Wang et al. compared MBCT and LKM versus a conventional psychological intervention. Participants were evaluated at 2, 4, 6, and 8 weeks after the completion of the intervention. At every time point, MBCT and LKM proved superior to controls in the degree of depression and rumination compared with baseline. Significant improvements in self‐acceptance and quality‐of‐life scores were seen at all time points.
Depression and mindfulness in cancer
The link between depression and cancer prognosis is well demonstrated. Clinical depression increases cancer mortality rates by up to 39%; even minor depressive symptoms may increase risk by 25%. Giese‐Davis et al. conducted a randomized trial of women with metastatic breast cancer who were receiving group therapy and showed that those with improved depression scores at 1 year postintervention had significantly better survival (53.6 months) than those with worsening scores (25.1 months).
Depression in patients with cancer stems from both psychosocial and biological factors. Psychosocial factors include the emotions and stress of an uncertain or bleak prognosis and the illness’s effects on the patient’s job, family, appearance, finances, and independence. Biological factors can include cortisol dysregulation and activation of inflammatory cytokines, causing serotonin depletion., , , Notably, these exact mechanisms promote tumor growth, progression, and metastasis., Therefore, interventions targeting depression in this population may also slow progression and improve survival.
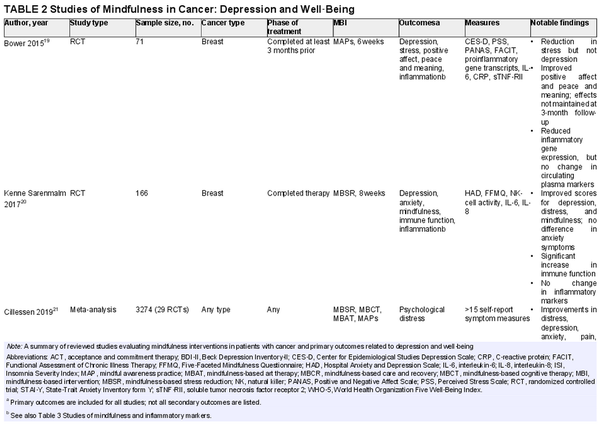
Scientific interest in the effect of MBIs on depression in patients with cancer has increased in recent years (Table 2)., , , , A meta‐analysis by Xunlin et al. showed that patients participating in an MBI had significantly lower anxiety, depression, fatigue, and perceived stress; the authors also reported significantly greater quality of life across all cancer types. Another meta‐analysis by Cillessen et al. showed a significant effect of MBIs on psychological distress, anxiety, depression, pain, and fatigue. Although the effect sizes were small in that analysis, larger effects were seen in younger patients, those with shorter time to follow‐up, and in studies with a passive control. Kenne Sarenmalm et al. randomized women to a formal MBSR group, a self‐guided MBSR group (active control), or a nonintervention group. Both the MBSR and active control groups had significant improvements in depression compared with the nonintervention group.
Some studies have demonstrated mixed results in the effectiveness of MBIs. Nissen et al. randomized depressed patients with breast cancer and prostate cancer to an internet‐based MBSR program or a wait‐list control. Outcomes were assessed at baseline, 5 weeks, 10 weeks, and 6 months after the intervention. Although significant improvements were noted for anxiety and depression in the MBSR group, these results were not maintained at the 6‐month follow‐up. This finding suggests that ongoing maintenance of mindfulness practice may be needed for the benefit to persist. An RCT by Bower et al. involving women with breast cancer younger than 50 years found that patients participating in a 6‐week MBI had a significant reduction in perceived stress, fatigue, and sleep disturbance and a significant increase in positive affect and peace and meaning. However, no significant decrease was noted in depressive symptoms. As with the previous study, the effects were not maintained at a 3‐month follow‐up assessment.
Depression, neuroinflammation, and biomarkers
Studies measuring depressive symptoms typically use validated but subjective questionnaires, such as the Center for Epidemiologic Studies Depression Scale,, the Beck Depression Inventory,, the Hospital Anxiety and Depression Scale,, , or the Hamilton Depression Rating Scale., This variability presents a difficulty in generalizing results across studies. Also, the subjective nature of these measures and the inability to blind participants to psychological interventions raise the concern of bias. Participants may think they are less depressed because they have completed an intervention with obvious psychological intent. However, some biomarkers that are increased in inflammatory and chronic disease states are also known to be elevated in depressive disorders. Although not specific, these markers may be useful as an adjunct to the questionnaires in determining the effectiveness of psychological interventions. Interleukin‐6 (IL‐6), tumor necrosis factor‐α (TNF‐α), and cortisol have all been implicated in depression, , , and correlate with the severity of symptoms.
IL‐6 is a glycoprotein that moderates the immune system as a regulator of B‐cell differentiation. Exposure to repeated psychological stress increases the expression of IL‐6 from immune cells. This and other proinflammatory cytokines cross the blood–brain barrier and cause neuroinflammation, influencing behavior., IL‐6 has been linked with vegetative symptoms and proposed as a biomarker for depression with 79% sensitivity and 87% specificity. TNF‐α, another inflammatory cytokine generated by immune cells, is also found at higher serum levels in patients with major depressive disorder. In the cytokine hypothesis of depression described by Jeon and Kim, stress and sympathetic nervous system activation stimulate the release of these and other cytokines, which then direct a pathway leading to the depletion of serotonin and other neurotransmitters that are important for mood stability., ,
In addition to cytokine activation, dysregulation of the hypothalamus‐pituitary‐adrenal (HPA) axis is another mechanism widely implicated in the neurobiology of depression. Chronic psychological stress activates the HPA axis and the sympathetic nervous system, leading to the release of glucocorticoids such as cortisol., Cortisol also depletes serotonin by stimulating re‐uptake. Repeated stress and prolonged cortisol exposure activate the immune response in a maladaptive way, reducing the sensitivity of immune cells to anti‐inflammatory mechanisms., Thus the cytokine and cortisol pathways act synergistically to promote mood imbalance.
Several studies support the link between inflammation and depression. In a cross‐sectional study of males with various levels of depression, Jia et al. measured the correlation between serum cortisol, inflammatory cytokines, and depression. Participants in the depression group were found to have significant elevations of cortisol and TNF‐α compared with nondepressed participants. Those investigators also found that serum cortisol levels correlated with depression severity, allowing them to differentiate between mild, moderate, and severe depression. Heinze et al. and Capuron et al. demonstrated that patients with renal cell carcinoma or malignant melanoma who were receiving cytokine therapy, such as IL‐2 or interferon‐α, were at risk for the development of severe depressive symptoms during the course of therapy. Other studies demonstrated an improvement in depressive symptoms in patients taking anti‐inflammatory therapies. McIntyre et al. assessed the efficacy of infliximab, a monoclonal antibody targeting TNF‐α, in the treatment of depression and found that patients who reported childhood trauma experienced a reduction >50% in depressive symptoms after 12 weeks of treatment. A meta‐analysis by Kohler‐Forsberg and colleagues showed that anti‐inflammatories, such as nonsteroidal anti‐inflammatories, corticosteroids, statins, and cytokine inhibitors, reduced depressive symptoms, both as an adjunct to standard antidepressants and as monotherapy. Adjunctive therapy with anti‐inflammatories also improved response and remission rates compared with placebo.
Inflammatory pathways in cancer
The significance of these neuroinflammatory mechanisms in depression cannot be underestimated when one considers the role of these same pathways in cancer progression and prognosis. There is substantial evidence that cytokines and glucocorticoids are associated with a poorer prognosis in patients with cancer., , , , , , , IL‐6 and TNF‐α are involved in all stages of tumor development, from formation to progression and metastasis. This is largely because of their role in vascular permeability, cellular adhesion, and angiogenesis, processes required for tumor growth and spread., , , ,
Cortisol abnormalities may block pathways involved in DNA repair and stimulate cancer cell growth. In addition, stress‐induced chronic glucocorticoid exposure and sympathetic nervous system activity stimulate a shift to myeloid cell production in the bone marrow. This may explain why higher levels of IL‐6, TNF‐α, and other cytokines increase acute myeloid leukemia aggressiveness. These shared inflammatory mechanisms of cancer and depression have led researchers to note that depressive symptoms often precede a cancer diagnosis. This phenomenon has been particularly noted for pancreas and lung cancer and cancers causing malignant hypercalcemia.
Several studies have demonstrated the correlation between these biomarkers and cancer prognosis. Tripsianis et al. completed a longitudinal study evaluating the prognosis and characteristics of patients with breast cancer relative to IL‐6 and TNF‐α levels. High IL‐6 levels were associated with lymphovascular invasion, advanced stage, and greater than three positive lymph nodes. High TNF‐α levels were associated with the same findings, in addition to poorly differentiated tumors. Finally, there was a significant decrease in survival time among patients who had high IL‐6 levels compared with those who had low or no IL‐6 expression (39 vs. 62 months); findings were similar for TNF‐α (45 vs. 61 months).
In another study demonstrating the importance of HPA‐axis dysregulation in ovarian cancer outcomes, participants provided salivary cortisol samples for 3 days before starting treatment and were prospectively followed. Ascites fluid was sampled for IL‐6 levels in patients with advanced stages. A one standard deviation increase in night cortisol was correlated with a 46% greater likelihood of death, and IL‐6 levels were correlated with cortisol variables.
Given the shared inflammatory mechanisms implicated in both depression and cancer, it is no surprise that depression in these individuals is intensified because psychological stress combined with cancer‐related inflammatory pathways may have a synergistic effect on cognitive symptoms. Unfortunately, some treatments enhance cytokine production by noncancerous cells, promoting further inflammation. Tissue destruction from surgery, chemotherapy, or radiation leads to damage‐associated molecular patterns on injured tissue, stimulating the production of cytokines., The therapies discussed previously for renal cell cancer and melanoma involve direct infusion of cytokines to promote tumor destruction through the inflammatory response., If treatments worsen the inflammatory response, it is essential to consider additional therapies that counteract this process while keeping the oncolytic mechanisms intact.
Two additional factors deserving inclusion in any discussion of inflammation, depression, and cancer outcomes are pain and social isolation (Figure 1).
Pain, opioids, and inflammation
It is estimated that up to 50% of patients living with cancer experience moderate‐to‐severe pain. Cancer‐related pain comes in many forms, including acute pain, which is associated with immediate therapies (e.g., surgery, chemotherapy, radiation); chronic pain, which is related to cancer progression, ongoing therapy, or pre‐existing pain syndromes; pain related to a history of drug addiction; or end‐stage pain. Both pain and its conventional therapies contribute to a proinflammatory state. Pain triggers a stress response through HPA‐axis dysfunction and excessive glucocorticoid secretion, promoting immunosuppression and tumor progression. Opioids have long been a mainstay of treatment for all forms of cancer‐related pain., One of the concerns arising from prolonged treatment is tolerance, or the reduction in efficacy requiring steadily larger doses to maintain therapeutic effect. Inflammatory mechanisms of opioid tolerance have been well described in the literature., , Opioids bind to glycoproteins on toll‐like receptor 4, an immune receptor found on microglia., , This signaling activates an inflammatory response, resulting in the release of cytokines, including TNF, IL‐1, and IL‐6., , The role of these cytokines in neuroinflammation and opioid use is highly complex, but their release increases exponentially with repeated opioid use and results in an increase in the activity of pain receptors, thereby inducing tolerance.
In addition to the detrimental effects of inflammation discussed above, there remain other concerns related to chronic opioid use and tolerance in patients living with cancer. Preclinical and retrospective studies suggest a possible link between opioid use and cancer metastasis or progression., Possible mechanisms proposed include the activation of μ opioid receptors found in malignant cells and the stimulation of cell proliferation and angiogenesis. Some opioids have been shown to inhibit the activity and cytotoxicity of immune cells. Notably, although several retrospective studies have shown that opioid use is associated with decreased survival in several cancer types,, , , this link has not been confirmed through prospective clinical trials, and opioids remain a primary component of cancer pain management., , , Still, the inflammatory nature of pain and opioid use requires further investigation into their potential role in depression and poor outcomes. Additional concerns remain regarding the adverse effects of opioid use in patients with cancer, including gastrointestinal side effects and the risks of abuse, diversion, and overdose. Thus interventions that could reduce pain and inflammation while potentially reducing opioid use may be valuable additions to a cancer treatment program.
Social isolation
It is well established that social isolation is associated with depression and higher levels of psychological distress., The current review has already explored the effects of psychological distress on inflammatory pathways and cancer prognosis. Animal and human studies provide further evidence of the effect of social isolation on cancer progression and survival through inflammatory pathways. In numerous studies, mice exposed to social isolation demonstrate cellular alterations that promote tumor progression, including angiogenesis, gene expression, and neuroendocrine and immune responses., A large cross‐sectional study by Hafner et al. found a synergistic relationship between depression and social isolation on IL‐6 and C‐reactive protein levels in men. Prospective cohort studies demonstrate an association between a poor social network or poor social support and cancer survival. Social support is generally defined in the literature as the presence of friends, living children, and close relatives and having someone with whom to talk about their illness and personal problems. Kroenke et al. found a 66% higher all‐cause mortality and a two‐fold increase in cancer‐related mortality among women with breast cancer who reported low social support. In a meta‐analysis by Pinquart and Duberstein, high levels of perceived social support, larger network size, and married status were associated with lower mortality in patients with cancer. Other studies demonstrated similar findings., It is possible that this association is caused by the lack of caregiver support where such assistance is vital (medication management, transportation to appointments, etc.). The mortality risk may also be compounded by HPA‐axis reactivity and neuroinflammation associated with psychosocial stress. These mechanisms remain speculative, and the known associations between social isolation, stress, and cancer mortality warrant closer attention.
Mindfulness and inflammation, pain, and social functioning
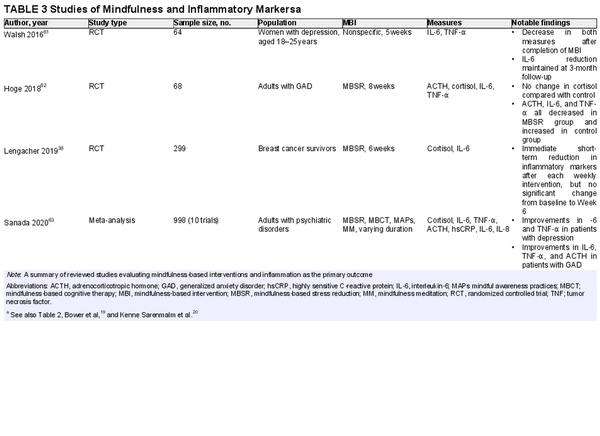
The literature demonstrates recent interest in the effect of mindfulness on inflammation (Table 3)., , , , , Lengacher et al. examined the effects of a 6‐week MBSR program on salivary cortisol and IL‐6 levels in breast cancer survivors. The results of that RCT showed positive short‐term effects, with a significant decrease in both markers immediately after the intervention in Weeks 1 and 6 (compared with immediately before the same session). However, they did not demonstrate a significant difference from baseline to Week 6. The authors noted that earlier studies found contradictory results, with some reports suggesting a significant decrease over time, whereas others did not. Another RTC by Hoge and colleagues measured adrenocorticotropic hormone (ACTH), cortisol, IL‐6, and TNF‐α before and after an 8‐week MBSR program in adults with generalized anxiety disorder. ACTH, IL‐6, and TNF‐α decreased after 8 weeks in the MBSR group, whereas they all increased in the control group. There was no change in cortisol compared with the control group, suggesting that ACTH may be a more sensitive measure of HPA‐axis dysfunction than cortisol. An earlier RCT by Walsh et al. demonstrated a significant decrease in IL‐6 and TNF‐α in depressed women undergoing a 5‐week MBI compared with a control group. The IL‐6 reduction was maintained at a 3‐month follow‐up assessment, with a more substantial effect in patients who had higher baseline depressive symptoms. Finally, in a recent meta‐analysis of 10 trials of MBIs in patients who had psychiatric disorders, Sanada and colleagues found that MBIs demonstrated significant reductions in cortisol, IL‐6, and TNF‐α.
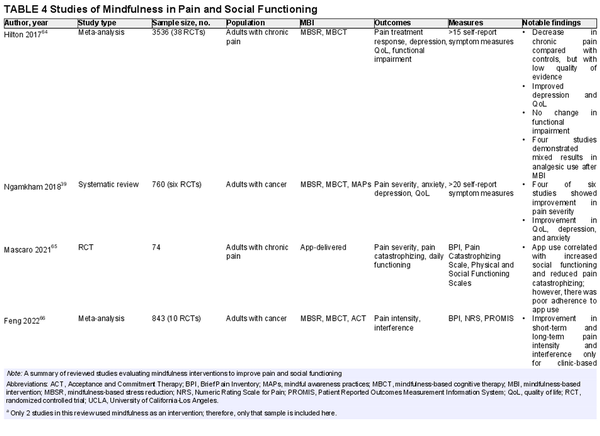
MBAs have also shown modest potential as modalities for pain and perceived social support, two factors associated with inflammation and possibly survival (Table 4)., , , , Ngamkham et al. conducted a systematic review and found that MBIs resulted in a statistically significant improvement in pain severity in four of six studies included. The authors propose that mindfulness helps to turn attention away from unpleasant symptoms, thereby improving pain tolerance. Other evidence presented in their review suggests that a reduction in inflammation may also play a role; therefore, a reduction in opioid tolerance may be an additional desired effect.
A meta‐analysis of 30 RCTs by Hilton et al. showed a small benefit of MBIs on chronic pain compared with treatment as usual, passive controls, and education/support groups. Only four studies in that meta‐analysis reported on analgesic use as an outcome, and results were mixed, with two studies showing a decrease in analgesic use after an MBI. Another recent meta‐analysis by Feng and colleagues demonstrated that MBIs were associated with significant improvements in pain intensity among patients with cancer at both short‐term and long‐term follow‐up, albeit with a small effect size. The improvements were more significant in face‐to‐face settings than with internet‐based or remote delivery. Still, there may be a role for app‐delivered MBIs in pain reduction. Mascaro et al. conducted an RCT to evaluate the effects of 6 weeks of self‐guided, informal mindfulness app use on pain severity and pain catastrophizing. Participants who used the app reported significantly less helplessness (on a subscale of pain catastrophizing). There was no significant change in pain severity scores in the mindfulness group, which could have been because of poor adherence to recommended app use duration and frequency. However, an interesting finding was that the mindfulness group reported deterioration in physical functioning but improved social functioning. The authors suggest that mindfulness allows people to be more engaged with their social lives despite their pain.
Although social isolation is associated with increased cancer mortality, few studies have explored MBIs as a social intervention. In a review of studies investigating interventions for reducing loneliness, Veronese et al. identified mindfulness and meditation as positive factors, but their findings were based on only two studies and had a low quality of evidence. Mindfulness may reduce ruminative thoughts that can precipitate feelings of loneliness and isolation. Group‐based MBIs may also offer a sense of community to those otherwise isolated. This area of study is limited and should be further explored to better understand the social benefits of MBIs, particularly in patients with cancer who rely so closely on their social network.
Discussion
This review examines the links between depression, inflammation, and prognosis and the benefits of MBIs in treating depression in patients with cancer (Figure 1). The evidence suggests that mindfulness may treat depression by targeting the very inflammatory pathways that correlate with a worse prognosis in cancer. There may also be an indirect anti‐inflammatory effect through the benefits on pain and social functioning. Furthermore, if depression and inflammation are both inversely correlated with survival, MBIs may offer more potential for prolonging life while also improving its quality.
Despite this evidence, there are some shortcomings with the current research. For example, in a review investigating fidelity in MBIs by using the National Institutes of Health Behavior Change Consortium guidelines, Kechter and colleagues found that only 12% of identified efficacy trials among adult participants described treatment fidelity. In addition, there are differences in the quality of programs, depending on the level of experience of providers and the content of sessions. Lutz et al. describe differences in content based on whether programs teach focused attention (maintain focus on a particular sensation, sound, or image) and open monitoring (broadening the attentional field without a focus, then releasing attention gently and without judgment). Finally, because many mindfulness interventions are relatively short‐term and lack long‐term follow‐up, there is evidence that initial practice of mindfulness can contribute to an increase in dysphoric affect because of increased awareness of internal thoughts and emotions, thereby contributing to mixed results related to improvements in depressive symptoms.
Nonetheless, as scientific knowledge related to the benefits of MBIs continues to grow and disseminate, more cancer centers are implementing formal mindfulness programs as part of an integrative psychosocial program. The growing interest in these programs will hopefully stimulate further research because no studies have measured their effect on survival. Most studies also do not measure patients’ symptoms or inflammatory markers long after the intervention; the longest follow‐up period reviewed here is 6 months. In this case, patients would be encouraged to commit to an ongoing, independent practice, which may prove challenging. It is also unclear whether such informal and less intensive practice would yield the same benefits as the short‐term studies discussed here.
There are some barriers to consider when implementing an MBI in cancer care. Patients may be hesitant to participate if they do not see a direct connection between the exercises and their disease or treatments. Treatment regimens may already be intense, and patients may be unwilling to add more complexity to their schedules. Lengthy hospitalizations in some cases would make regular, formal participation challenging, although the increased use of telemedicine can help expand access to these patients. These programs also require trained facilitators, which typically is not a concern for large academic centers but perhaps is less feasible for community‐based facilities. Furthermore, although some insurance companies cover these interventions as a form of group therapy, the programs may be too costly for individuals without coverage. Finally, programs will need to address gaps in utilization of mindfulness programs based on sex, education, and cultural background. This is based on evidence that men are one half as likely as women to practice mindfulness. Individuals with lower levels of education are also less likely to practice mindfulness, and there is low engagement in communities of color, particularly Black and Hispanic or Latino adults, largely because of stigma, limited accessibility, and a tendency of American‐based mindfulness programs to ignore the importance of cultural background., Despite these potential limitations, the devastation of a cancer diagnosis, coupled with the consequences of comorbid depression on survival and quality of life, warrants more attention to this field. The available literature demonstrates promise for MBIs as an intervention to prolong survival in patients with cancer. Research in this area is rapidly evolving, and future efforts should focus on longitudinal RTCs to fully evaluate the potential impact of MBIs on cancer survival, with emphasis on depression, pain, inflammation, and social functioning as mediating variables. [Black Square]
Conflicts of interest
The authors made no disclosures.
Acknowledgements
The authors thank Randy Danielsen PhD, PA‐C, for providing substantial guidance and encouragement in the creation of this article.
References
- 1. American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2019–2021. American Cancer Society; 2019. Accessed August 10, 2021. cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/cancer‐treatment‐and‐survivorship‐facts‐and‐figures/cancer‐treatment‐and‐survivorship‐facts‐and‐figures‐2019‐2021.pdf
- 2. Ahmad FB, Cisewski JA, Minino A, Anderson RN. Provisional mortality data—United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(14):519–522. doi:10.15585/mmwr.mm7014e1
- 3. Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. doi:10.1016/j.ctrv.2016.11.004
- 4. Aldea M, Craciun L, Tomuleasa C, Crivii C. The role of depression and neuroimmune axis in the prognosis of cancer patients. J BUON. 2014;19(1):5–14.
- 5. Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101–111. doi:10.1093/annonc/mdx526
- 6. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018:k1415. doi:10.1136/bmj.k1415
- 7. Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (review). Oncol Lett. 2015;9(4):1509–1514. doi:10.3892/ol.2015.2944
- 8. Kabat‐Zinn J. Full Catastrophe Living (Revised Edition): Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Bantam Books; 2013.
- 9. Felsted KF. Mindfulness, stress, and aging. Clin Geriatr Med. 2020;36(4):685–696. doi:10.1016/j.cger.2020.06.010
- 10. UMass Memorial Health . Center for Mindfulness. Accessed August 9, 2021. http://www.ummhealth.org/center‐mindfulness
- 11. Crane RS, Brewer J, Feldman C, et al. What defines mindfulness‐based programs? The warp and the weft. Psychol Med. 2017;47(6):990–999. doi:10.1017/s0033291716003317
- 12. Hoffman S, Gomez A. Mindfulness‐based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739–749. doi:10.1016/j.psc.2017.08.008
- 13. Wang Y, Fu C, Liu Y, et al. A study on the effects of mindfulness‐based cognitive therapy and loving‐kindness meditation on depression, rumination, mindfulness level and quality of life in depressed patients. Am J Transl Res. 2021;13(5):4666–4675.
- 14. Eisendrath SJ, Gillung E, Delucchi KL, et al. A randomized controlled trial of mindfulness‐based cognitive therapy for treatment‐resistant depression. Psychother Psychosom. 2016;85(2):99–110. doi:10.1159/000442260
- 15. Querstret D, Morison L, Dickinson S, Cropley M, John M. Mindfulness‐based stress reduction and mindfulness‐based cognitive therapy for psychological health and well‐being in non‐clinical samples: a systematic review and meta‐analysis. Int J Stress Manag. 2020;27(4):394–411. doi: 10.1037/str0000165
- 16. Burgess EE, Selchen S, Diplock BD, Rector NA. A brief mindfulness‐based cognitive therapy (MBCT) intervention as a population‐level strategy for anxiety and depression. J Cogn Ther. 2021;14(2):380–398. doi:10.1007/s41811‐021‐00105‐x
- 17. Giese‐Davis J, Collie K, Rancourt KMS, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. doi:10.1200/jco.2010.28.4455
- 18. Troubat R, Barone P, Leman S, et al. Neuroinflammation and depression: a review. Eur J Neurosci. 2021;53(1):151–171. doi:10.1111/ejn.14720
- 19. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–1240. doi:10.1002/cncr.29194
- 20. Kenne Sarenmalm E, Martensson LB, Andersson BA, Karlsson P, Bergh I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017;6(5):1108–1122. doi:10.1002/cam4.1052
- 21. Cillessen L, Johannsen M, Speckens AEM, Zachariae R. Mindfulness‐based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta‐analysis of randomized controlled trials. Psychooncology. 2019;28(12):2257–2269. doi:10.1002/pon.5214
- 22. Nissen ER, O’Connor M, Kaldo V, et al. Internet‐delivered mindfulness‐based cognitive therapy for anxiety and depression in cancer survivors: a randomized controlled trial. Psychooncology. 2020;29(1):68–75. doi:10.1002/pon.5237
- 23. Xunlin N, Lau Y, Klainin‐Yobas P. The effectiveness of mindfulness‐based interventions among cancer patients and survivors: a systematic review and meta‐analysis. Support Care Cancer. 2020;28(4):1563–1578. doi:10.1007/s00520‐019‐05219‐9
- 24. Jia Y, Liu L, Sheng C, et al. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J Nerv Ment Dis. 2019;207(4):271–276. doi:10.1097/nmd.0000000000000957
- 25. Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17(8):497–511. doi:10.1038/nrn.2016.69
- 26. Yao X, Huang J, Zhong H, et al. Targeting interleukin‐6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–139. doi:10.1016/j.pharmthera.2013.09.004
- 27. Asslih S, Damri O, Agam G. Neuroinflammation as a common denominator of complex diseases (cancer, diabetes type 2, and neuropsychiatric disorders). Int J Mol Sci. 2021;22(11):6138. doi:10.3390/ijms22116138
- 28. Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J Psychiatry. 2016;6(3):283–293. doi:10.5498/wjp.v6.i3.283
- 29. Heinze S, Egberts F, Rotzer S, et al. Depressive mood changes and psychiatric symptoms during 12‐month low‐dose interferon‐alpha treatment in patients with malignant melanoma: results from the multicenter DeCOG trial. J Immunother. 2010;33(1):106–114. doi:10.1097/cji.0b013e3181b8bdb9
- 30. Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa‐2b therapy. J Clin Oncol. 2000;18(10):2143–2151. doi:10.1200/jco.2000.18.10.2143
- 31. McIntyre RS, Subramaniapillai M, Lee Y, et al. Efficacy of adjuctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(8):783–790. doi:10.1001/jamapsychiatry.2019.0779
- 32. Kohler‐Forsberg O, Lydholm CN, Hjorthoj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti‐inflammatory treatment on major depressive disorder or depressive symptoms: meta‐analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404–419. doi:10.1111/acps.13016
- 33. Binder S, Luciano M, Horejs‐Hoeck J. The cytokine network in acute myeloid leukemia (AML): a focus on pro‐ and anti‐inflammatory mediators. Cytokine Growth Factor Rev. 2018;43:8–15. doi:10.1016/j.cytogfr.2018.08.004
- 34. Tripsianis G, Papadopoulou E, Romanidis K, et al. Overall survival and clinicopathological characteristics of patients with breast cancer in relation to the expression pattern of HER‐2, IL‐6, TNF‐α and TGF‐β1. Asian Pac J Cancer Prev. 2013;14(11):6813–6820. doi:10.7314/apjcp.2013.14.11.6813
- 35. Tripsianis G, Papadopoulou E, Anagnostopoulos K, et al. Coexpression of IL‐6 and TNF‐α: prognostic significance on breast cancer outcome. Neoplasma. 2014;61(2):205–212. doi:10.4149/neo_2014_026
- 36. Schrepf A, Thaker PH, Goodheart MJ, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256–267. doi:10.1016/j.psyneuen.2015.01.010
- 37. Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73(9):724–730. doi:10.1097/psy.0b013e318235be76
- 38. Lengacher CA, Reich RR, Paterson CL, et al. A large randomized trial: effects of mindfulness‐based stress reduction (MBSR) for breast cancer (BC) survivors on salivary cortisol and IL‐6. Biol Res Nurs. 2019;21(1):39–49. doi:10.1177/1099800418789777
- 39. Ngamkham S, Holden JE, Smith EL. A systematic review: mindfulness intervention for cancer‐related pain. Asia Pac J Oncol Nurs. 2019;6(2);161–169. doi:10.4103/apjon.apjon_67_18
- 40. Manchikanti L, Manchikanti K, Kaye A, Kaye A, Hirsch J. Challenges and concerns of persistent opioid use in cancer patients. Expert Rev Anticancer Ther. 2018;18(7):705–718. doi:10.1080/14737140.2018.1474103
- 41. Ramirez M, Rangel F, Cata J. Perioperative pain, analgesics and cancer‐related outcomes: where do we stand? Pain Manag. 2022;12(2):229–242. doi:10.2217/pmt‐2021‐0070
- 42. Edison L, Murphy A. Inflammatory mediators of opioid tolerance: implications for dependency and addiction. Peptides. 2019;115:51–58. doi:10.1016/j.peptides.2019.01.003
- 43. Zhou J, Ma R, Jin Y, et al. Molecular mechanisms of opioid tolerance: from opioid receptors to inflammatory mediators. Exp Ther Med. 2021;22(3):1004. doi:10.3892/etm.2021.10437
- 44. Hutchinson M, Benjamen C, Lewis S, et al. Proinflammatory cytokines oppose opioid‐induced acute and chronic analgesia. Brain Behav Immun. 2008;22(8):1178–1189. doi:10.1016/j.bbi.2008/05.004
- 45. Lucia M, Luca T, Federica D, et al. Opioids and breast cancer recurrence: a systematic review. Cancers (Basel). 2021;13(21):5499. doi:10.3390/cancers13215499
- 46. Zheng J, He J, Wang W, et al. The impact of pain and opioids use on survival in cancer patients: results from a population‐based cohort study and a meta‐analysis. Medicine. 2020;99(9):e19306. doi:10.1097/md.0000000000019306
- 47. Maher D, Wong W, White P, et al. Association of increased postoperative opioid administration with non‐small‐cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113(1):i88–i94. doi:10.1093/bja/aeu192
- 48. Cata J, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non‐small cell lung cancer resection. Cancer Med. 2014;3(4):900–908. doi:10.1002/cam4.236
- 49. Oh TK, Jeon JH, Lee JM, et al. Association of high‐dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: reinterpreting ERAS protocols for long‐term oncologic surgery outcomes. Dis Esophagus. 2017;30:1–8. doi:10.1093/dote/dox074
- 50. Rangel F, Auler J Jr, Carmona M, et al. Opioids and premature biochemical recurrence of prostate cancer: a randomized prospective clinical trial. Br J Anaesth. 2021;136(5):931–939. doi:10.1016/j.bja.2021.01.031
- 51. Cronin‐Fenton D, Heide‐Jorgensen U, Ahern T, et al. Opioids and breast cancer recurrence: a Danish population‐based cohort study. Cancer. 2015;121(19):3507–3514. doi:10.1002/cncr.29532
- 52. Matthews T, Danese A, Wertz J, et al. Social isolation, loneliness and depression in young adulthood: a behavioral genetic analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(3):339–348. doi:10.1007/S00127‐016‐1178‐7
- 53. Taylor H, Taylor R, Nguyen A, Chatters L. Social isolation, depression, and psychological distress among older adults. J Aging Health. 2018;30(2):229–246. doi:10.1177/0898264316673511
- 54. Powell N, Tarr A, Sheridan J. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(suppl):41–47. doi:10.1016/j.bbi.2012.06.015
- 55. Verza F, Valente V, Oliveira L, et al. Social isolation stress facilitates chemically induced oral carcinogenesis. PLoS One. 2021;16(1):e0245190. doi:10.1371/journal.pone.0245190
- 56. Hafner S, Emeny R, Lacruz M, Baumert J, Herder C, Koenig W. Association between social isolation and inflammatory markers in depressed and non‐depressed individuals: results from the MONICA/KORA study. Brain Behav Immun. 2011;25(8):1701–1707. doi:10.1016/j.bbi.2011.06.017
- 57. Kroenke C, Kubzansky L, Schernhammer E, Holmes M, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Am J Clin Oncol. 2006;24(7):1105–1111. doi:10.1200/jco.2005.04.2846
- 58. Reynolds P, Boyd PT, Blacklow R, et al. The relationship between social ties and survival among black and white breast cancer patients: National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1994;3(3):253–259.
- 59. Pinquart M, Duberstein P. Associations of social networks with cancer mortality: a meta‐analysis. Crit Rev Oncol Hematol. 2010;75(2):122–137. doi:10.1016/j.critrevonc.2009.06.003
- 60. Sprehn G, Chambers J, Saykin A, Konski A, Johnstone P. Decreased cancer survival in individuals separated at time of diagnosis. Cancer. 2009;115(21):5108–5116. doi:10.1002/cncr.24547
- 61. Walsh E, Eisenlohr‐Moul T, Baer R. Brief mindfulness training reduces salivary IL‐6 and TNF‐α in young women with depressive symptomatology. J Consult Clin Psychol. 2016;84(10):887–897. doi:10.1037/ccp0000122
- 62. Hoge EA, Bui E, Palitz SA, et al. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018;262:328–332. doi:10.1016/j.psychres.2017.01.006
- 63. Sanada K, Montero‐Marin J, Barcelo‐Soler A, et al. Effects of mindfulness‐based interventions on biomarkers and low‐grade inflammation in patients with psychiatric disorders: a meta‐analytic review. Int J Mol Sci. 2020;21(7):2484. doi:10.3390/ijms21072484
- 64. Hilton L, Hempel S, Ewing B, et al. Mindfulness meditation for chronic pain: systematic review and meta‐analysis. Ann Behav Med. 2017;51(2):199–213. doi:10.1007/S12160‐016‐9844‐2
- 65. Mascaro J, Singh V, Wehrmeyer K, et al. Randomized, wait‐list controlled pilot study of app‐delivered mindfulness for patients reporting chronic pain. Pain Rep. 2021;6(1):e924. doi:10.1097/pr9.0000000000000924
- 66. Feng B, Hu X, Lu W, Wang Y, Ip W. Are mindfulness treatments effective for pain in cancer patients? A systematic review and meta‐analysis. Eur J Pain. 2022;26(1):61–76. doi:10.1002/ejp.1849
- 67. Veronese N, Galvano D, D’Antiga F, et al. Interventions for reducing loneliness: an umbrella review of intervention studies. Health Soc Care Community. 2021;29(5):e89–e96. doi:10.1111/hsc.13248
- 68. Brown SL, Hughes M, Campbell S, Cherry MG. Could worry and rumination mediate relationships between self‐compassion and psychological distress in breast cancer survivors? Clin Psychol Psychother. 2020;27(1):1–10. doi:10.1002/cpp.2399
- 69. Kechter A, Amaro H, Black DS. Reporting of treatment fidelity in mindfulness‐based intervention trials: a review and new tool using NIH Behavior Change Consortium guidelines. Mindfulness. 2019;10:215–233. doi:10.1007/s12671‐018‐0974‐4
- 70. Lutz A, Slagter H, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi:10.1016/j.tics.2008.01.005
- 71. Davidson R, Kaszniak A. Conceptual and methodological issues in research on mindfulness and meditation. Am Psychol. 2015;70(7):581–592. doi:10.1037/a0039512
- 72. Olano HA, Kachan D, Tannenbaum SL, Mehta A, Annane D, Lee DJ. Engagement in mindfulness practices by U.S. adults: sociodemographic barriers. J Altern Complement Med. 2015;21(2):100–102. doi:10.1089/acm.2014.0269
- 73. Prouix J, Croff R, Oken B, Aldwin CM, Fleming C, Bergen‐Cico D. Considerations for research and development of culturally relevant mindfulness interventions in American minority communities. Mindfulness. 2018;9:361–370. doi:10.1007/s12671‐017‐0785‐z