Introduction
The addition of phosphates to topical fluoride (F) products has shown to increase the effects of F against demineralization in vitro [Takeshita et al., 2015] and in situ [Emerenciano et al., 2018]. Recently, several studies have shown increased effectiveness of 500 or 1,100 ppm F toothpastes supplemented with phosphates on the prevention of enamel demineralization and on the enhancement of remineralization, in comparison with their counterparts without phosphates [Moretto et al., 2010; do Amaral et al., 2013; Zaze et al., 2014; de Castro et al., 2015; Takeshita et al., 2015; Freire et al., 2016; Takeshita et al., 2016; Danelon et al., 2017]. The hypotheses on the effects of phosphates on the reduction of enamel mineral loss are mainly based on their ability to bind to hydroxyapatite hydroxyl groups, forming a layer that would retain calcium (Ca), phosphate (PO4), and F and increase their availability during de- and remineralization [do Amaral et al., 2013; de Castro et al., 2015; Takeshita et al., 2015; Takeshita et al., 2016; Danelon et al., 2017]. These hypotheses were considered for sodium trimetaphosphate (TMP) and calcium glycerophosphate (CaGP). Given that TMP and CaGP are inorganic cyclic and organic phosphates (phospholipid), respectively, their interactions with the enamel surface may differ. In this sense, it is essential to evaluate their interactions with the enamel surface in an environment rich in Ca2+ and PO43− (simulating the salivary environment) to corroborate the scientific evidence supporting the hypotheses raised.
Nonetheless, it is known that the surface properties of a substrate and its interaction with the environment can be analyzed using surface free energy (γs). The method uses principles of thermodynamics, and, depending on the approach, it is possible to demonstrate the reactive forces (nonpolar and/or polar) [Comyn, 1992; Ueta, 2016] that are involved in the interaction between enamel and phosphates [Neves et al., 2018] and ascertain their effects on donor or recipient surface electrons, which can change their reactivity with the environment. A previous study has shown that sodium hexametaphosphate treatment increases electron donor sites and leads to higher adsorption of calcium and phosphate to the enamel [Neves et al., 2018].
Based on the above, the aim of the present in vitro study was to analyze γs of the dental enamel after treatment with different concentrations of TMP or CaGP and exposure or nonexposure to solutions containing Ca2+ and PO43−, as well as to determine the adsorption of TMP, CaGP, Ca2+, and PO43− on dental enamel. The null hypothesis was that γs of enamel would not change after treatment with TMP or CaGP, and the exposure to solutions containing Ca2+ and PO43− would not influence their adsorption to the enamel.
Materials and Methods
Enamel Block Preparation
Bovine incisor teeth were extracted from 2 to 3 cattle of the same breed (Nelore Cattle, Brazil) and maintained in formalin solution (pH 7.0) for 1 month. Afterward, enamel blocks (4 mm × 4 mm, n = 192, 24 blocks/group) were obtained from the vestibulo-cervical region of the teeth, and their enamel surfaces were sequentially polished using water-cooled silicon carbide paper disks (600, 800, and 1,200 grit; Extec, Enfield, CT, USA) and a grinding polisher (Vector-Phoenix Beta; Buehler, Lake Bluff, IL, USA). Final polishing was performed with a felt disk (Polishing Pads; LAP MASTER FNL-A008, Mount Prospect, IL, USA) moistened in an aqueous diamond suspension (0.25 μm; Extec, Enfield, CT, USA). Between each disk and at the end, the blocks were cleaned by ultrasound (Unique USC 1400; Indaiatuba, SP, Brazil) at 40 Hz in deionized water for 20 min. Enamel blocks with flat surfaces and without cracks and hypoplasia were utilized in the experiment.
Solutions and Treatment of Enamel Blocks
Solutions containing TMP (CAS 7785-84-4; Sigma-Aldrich Co., St. Louis, MO, USA) were prepared at concentrations of 1% (pH 6.04), 3% (pH 6.21), 9% (pH 5.89) [Amaral et al., 2018], and 0% (without TMP, distilled/deionized water). CaGP-containing solutions (β-isomer, CAS 58409-70-4; Sigma-Aldrich Chemistry, St. Louis, MO, USA) were formulated at concentrations of 0.25% (pH 7.52), 0.5% (pH 7.78), 1.0% (pH 9.22) [Zaze et al., 2014], and 0% (without CaGP, water). Solutions containing Ca2+ and PO43− (1.25 mmol Ca [NO3]2·4H2O and 3.5 mmol/L NaH2PO4·2H2O, pH 5.33; Sigma-Aldrich) were prepared [ten Cate, 2012]. The blocks (n = 24/group) were immersed in individual vials containing 1 mL of a treatment solution (TMP or CaGP) under constant agitation for 2 min (THE-420 Shaker Table Orbital; Tecnal, Piracicaba, SP, Brazil). Subsequently, they were gently washed with deionized water for 30 s and dried with a paper towel. Twelve blocks were stored in an environment with a relative humidity of 44 ± 6% and a temperature of 23°C (HTH-240 Digital Thermo-Hygromer; Hikari, São Paulo, SP, Brazil). The remaining blocks (n = 12/group) were immersed in 1 mL of a solution containing Ca2+ and PO43− (CaPO4-containing solution) while stirring for 2 min, washed with water, and dried as described above.
Determination of TMP, CaGP, Ca2+, and PO43− in the Solutions
TMP and CaGP were measured after acid hydrolysis by phosphorus (P) determination. For TMP analysis, 0.1 mL aliquots of samples were added to 100 μL of 1.0 mol/L hydrochloric acid and heated in a boiling bath at 100°C for 1 h. Subsequently, 50 μL molybdate and 20 μL reducing reagent were added to 100 μL aliquots of the samples and standards (1.5, 3, 6, 12, and 24 μg P/mL). P was determined by the colorimetric method (at 660 nm wavelength) [Fiske & Subbarow, 1925] using 96-well plates and a spectrophotometer (Microplate Spectrophotometer EON; Biotek, Winooski, VT, USA). For CaGP analysis, 100 μL aliquots of samples were added to 20 μL 0.05 mol/L sulfuric acid and 20 μL 1% periodic acid and heated in a boiling bath at 100°C for 1 h. After cooling, 800 μL of deionized water was added. Subsequently, 55 μL aliquots of the samples and standards (1.5, 3, 6, 12, and 24 μg P/mL) were pipetted into 96-well plates (Kasvi; São Jose dos Pinhais, PR, Brazil), and 10 μL of 8% sodium sulfite and 5 μL of 7% sodium molybdate were added. After homogenization of the reagents, 5 μL of 1% hydroquinone was added [Anderson et al., 1977]. The plate was incubated for 30 min at 37°C, and the volume of each well was adjusted with 175 μL of deionized water. P was determined using the wavelength colorimetric method at 640 nm using a plate reader (Microplate Spectrophotometer EON; Biotek). Ca analysis was performed using the Arsenazo III colorimetric method [Vogel et al., 1983] with a 650-nm wavelength microplate reader. Aliquots of 5 μL were collected from the samples, and 50 μL deionized water, 50 μL Arsenazo, and the standards (40, 80, 120, 160, and 200 μg Ca/mL) were added. The P concentrations of the CaPO4-containing solutions were determined, as described above, for TMP dosing without hydrolysis. The dosages were determined from the solutions before and after the enamel surface treatment in duplicate. Enamel adsorption was calculated as the difference (Δ µg) between the final and initial TMP, CaGP (GP), Ca2+ from CaGP (Ca2+-GP), Ca2+, and PO43− concentrations in the solutions; negative values indicated the decrease in the solutions due to the enamel adsorption.
Surface and Interaction Free Energy Analysis
To evaluate the mechanism of TMP and CaGP interaction with the enamel surface, all blocks were analyzed for surface free energy (γs) and apolar (γsLW: Lifshitz-van der Waals) and polar (γsAB: acid/base) components. Measurements were performed with an automatic goniometer (DSA100S; KRÜSS GMBH, Hamburg, Germany) using 3 liquid probes with known surface energy: deionized water (polar), di-iodomethane (nonpolar), and ethylene glycol (polar with acid and base components). Prior to the measurements, the blocks were kept in air for 45 min to stabilize the treatment layer [van der Mei et al., 2002]. For contact angle determination, a 0.5 μL drop of each liquid was dispensed into a different quadrant on the enamel surface using a glass syringe (500 μL) and a 0.5-mm-gauge needle. After 1 s after drop deposition, the contact angles (right and left) were measured using a CCD camera for image capture and the Laplace-Young shape analysis method (Drop Shape Analysis DSA4 Software, version 2.0-01; Krüss). The contact angles of each drop were measured 5 times over 5 s at a temperature of 23°C and a relative humidity of 44 ± 6% (HTH-240 Digital Thermo-Hygrometer; Hikari, São Paulo, SP, Brazil) [van der Mei et al., 2002; Harnett et al., 2007]. γsLW, γsAB, and acid (γs+, donor component) and base (γs−, receptor component) parameters of surface free energy (mN/m) were calculated according to the model of Van Oss, Chaudhury, and Good [van Oss, 1995; Chaudhury, 1996; van der Mei et al., 2002; Knorr et al., 2005; Harnett et al., 2007]. The interaction free energy (ΔGiwi) was also calculated to determine the hydrophobicity/hydrophilicity of the enamel surface: ΔGiwi > 0 indicates a hydrophilic surface, and ΔGiwi < 0 indicates a hydrophobic surface [Harnett et al., 2007]. ΔGiwi = −2(√γsLW − √γwLW)2 − 4(√γs+γs− + √γw+γw− − √γs+γw− − √γs−γw+) [Harnett et al., 2007].
Statistical Analysis
For data analysis, the TMP and CaGP concentrations and exposure to CaPO4-containing solutions were considered as variation factors. The surface and interaction free energy values (mN/m), the polar and nonpolar components, and the adsorption values (Δ µg) of TMP, GP, Ca2+-GP, Ca2+, and PO43− were considered as variables. After normality and homogeneity tests, data were submitted to 2-way (surface free energy analysis) and 1-way (solutions analysis) analysis of variance followed by the Student-Newman-Keuls test. The Ca2+-GP and PO43− data from CaGP experiments were heterogeneous, and they were submitted to Kruskal-Wallis and Student-Newman-Keuls tests. Pearson or Spearman correlation tests were applied to the variables [Dancey & Reidy, 2011]. The statistical program SigmaPlot version 12.0 was used, and statistical significance was set at 5%.
Results
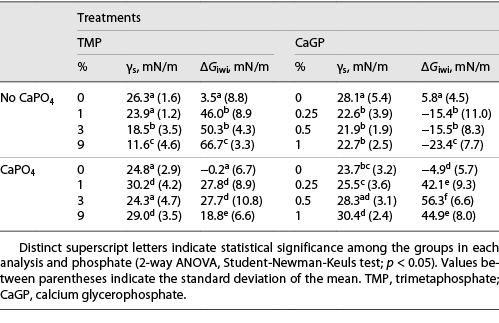
TMP treatment (1%, 3%, and 9%) of enamel led to a decrease in γs and an increase in ΔGiwi (p < 0.001), showing a dose-response relationship (Table 1). The values of γsAB, γs−, and ΔGiwiAB followed this trend (Fig 1a, b, c). TMP adsorption to enamel showed a dose-response relationship with concentrations in the treatment solutions (Fig. 1d) and strong correlation (Spearman) with the variables (p < 0.001): γs (r = 0.852), γsAB (r = 0.845), γs− (r = 0.904), ΔGiwi (r = −0.862), and ΔGiwiAB (r = −0.872). When TMP-pretreated enamels were exposed to CaPO4-containing solutions, there was an increase in γs values and a decrease in ΔGiwi values (p < 0.001). The γsAB values were less negative, and γs− and ΔGiwiAB values decreased (p < 0.001). Ca2+ adsorption for 1%, 3%, and 9% TMP increased compared to 0% TMP (p < 0.001), but the differences among them were not significant (p ≥ 0.228). For PO43−, adsorption raised with increasing percentage of TMP in the solutions (p < 0.001). There was a strong correlation (Pearson) between Ca2+ and PO43−: γs− (r = −0.858 and r = −0.888), ΔGiwi (r = −0.885 and r = −0.859), and ΔGiwiAB (r = −0.894 and r = −0.861).
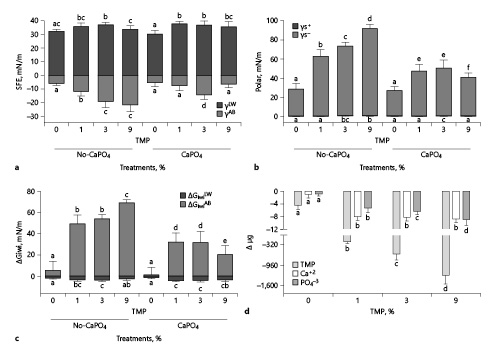
Fig. 1
Mean values of the apolar (γLW: Lifshitz-van Der Waals) and polar (γsAB: Lewis acid-base) components of surface free energy (a) and the acid (γs+: receptor of electrons) and base (γs−: donor of electrons) components of polar energy (b) stratified by %TMP and exposure to CaPO4-containing solution. The mean values of the apolar (ΔGiwiLW) and polar (ΔGiwiAB) components of the interaction free energy (c). Mean values of TMP, Ca2+, and PO43− adsorption to enamel stratified by %TMP (d). The vertical bars indicate standard deviations. Distinct letters indicate a significant difference between groups in each analysis (n = 12; Student-Newman-Keuls; p < 0.05). TMP, trimetaphosphate.
For enamel treated with 0.25%, 0.5%, and 1% CaGP, γs (Table 1) and γsLW (Fig. 2a) values decreased when compared to 0% CaGP (p < 0.001). No difference was found among treatments containing CaGP (γs: p ≥ 0.599; and γsLW: p ≥ 0.784). However, γsAB values did not change with the variation in the percentages of CaGP (p ≥ 0.677). The γs− values (Fig. 2b) for 0.25%, 0.5%, and 1% CaGP were lower than those of 0% CaGP (p < 0.001); there were no significant differences among them (p ≥ 0.111). ΔGiwi for 0.25%, 0.5%, and 1% CaGP (Table 1) had more negative values than those of 0% CaGP (p < 0.001) as well as its polar component (ΔGiwiAB; Fig. 2c). CaGP adsorption to enamel (Fig. 2d) increased according to its concentration in the treatment solutions (p < 0.001). After exposure to CaPO4-containing solutions, 0.25%, 0.5%, and 1% CaGP-pretreated enamel showed an increase in γs, γsLW, γsAB, and γs− and positive ΔGiwi and ΔGiwiAB values (p < 0.001). However, for 0% CaGP, there was a decrease in γs and γs− and negative ΔGiwi and ΔGiwiAB values (p < 0.001). There was no adsorption of Ca2+ on enamel for 0.25%, 0.5%, and 1% CaGP and small PO43− adsorption on enamel (Fig. 2d).
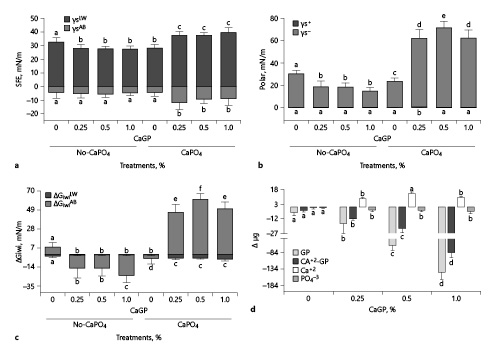
Fig. 2
Mean values of the apolar (γLW: Lifshitz-van Der Waals) and polar (γsAB: Lewis acid-base) components of surface free energy (a) and the acid (γs+: receptor of electrons) and base (γs−: donor of electrons) components of polar energy (b) stratified by GP and exposure to CaPO4-containing solution. The mean values of the apolar (ΔGiwiLW) and polar (ΔGiwiAB) components of the interaction free energy (c). Mean values of GP, Ca2+-GP (Ca2+ from GP), Ca2+, and PO43− adsorption to enamel stratified by GP (d). The vertical bars indicate standard deviations. Distinct letters indicate significant difference between groups in each analysis (n = 12; Student-Newman-Keuls; p < 0.05). GP, glycerophosphate; CaGP, calcium glycerophosphate.
Discussion/Conclusion
Since the enamel surface does not have characteristics that favor calcium and phosphate adsorption [Neves et al., 2018], this study evaluated the effect of CaGP and TMP treatment at different concentrations on the surface free energy as well as calcium and phosphate adsorption to bovine enamel. As noted earlier in this study [Neves et al., 2018], the flattened enamel surface (no-TMP and CaGP) was slightly hydrophilic as γs was <30 mN/m (Table 1) [Knorr et al., 2005], ΔGiwi was slightly >0, and γs− was close to 28.5 mN/m (Fig. 1, 2) [van Oss, 1995; Harnett et al., 2007], with values of γs+ close to zero. Moreover, bovine enamel can be a suitable surrogate for human enamel because it resembles the ultrastructural architecture, mineral composition [Nogueira et al., 2014; Teruel et al., 2015], and lubricity conditions [Reeh et al., 2015]. Given that the acid-base theoretical approach was used in this study [van Oss, 1995; Harnett et al., 2007], γs is strongly influenced by its components γsLW and γsAB. Thus, the outcome may differ from that of other studies that used different theoretical approaches to obtain γs. In addition, the acid (γs+)/base (γs−) forces indicate whether a surface is more hydrophobic or hydrophilic since dispersive forces (nonpolar energy) are always present [van Oss, 1995; Harnett et al., 2007]. The results showed that TMP treatment favored Ca2+ and PO43− adsorption by reducing γs, with a dose-response relationship between TMP concentrations in solutions, γsAB, and γs−. Treatment with CaGP solutions showed that CaGP was adsorbed to enamel, reducing γs due to lower γsLW values. Thus, the null hypothesis was rejected.
Considering the abovementioned studies, which assessed only ΔGiwi, it can be stated that TMP treatment led to a pronounced increase in the hydrophilic character of the enamel surface, making it prone to wetting since it was strongly influenced by its acid-base component (ΔGiwiAB; Fig. 1c) [Van Oss, 1995; Harnett et al., 2007]. Furthermore, in line with these trends, the values of γs− >28 mN/m were observed (Fig. 1b), indicating a hydrophilic surface after TMP treatment [Olsson et al., 1992; van Oss, 1995]. TMP-untreated enamel adsorbed 4× less calcium than the TMP-treated surface (Fig. 1d). This indicated a minimal binding of phosphate ions to the enamel surface [Harding et al., 2005; Vandiver et al., 2005], given that the values of the polar component (γsAB) and its parameters (γs+; γs−) did not change. It can be concluded, therefore, that the enamel does not favor Ca2+ and PO43− adsorption. Nonetheless, in the oral environment, proline- and tyrosine-rich protein (acquired film) adsorption to the enamel facilitates the binding of Ca2+ and PO43− from saliva [Buzalaf et al., 2012]. Ca2+ adsorption without a change in the surface charge probably occurs by displacing 2 surface H+ ions with 1 Ca2+ [Harding et al., 2005].
The data show that TMP gets adsorbed by enamel (Fig. 1d) even though both are negatively charged through the interaction between the anionic sites of TMP and hydroxyapatite hydroxyl groups [Delbem et al., 2014; Amaral et al., 2018]. Consequently, enamel surfaces acquire more electron donor sites through TMP adsorption (Fig. 1b, d). Thus, they may be partially or totally neutralized by Ca2+ or CaH2PO4+ present in CaPO4-containing solutions [Neves et al., 2018]. It can be observed that more Ca2+ was adsorbed to the enamel than PO43− (Fig. 1d), which may be explained by the reduction of γs− and less negative values for γsAB (Fig. 1a, b). Since Ca2+ and PO43− nucleation occurs in the adsorbed TMP layer, there was an oversaturation of these ions close to the enamel surface. Thus, precipitation did not occur directly on the enamel; this kept the surface pores open and facilitated the diffusion of ions into enamel [Takeshita et al., 2011; Takeshita et al., 2015; Takeshita et al., 2016; Emerenciano et al., 2018]. It is reasonable to assume that the pattern found in the in situ studies [Takeshita et al., 2015; Takeshita et al., 2016; Emerenciano et al., 2018] that showed higher Ca content in enamel in the presence of TMP is a consequence of this mechanism. The results of the present study provide new insights to help explain the mechanism of TMP in reducing demineralization and increasing deep remineralization in the enamel [Takeshita et al., 2011; de Castro et al., 2015; Takeshita et al., 2015; Takeshita et al., 2016; Danelon et al., 2017; Emerenciano et al., 2018], thus confirming the hypotheses of previous studies.
Unlike TMP, CaGP treatment of enamel led to a hydrophobic surface as there was a reduction in γs due to the reduction in γsLW values (20% reduction) and no significant change in γsAB values (Fig. 2a). The results of γs− confirm these findings as there was a 43% reduction in their values (<28.5 mN/m) and the negative values of ΔGiwi that reinforced the enamel surface as hydrophobic (Fig. 2b, c) [van Oss, 1995] after CaGP treatment, regardless of the concentration used. Van Der Waals interactions occur between all atoms and molecules (nonpolar energy). Therefore, there are no surface energy substances with only polar energy. However, some surfaces may contain no polar energy, such as hydrocarbons. In enamel, most of the surface energy comes from its apolar component and smaller polar component. CaGP is a phospholipid composed of glycerol, which has a functional group of alcohol and phosphate (polar). Although the glycerol moiety contains hydrocarbons (nonpolar), it also contains oxygen that adds a polar component to the molecule. Therefore, water and polar molecules have greater difficulty adhering to this surface. This suggests that the nonchange in polar energy values after treatments with CaGP and CaPO4-containing solutions is due to the enamel surface energy.
The structural arrangement of CaGP adsorbed to the enamel surface, considering that the union between glycerol and phosphate is the stable part of the molecule, produces a shell arrangement of the molecule in the enamel [Inoue et al., 1992]. Thus, a film forms with this characteristic that leads to less apolar energy when the enamel is treated with solutions containing CaGP. This explains the reduction in enamel surface energy as the polar component did not change after treatments. Nevertheless, there was a decrease in electron donor sites, the basic component of polar energy (Fig. 2b). Lower values of the basic component of polar energy may indicate that the CaGP shell arrangement reacts with enamel hydroxyapatite so that the nonpolar part of the molecule (glycerol) faces the enamel surface, and the polar part (phosphate) to the outside with Ca atoms arranged superficially in the CaGP layer allows for adsorption by the enamel. Considering these data, CaGP-only treatments produce a more hydrophobic surface that does not favor cell or protein adhesion to the enamel surface or Ca2+ or CaH2PO4+ adsorption (Fig. 2d).
The bond between the calcium and phosphate group of the CaGP structure is considered to be the unstable part of the molecule [Inoue et al., 1992]. This suggests that this instability favors the release of Ca2+ to the medium (Fig. 2d), leaving free anionic phosphate groups. Thus, this explains the increase in electron donor sites after treatment with CaPO4 solution and the increase in Ca2+ in the CaPO4 solution (Fig. 2b, d). Thus, the CaGP layer adsorbed to enamel would function only as a calcium source, favoring the reduction of mineral loss in caries processes, as observed in in vitro [Zaze et al., 2014] and in situ [do Amaral et al., 2013] studies. In these studies, the content of Ca2+ and PO43− in enamel increased with dentifrice formulations containing CaGP [do Amaral et al., 2013], as well as the amount of these ions in the biofilm [do Amaral et al., 2013]. Nevertheless, after Ca2+ release, the surface presents more electron donor sites, which may favor the Ca2+ and PO43− nucleation to enamel, being in an environment rich with these ions.
This study provides new insights into the mechanisms of action of TMP and CaGP on enamel de- and remineralization through the surface interactions between these phosphate molecules and enamel, a medium containing calcium and phosphate. Although both phosphates reduce enamel γs, it occurs in different ways. TMP, as it is an inorganic phosphate, makes γsAB more negative and increases γs−, which leads to greater calcium and phosphate adsorption. The present data provide evidence for our previous theory derived from in vitro studies [de Castro et al., 2015; Danelon et al., 2017; Gonçalves et al., 2021] which reported that TMP is adsorbed onto enamel and that this TMP layer can lead to greater adsorption of Ca2+ and PO43− compared to enamel. The enamel with the TMP-CaPO4 layer presented higher calcium and phosphate contents [Gonçalves et al., 2021] and less cariogenic demineralization than untreated enamel [Takeshita et al., 2011; de Castro et al., 2015; Danelon et al., 2017; Gonçalves et al., 2021]. CaGP, an organic phosphate with nonpolar and polar grouping, decreases γsLW and γs−, but γs− increases after Ca2+ release from the molecule. The results confirm the previous hypothesis that CaGP is adsorbed by the enamel [Zaze et al., 2014] and provides an environment with a greater amount of calcium and phosphate [do Amaral et al., 2013] and smaller enamel subsurface lesions [do Amaral et al., 2013; Zaze et al., 2014].
The present study contributes to a better understanding of the mechanism of action of TMP and CaGP under in vitro [Takeshita et al., 2011; Zaze et al., 2014; de Castro et al., 2015; Danelon et al., 2017; Gonçalves et al., 2021] and in situ [ do Amaral et al., 2013; Takeshita et al., 2015; Takeshita et al., 2016; Emerenciano et al., 2018] conditions. However, the results should be carefully evaluated, since the model did not factor in the presence of saliva. The treatment was performed once using aqueous solutions without the complex formulations tested in previous studies. Despite not providing direct clinical relevance, the present data provide the foundation and serve as a guide for future studies. In addition, the association between fluoride and its effect on γs and enamel adsorption should be investigated in order to improve our understanding of the mechanism of action of these phosphates.
TMP treatment made the enamel more hydrophilic, increased electron donor sites (γs−), and decreased enamel γs, favoring higher adsorption of calcium and phosphate to the enamel surface. CaGP formed a layer on the enamel surface, making it hydrophobic, reducing γs and γsLW, which did not favor Ca2+ and PO43− adsorption.
Acknowledgments
The authors thank CNPq (National Council for the Development of Science and Technology) grant #308981/2014-6 and high school scientific initiation scholarship for the third author #167780/2017-4 and CAPES (Higher Education Personal Improvement Coordination, grant #88887.068358/2014-00) for financial support of research. The authors thank JBS/FRIBOI slaughterhouse (Andradina, SP, Brazil) for the donation of bovine teeth.
Statement of Ethics
The research was ethically conducted in accordance with the world guides and Brazilian guidelines to the Animal Research (Law No. 11.794, October 8, 2008). Thus, no approval was required as it did not involve animal experiments, that is, procedures performed on live animals, but used bovine teeth obtained from slaughterhouses (JBS/FRIBOI; Andradina, SP, Brazil). In these cases, there is no indication or legal guideline for submission to the animal ethics committee.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by CNPq (National Council for the Development of Science and Technology, grant 308,981/2014-6) and CAPES (Higher Education Personal Improvement Coordination, grant 88887.068358/2014-00). The funding agencies had no role in the experimental design, data collection and analysis, publication decision, or manuscript preparation.
Author Contributions
Emanuelle Karine Prado Nalin, Emanuel Soares da Silva, and Marcele Danelon participated in the study design, performed the laboratory experiments, organized the data for statistical analysis, and contributed to the preparation of the manuscript. Juliano Pelim Pessan participated in the study design and was involved in the preparation of the manuscript. Thayse Yumi Hosida organized the data for statistical analysis and contributed to the preparation of the manuscript. Marcele Danelon participated in the study design and contributed to the preparation of the manuscript. Alberto Carlos Botazzo Delbem conceived the study idea, participated in the study design, supervised the laboratory experiments, organized the data for statistical analysis, and contributed to the preparation of the manuscript. All authors performed the revision and approval of the final version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Amaral JG, Pessan JP, Souza JAS, Moraes JCS, Delbem ACB. Cyclotriphosphate associated to fluoride increases hydroxyapatite resistance to acid attack. J Biomed Mater Res B Appl Biomater. 2018;106:2553–64. http://dx.doi.org/10.1002/jbm.b.34072.
- 2. Anderson W, Dingwall D, Stephen KW. Dissolution of two commercial preparations of calcium glycerophosphate in human saliva. Arch Oral Biol. 1977;22:159–62. http://dx.doi.org/10.1016/0003-9969(77)90148-0.
- 3. Buzalaf MA, Hannas AR, Kato MT. Saliva and dental erosion. J Appl Oral Sci. 2012;20:493–502. http://dx.doi.org/10.1590/s1678-77572012000500001.
- 4. Chaudhury MK. Interfacial interaction between low-energy surfaces. Mater Sci Eng R Rep. 1996;16(3):97–159. http://dx.doi.org/10.1016/0927-796x(95)00185-9.
- 5. Comyn J. Contact angles and adhesive bonding. Int J Adhes Adhes. 1992;12(3):145–9. http://dx.doi.org/10.1016/0143-7496(92)90045-w.
- 6. Dancey CP, Reidy J. Statistics without maths for psychology. 5rd ed.London: Pearson; 2011.
- 7. Danelon M, Pessan JP, Souza-Neto FN, de Camargo ER, Delbem AC. Effect of fluoride toothpaste with nano-sized trimetaphosphate on enamel demineralization: An in vitro Study. Arch Oral Biol. 2017;78:82–7. http://dx.doi.org/10.1016/j.archoralbio.2017.02.014.
- 8. de Castro LP, Delbem AC, Danelon M, Passarinho A, Percinoto C. In vitro effect of sodium trimetaphosphate additives to conventional toothpastes on enamel demineralization. Clin Oral Investig. 2015;19:1683–7. http://dx.doi.org/10.1007/s00784-015-1446-z.
- 9. Delbem AC, Souza JA, Zaze AC, Takeshita EM, Sassaki KT, Moraes JC. Effect of trimetaphosphate and fluoride association on hydroxyapatite dissolution and precipitation in vitro. Braz Dent J. 2014;25:479–84. http://dx.doi.org/10.1590/0103-6440201300174.
- 10. do Amaral JG, Sassaki KT, Martinhon CC, Delbem AC. Effect of low-fluoride dentifrices supplemented with calcium glycerophosphate on enamel desmineralization in situ. Am J Dent. 2013;26:75–80.
- 11. Emerenciano NG, Botazzo Delbem AC, Pessan JP, Nunes GP, Souza Neto FN, de Camargo ER, et al. In situ effect of fluoride toothpaste supplemented with nano-sized sodium trimetaphosphate on enamel demineralization prevention and biofilm composition. Arch Oral Biol. 2018;96:223–9. http://dx.doi.org/10.1016/j.archoralbio.2018.09.019.
- 12. Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66(2):375–400. http://dx.doi.org/10.1016/s0021-9258(18)84756-1.
- 13. Freire IR, Pessan JP, Amaral JG, Martinhon CC, Cunha RF, Delbem AC. Anticaries effect of low-fluoride dentifrices with phosphates in children: a randomized, controlled trial. J Dent. 2016;50:37–42. http://dx.doi.org/10.1016/j.jdent.2016.04.013.
- 14. Gonçalves FMC, Delbem ACB, Gomes LF, Emerenciano NG, dos Passos Silva M, Cannon ML, et al. Combined effect of casein phosphopeptide-amorphous calcium phosphate and sodium trimetaphosphate on the prevention of enamel demineralization and dental caries: an in vitro Study. Clin Oral Invest. 2021;25(5):2811–20. http://dx.doi.org/10.1007/s00784-020-03597-7.
- 15. Harding IS, Rashid N, Hing KA. Surface charge and the effect of excess calcium ions on the hydroxyapatite surface. Biomaterials. 2005;26:6818–26. http://dx.doi.org/10.1016/j.biomaterials.2005.04.060.
- 16. Harnett EM, Alderman J, Wood T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf B Biointerfaces. 2007;55:90–7. http://dx.doi.org/10.1016/j.colsurfb.2006.11.021.
- 17. Inoue M, In Y, Ishida T. Calcium binding to phospholipid: structural study of calcium glycerophosphate. J Lipid Res. 1992;33:985–94. http://dx.doi.org/10.1016/s0022-2275(20)41414-2.,
- 18. Knorr SD, Combe EC, Wolff LF, Hodges JS. The surface free energy of dental gold-based materials. Dent Mater. 2005;21:272–7. http://dx.doi.org/10.1016/j.dental.2004.06.002.
- 19. Moretto MJ, Magalhães AC, Sassaki KT, Delbem AC, Martinhon CC. Effect of different fluoride concentrations of experimental dentifrices on enamel erosion and abrasion. Caries Res. 2010;44:135–40. http://dx.doi.org/10.1159/000302902.
- 20. Neves JG, Danelon M, Pessan JP, Figueiredo LR, Camargo ER, Delbem ACB, et al. Surface free energy of enamel treated with sodium hexametaphosphate, calcium and phosphate. Arch Oral Biol. 2018;90:108–12. http://dx.doi.org/10.1016/j.archoralbio.2018.03.008.
- 21. Nogueira BCL, Fernandes PM, Paiva ACJ, Fagundes NCF, Teixeira FB, Lima RR. Avaliação comparativa da ultraestrutura e propriedades físicas do esmalte bovino, bubalino e humano. Pesq. Vet. Bras. 2014;34(5):485–90. http://dx.doi.org/10.1590/s0100-736x2014000500017.
- 22. Olsson J, van der Heijde Y, Holmberg K. Plaque formation in vivo and bacterial attachment in vitro on permanently hydrophobic and hydrophilic surfaces. Caries Res. 1992;26:428–33. http://dx.doi.org/10.1159/000261482.
- 23. Reeh ES, Douglas WH, Levine MJ. Lubrication of human and bovine enamel compared in an artificial mouth. Arch Oral Biol. 1995;40:1063–72. http://dx.doi.org/10.1016/0003-9969(95)00031-j.
- 24. Takeshita EM, Exterkate RA, Delbem AC, ten Cate JM. Evaluation of different fluoride concentrations supplemented with trimetaphosphate on enamel de- and remineralization in vitro. Caries Res. 2011;45:494–7. http://dx.doi.org/10.1159/000331209.
- 25. Takeshita EM, Danelon M, Castro LP, Sassaki KT, Delbem AC. Effectiveness of a toothpaste with low fluoride content combined with trimetaphosphate on dental biofilm and enamel demineralization in situ. Caries Res. 2015;49(4):394–400. http://dx.doi.org/10.1159/000381960.
- 26. Takeshita EM, Danelon M, Castro LP, Cunha RF, Delbem AC. Remineralizing potential of a low fluoride toothpaste with sodium trimetaphosphate: an in situ Study. Caries Res. 2016;50:571–8. http://dx.doi.org/10.1159/000449358.
- 27. ten Cate JM. The role of saliva in mineral equilibria – caries, erosion and calculus formation. In: Edgar WM, Dawes C, O’Mullane DM, editors. Saliva and oral health: an essential overview for the health professional. England: Stephen Hancocks Ltd; 2012. Vol. 4. p. 135–50.
- 28. Teruel JD, Alcolea A, Hernández A, Ruiz AJ. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol. 2015;60:768–75. http://dx.doi.org/10.1016/j.archoralbio.2015.01.014.
- 29. Ueta H, Tsujimoto A, Barkmeier WW, Oouchi H, Sai K, Takamizawa T, et al. Influence of an oxygen-inhibited layer on enamel bonding of dental adhesive systems: surface free-energy perspectives. Eur J Oral Sci. 2016;124:82–8. http://dx.doi.org/10.1111/eos.12231.
- 30. Vandiver J, Dean D, Patel N, Bonfield W, Ortiz C. Nanoscale variation in surface charge of synthetic hydroxyapatite detected by chemically and spatially specific high-resolution force spectroscopy. Biomaterials. 2005;26:271–83. http://dx.doi.org/10.1016/j.biomaterials.2004.02.053.
- 31. van Oss CJ. Hydrophobicity of biosurfaces - origin, quantitative determination and interaction energies. Colloids and Surfaces B: Biointerfaces. 1995;5(3-4):91–110. http://dx.doi.org/10.1016/0927-7765(95)01217-7.
- 32. van der Mei HC, White DJ, Kamminga-Rasker HJ, Knight J, Baig AA, Smit J, et al. Influence of dentifrices and dietary components in saliva on wettability of pellicle-coated enamel in vitro and in vivo. Eur J Oral Sci. 2002;110:434–8. http://dx.doi.org/10.1034/j.1600-0722.2002.21341.x.
- 33. Vogel GL, Chow LC, Brown WE. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983;17:23–31. http://dx.doi.org/10.1159/000260645.
- 34. Zaze AC, Dias AP, Sassaki KT, Delbem AC. The effects of low-fluoride toothpaste supplemented with calcium glycerophosphate on enamel demineralization. Clin Oral Investig. 2014;18:1619–24. http://dx.doi.org/10.1007/s00784-013-1140-y.