Introduction
Streptococcus mutans is consistently regarded as the primary pathogen of human dental caries [Selwitz et al., 2007; Lamont et al., 2018]. Exopolysaccharide (EPS), the important component of self-defense in cariogenic biofilm communities, is the vital virulence factor of S. mutans [Lei et al., 2020]. The water-soluble EPS is regarded to be responsible for energy resource. Meanwhile, the water-insoluble one was responsible for 3 dimensional (3D) structure of dental plaque [Hamada and Slade, 1980; Koo et al., 2013]. EPS is polymerized by multiple monosaccharide constituents, which play important roles in modulating cariogenicity of S. mutans, including α-monosaccharides and β-monosaccharides [Ebisu et al., 1975; Bowen and Koo, 2011; Zeng et al., 2018]. Several kinds of enzymes participate in the carbohydrate metabolism by transferring or cleaving different glycosidic bonds to produce or degrade EPS. For example, the well-known glucosyltransferase family of S. mutans, encoded by gtfB/C/D genes, is one of the enzymes, in which the GtfB has the ability to identify α-1,3-glycosidic linkages of water-insoluble glucans, and the GtfD could transfer α-1,6-glycosidic linkages in water-soluble glucans [Castillo Pedraza et al., 2020]. It also has been known that a system of carbohydrate reservoir closely related to fructosyltransferase, which synthesizes β(2,1)/β(2,6)-linked fructans [Lei et al., 2015]. Therefore, it is hypothesized that both α-glycosidic bonds and β-glycosidic bonds work federatively in contributing to the architecture and pathogenicity of S. mutans biofilm. The anomeric protons on C1 and C5 are on the same side in α-glycosidic bond while the anomeric protons on C1 and C5 are on the different sides in β-glycosidic bond, and this configuration makes β-monosaccharides more stable [Synytsya and Novak, 2013; Zhou et al., 2019]. Nevertheless, the information about the effects of glycosidic bonds on the biological characteristics of biofilm of S. mutans is still limited.
Ribonuclease III (RNase III), widely identified in bacteria and eukaryotes, is instrumental in RNA maturation by cleaving double-stranded RNA and thus control gene expression [Court et al., 2013; Aguado et al., 2017]. Several studies have illuminated that RNase III can modulate the metabolism of bacteria, such as influencing enterobactin production of Escherichia coli or cell division in Corynebacterium glutamicum [Lim et al., 2015; Maeda et al., 2016]. We have detected that the rnc gene, a kind of regulatory gene encoding RNase III of S. mutans, affects the EPS synthesis and biofilm forming at post-transcriptional level by microRNA-size small RNAs binding to the vicR gene and by vicR antisense RNA mitigating the functions of vicR [Mao et al., 2016, 2018; Lei et al., 2018, 2020]. VicR is a response regulator of the VicRK signal transduction system, which participates in EPS synthesis [Senadheera et al., 2005]. However, whether the regulatory effects of rnc gene on biofilm matrix of S. mutans are associated with the microstructure of EPS needs further elucidation.
In order to explore the microstructure of EPS produced by S. mutans, we have established a series of glycomics protocols, including gas chromatography-mass spectrometer (GC-MS) and 1H nuclear magnetic resonance (1H-NMR) [Lei et al., 2015]. In the present study, the role of rnc gene playing in modulating the micromolecular structure of the extracellular polysaccharide of S. mutans was investigated by these glycomics methods. The microstructural properties of EPS were ascertained using GC-MS for monosaccharide unit and proportion analysis, infrared radiation (IR) for functional groups verification, and 1H-NMR for hydrogen bond assessment. We found that the rnc gene regulated the microstructure of EPS by affecting the isomerization of β-glycosidic bonds. The results of the present study were conducive to further unveiling the mechanism of rnc gene in modulating EPS production and biofilm formation of S. mutans, which may provide a new potential micromolecular target for caries prevention and treatment.
Materials and Methods
Bacterial Strains and Construction of Mutant Strains
The bacterial strains and plasmids used in this study are presented in Table 1. The S. mutans parent strain, UA159 (ATCC 700610) was kindly provided by the State Key Laboratory of Oral Diseases (Sichuan University, Chengdu, China). The rnc-mutant strain (Smurnc) was constructed via allelic exchange with an erythromycin cassette, as previously described [Lau et al., 2002; Mao et al., 2016, 2018]. The rnc gene overexpressing strain (Smurnc+) was established by transforming the S. mutans UA159 with recombinant plasmid pDL278 which contained rnc-coding sequence and its upstream promoter region, as described previously [Mao et al., 2016, 2018].
Biofilm Growth Condition
UA159, Smurnc+, and Smurnc were revived on brain heart infusion (BHI; Oxoid, Basingstoke, UK) agar plates with 1 mg/mL spectinomycin or 10 μg/mL erythromycin when necessary for 48 h under anaerobic condition (10% CO2, 80% N2, 10%, and H2) at 37°C. A single colony was selected from each plate and incubated in fresh BHI broth overnight anaerobically. Then the strains were diluted by 1:20 ratio into fresh BHI broth and cultured to the exponential phase. The bacterial solution would be further titrated to an OD600 nm of 0.5 by an ultraviolet spectrophotometer before use (Helsinki, Finland). Equal volumes of each strain suspensions were diluted in BHI broth containing 1% (w/v) sucrose and incubated anaerobically for 24-h biofilm formation at 37°C.
Extraction, Isolation, and Purification of EPS
We performed extraction, isolation, and purification of EPS of biofilm according to previous protocols with modification [Lei et al., 2015; Mao et al., 2018]. In brief, after 24 h incubation in T-75 culture flasks with vent cap (Corning, USA), the biofilm samples were washed gently by sterile phosphate buffered saline and then the biofilms were collected by a sterile cell scraper into a sterile centrifuge tube. Suspensions were centrifuged (2,422 g) for 15 min at 4°C. The water-soluble EPS was in the supernatants, and the water-insoluble glucans were in the precipitates. Then the precipitates were dissolved in 1 M NaOH (Sigma, USA) and incubated for 2 h with shaking (120 rpm) at 37°C, followed by collecting the supernatant after centrifuging. We used trichloroacetic acid (Sigma, USA) with final concentration of 20% (w/v) to precipitate proteins overnight at 4°C in both water-soluble and water-insoluble EPS solutions. After these mixed solutions being centrifuged (2,422 g) for 15 min at 4°C, we discarded the precipitates and neutralized the acid and base of the supernatant. Then the supernatant containing water-soluble or water-insoluble EPS was filtered via a 0.45-μm filter (Milipore, USA) and dialyzed via 2,000 Da molecular weight cut-off (Viskase, USA) against sterile deionized water for 24 h at 4°C. Finally, the retentate was lyophilized at −65°C (Foring, China).
Identification of Monosaccharide Constituents
Two micrograms of lyophilized EPS were resolved in 1 mL trifluoroacetic acid with concentration of 2 M to hydrolyze for 90 min. The samples were rotationally evaporated after adding 2 mL methanol into it. Subsequently, the residue was dissolved with 2 mL distilled water and 60 mg sodium borohydride for 8 h, followed by glacial acetic acid for neutralization. Then the samples were evaporated to produce a powder form which was further dried at 110°C. One milliliter of acetic acid anhydride was added for acylation at 100°C for 1 h. After cooling, the solution was added 3 mL of methylbenzene and concentrated to remove the excess acetic anhydride. Then the product was dissolved with 3 mL of chloroform and transferred to the separation funnel. The chloroform layer was dried with anhydrous sodium sulfate and quantified to 10 mL [Fujii et al., 2012; Lei et al., 2015].
The EPS used GC-MS analysis by a 30-m × 0.25-mm × 320-μm chromatographic column. The temperature condition was programmed as initial temperature of 120°C at a warming speed of 3°C/min to 250°C and kept for 5 min. Both the injector temperature and the detector temperature were 250°C. The carrier gas was helium and the flow rate was 1 mL/min. The location of the peak and the standard sugar samples as rhamnose (Rha), fucose (Fuc), arabinose, xylose, mannose (Man), glucose (Glc), and galactose (Gal) were detected and compared to obtain the content components under the same circumstance [Lei et al., 2015].
Functional Group of Monosaccharides
Under infrared lamp baking, 200 mg of dried potassium bromide powder and 1 mg of EPS samples were grinded in an agate mortar in the same direction to avoid structural damage. The powders were put into the smooth inner surface of a clean mold which was put into the tablet press in the next step and stayed under 20 MPa for 2 min to get a translucent plate. In this way, we obtained infrared spectrum scan results of EPS samples and created the graphics with a Fourier transform infrared spectrometer (Thermo, USA).
Hydrogen Bonding Displacement of Monosaccharides
Ten milligrams of isolated and lyophilized sample powders were put into 500 μL deuteroxide and added in the nuclear magnetic tube with sealing with a film (Parafilm, USA) to reduce volatilization. The hydrogen bond displacement of polysaccharides was detected by the Bruker AVANCEIII 600 MHz spectrometer (Bruker, Rheinstetten, Germany).
Characteristics of Bacterial Surface
The adhesive capacity of bacterial surface in biofilms was assessed by atomic force microscopy. Biofilms cultured on slides for 24 h were gently washed by PBS and then dried at room temperature. A cantilever probe (HYDRA-ALL-G-20; AppNANO, USA) was positioned over the center of a bacterium, and the measurement of adhesion between the bacterial cell and the probe was carried out by the Shimadzu STM9700 system (Shimadzu, Japan) in the contact mode [Ivanov et al., 2011].
β-Glycosidic Bonds Verification Test
To verify the appearance of β-monosaccharide constituents, we used 4 U/mL (0.08 mg/mL) β-glucanase (Yuanye, China) solutions to treat the biofilms for different duration before assessing its biological characteristics. Meanwhile, sterile deionized water was used to treat the biofilms for the same duration as controls. β-glucanase is a kind of hydrolytic enzyme that acts exclusively on β-polysaccharide to degrade glycosidic bonds to produce low molecular saccharide polymers [Lu et al., 2009; Honma et al., 2018].
Observation of Biofilm Morphology
Structure of biofilms with the enzyme treatment for 1, 4, 8, 16, and 24 h or without the enzyme treatment were assessed by scanning electron microscopy (FEI, USA). After being incubated on glass slides, biofilms were gently washed twice by PBS and fixed with 2.5% glutaraldehyde for 4 h at 4°C in the dark, then serially dehydrated by ethanol solutions (30%, 50%, 75%, 85%, 95%, and 99%), and coated with gold. The image collection of each biofilm was done at 1,000, 5,000, and 20,000 magnifications.
Distribution of the EPS in Biofilms
To test the changes of distribution and proportion of EPS, the biofilms were treated with β-glucanase for 24 h during biofilm formation. The EPS in the biofilms was stained by Alexa Fluor 647 (Invitrogen, USA) with a final concentration of 1 μL/mL during cultivation, and the bacteria were labeled by 2.5 μM SYTO 9 green fluorescent stain (Invitrogen, USA). Microscopic observation was conducted by the confocal laser scanning microscopy (Olympus, Japan). A 3D reconstruction of the biofilms was analyzed by Imaris 7.2.3 (Bitplane, Switzerland), and quantification of the EPS or bacteria biomass was performed via ImageJ and COMSTAT 2.1 (Technical University of Denmark) [Yang et al., 2019].
Assessment of Biofilm Formation
The biomass of the biofilms was quantified using the crystal-violet (CV) microtiter assay. In brief, after incubation for 24 h, nonadherent cells and media components were gently removed from the biofilms which were subsequently stained with 0.1% CV (w/v) for 15 min. After being carefully rinsed by submerging the plates in a tank of water, 1 mL 33% acetic acid was added to extract the dye under gentle shake at 37°C for 5 min. Finally, the absorbance of the acetic acid was recorded in optical density of 575 nm.
Alteration of EPS Production
The biofilms were cultivated in 6-well microtiter plates and were treated with β-glucanase for 24 h before measuring the EPS content by using the anthrone method. EPS extraction from S. mutans biofilms was performed as before. All the EPS solutions were diluted by 0.2% anthrone-sulfuric acid reagent with the final concentration of 25% (v/v), then the mixed samples were blended, and incubated at 95°C for 6 min. After being cooled down on the ice, the absorbance of each sample was measured in an optical density of 625 nm.
Statistical Analyses
Statistical analysis was performed using SAS 9.3 and validation = 0.05. Data normality and the homogeneity of variance were tested by using the Shapiro-Wilk method and Bartlett method, respectively. If the data follow normal distribution and conform to the homogeneity of variance, Student’s test or 1-way ANOVA with Fisher’s least significant difference multiple comparison test was used to examine the statistical significance; otherwise, the Kruskal-Wallis analysis and Nemenyi test were used.
Results
β-Glycosidic Bonds in Monosaccharide Composition of EPS of S. mutans
The monosaccharide composition analysis was performed via GC-MS combined with IR and 1H-NMR. The profile of GC-MS showed us the monosaccharide components that the water-soluble EPS from S. mutans mainly contained glucose, mannose and galactose with various molar ratios, and the lowest proportion of Glc (49.61% ± 2.18%) was observed in water-soluble EPS of the UA159 compared to the Smurnc or Smurnc+ strain (Fig. 1a–c). Notably, there were rhamnose and Fuc, instead of Gal in the water-insoluble EPS of Smurnc compared to those from the UA159 and Smurnc+ strains (Fig. 1d–f). When compared with the Smurnc or Smurnc+ strain, Man was absent in the water-insoluble EPS of UA159 (Fig. 1d–f).
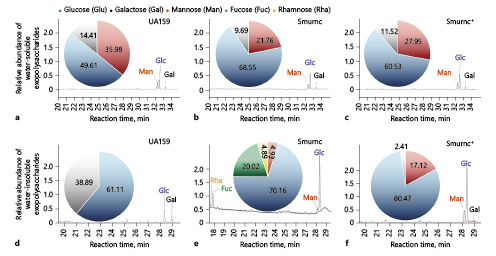
Fig. 1
Monosaccharide composition analysis of the EPS by GC-MS. a The GC/MS profile of the monosaccharide composition analysis showed water-soluble EPS in UA159 were Man, Glc, and Gal with a molar ratio of 35.98:49.61:14.41. b Water-soluble EPS in Smurnc were Man, Glc, and Gal with a molar ratio of 21.76:68.55:9.69. c Water-soluble EPS in Smurnc+ were Man, Glc, and Gal with a molar ratio of 27.95:60.53:11.52. d Water-insoluble EPS in UA159 were Glc and Gal with a molar ratio of 61.11:38.89. e Water-insoluble EPS in Smurnc were Rha, Fuc, Man, and Glc with a molar ratio of 4.89:20.02:4.93:70.16. f Water-insoluble EPS in Smurnc+ were Man, Glc, and Gal with a molar ratio of 17.12:80.47:2.41. EPS, exopolysaccharide; GC-MS, Gas chromatography-mass spectrometer; Smurnc, S. mutans rnc-deficient strain; Man, mannose; Fuc, fucose; Glc, glucose; Gal, galactose; Rha, rhamnose.
The profile of IR revealed the functional groups in EPS (Fig. 2a–f). The peaks at 3,378 cm−1, 3,420 cm−1, 3,406 cm−1, 3,407cm−1, and 3,438 cm−1 were the stretching vibration absorption peak of O-H [Mei et al., 2015], and the ones at 2,973 cm−1, 2,966 cm−1, 2,931 cm−1 and 2,935 cm−1 were the stretching vibration absorption peak of C-H [Li et al., 2011]. The peaks at 1,457 cm−1, 1,400 cm−1, and 1,342 cm−1 were the variable angle vibration of the C-H within the methyl and methylene [Du et al., 2016]. The peaks at 1,049 cm−1, 1,089 cm−1, and 1,042 cm−1 were caused by 2 kinds of C-O stretching vibrations, one of which was C-O-H, and the other was C-O-C of the pyranose ring [Wu et al., 2012]. The peak at 879 cm−1 and 880 cm−1 was caused by the variable angle vibration of epimeric C-H of β-end group, which usually presented the appearance of β-glycosidic bond (Fig. 2a, c, f) [Zhang et al., 2012]. The peaks at 836 cm−1 and 835 cm−1 were caused by the variable angle vibration of epimeric C-H of α-end group, which usually presented the appearance of α-glycosidic bond (Fig. 2b, d, e) [Zhao et al., 2005].
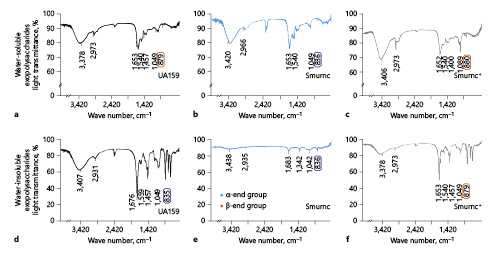
Fig. 2
β-Glycosidic bonds in monosaccharides of EPS was detected by IR. a The profile of IR showed water-soluble EPS had more β-glycosidic bonds (879 cm−1) in UA159. b More α-glycosidic bonds (836 cm−1) in Smurnc. c more β-glycosidic bonds (880 cm−1) in Smurnc+. d Water-insoluble EPS had more α-glycosidic bonds (835 cm−1) in UA159. e more α-glycosidic bonds (836 cm−1) in Smurnc. f More β-glycosidic bonds (879 cm−1) in Smurnc+. EPS, exopolysaccharide; IR, infrared radiation; Smurnc, S. mutans rnc-deficient strain.
Finally, we located hydrogen bond displacement (ppm) through the profile of 1H-NMR (Fig. 3). In this study, the most common lignans, such as Fuc, arabinose, xylose, Man, Glc, and Gal, in nature were selected as the control group, and deuterium water (δ4.7–4.8 ppm) was selected as the solvent according to solubility. The peaks of EPS in the UA159, Smurnc, or Smurnc+ were mainly distributed from δ4.5 to 5.5 ppm, and the results showed that there were more chemical shifts in the water-insoluble EPS of Smurnc than the UA159 and Smurnc+ strains.
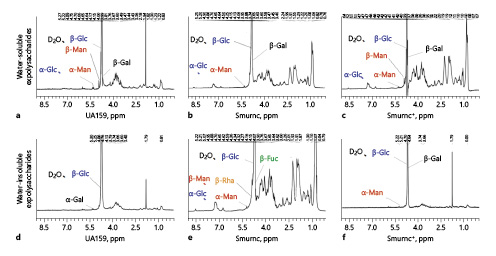
Fig. 3
Structural properties of the EPS of S. mutans were elucidated using 1H-NMR (600 MHz). a Combined with GC-MS and IR, the 1H-NMR spectrum demonstrated signal of β-Glc, β-Man, β-Gal, α-Glc, and α-Man in water-soluble EPS of UA159. b The signal of β-Glc, β-Gal, α-Glc, and α-Man in water-soluble EPS of Smurnc. c The signal of β-Glc, β-Gal, β-Man, α-Glc, and α-Man in water-soluble EPS of Smurnc+. d The signal of β-Glc and α-Gal in water-insoluble EPS of UA159. e The signal of α-Glc, α-Man, β-Glc, β-Man, β-Fuc, and β-Rha in water-insoluble EPS of Smurnc. f The signal of β-Glc, β-Gal, and α-Man in water-insoluble EPS of Smurnc+. EPS, exopolysaccharide; IR, infrared radiation; H-NMR, H nuclear magnetic resonance; GC-MS, Gas chromatography-mass spectrometer; Smurnc, S. mutans rnc-deficient strain; Man, mannose; Fuc, fucose; Glc, glucosse; Gal, galactose; Rha, rhamnose.
These aforementioned comprehensive analyses revealed the detailed micromolecular structure of EPS of S. mutans. We identified β-Glc, β-Man, and β-Gal in the water-soluble EPS of UA159 and Smurnc+, while there were α-Glc and α-Man in the water-soluble EPS from Smurnc (Fig. 1-3a–c). α-Configuration exists in the water-insoluble EPS of UA159 and Smurnc (α-Glc and α-Man) (Fig. 1-3d, e). Conversely, the primary constituents of the water-insoluble EPS from Smurnc+ were β-Glc and β-Gal (Fig. 1-3f). Taken together, the EPS of Smurnc was mainly composed of α-glycosidic bonds, whereas the β-glycosidic bonds were detected in the EPS of UA159 and Smurnc+, which implied that the deletion of rnc might affect the isomerization of glycosidic bonds in S. mutans.
Characteristics of the Biofilm Surface of S. mutans Were Associated with β-Glycosidic Bonds
The surface morphology and adhesion ability of UA159, Smurnc, and Smurnc+ were evaluated and quantitated by atomic force microscopy. Bacteria are evenly wrapped by abundant extracellular matrix in the UA159 biofilm (Fig. 4a, d). On the contrary, the deficiency of the rnc gene resulted in scanty EPS matrix and exposed bacterial cells in the biofilm, leading to the uneven biofilm surface compared to the UA159 (Fig. 4b, e), together with a significantly impaired adhesion force between the bacteria and the probe compared to that of the UA159 (p = 0.0026, Fig. 4g).
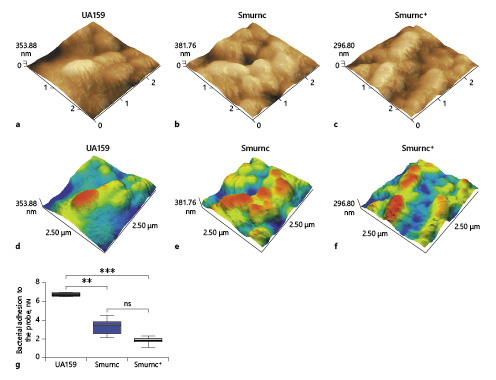
Fig. 4
Different characteristics of the biofilms surface were evaluated via AFM. a–f Bacteria are embraced by abundant extracellular matrixes in the UA159 biofilm, whereas the Smurnc biofilm had scanty EPS matrix and uneven surface. g The UA159 showed the highest average adhesive force (6.765 ± 0.159 nN) while the Smurnc showed lower average adhesive force (3.37 ± 0.72 nN). The Smurnc+ showed the lowest average adhesive force (1.85 ± 0.32 nN). Each sample was randomly taken 10 biological replicates and was presented as the mean ±SD. **p < 0.01, ***p < 0.001. AFM, atomic force microscopy; EPS, exopolysaccharide; Smurnc, S. mutans rnc-deficient strain; SD, standard deviation.
β-Glucosidic Bonds Existed in EPS and Participated in Modulating the Biofilms
To explore the biological functions of β-glucosidic bonds in EPS, we observed the morphology of EPS matrix treated with β-glucanase. When there was no enzyme treated, the bacterial cells were surrounded by the enriched EPS matrix to form the 3D structure in the UA159 biofilm; however, the biofilm of Smurnc had scattered microcolonies with decreased matrix compared to the UA159. Smurnc+ appeared to gather into condensed bacterial clusters, and the exopolysaccharide matrix in biofilm was less than UA159. Biofilms of both UA159 or rnc mutant strains seemed to be devoid of a distinct 3D structure and had a less condensed EPS matrix after enzyme digestion (Fig. 5), which suggested that β-glucosidic bonds may play a crucial role in the establishment of EPS architecture. To visualize. the productions and distributions of EPS in S. mutans biofilm, the double labeling of both bacteria (green) and EPS (red) was applied. Quantitative biomass calculation showed that UA159 and Smurnc+ produced more EPS than Smurnc in enzyme-untreated biofilms, but there was not any significant difference in bacterial biomass between those groups (Fig. 6d); this is in line with the results of crystal-violet staining which affirmed that the biofilm formation of Smurnc became diminished when compared to the UA159 or Smurnc+, primarily due to the restrained synthesis of EPS (Fig. 6e). EPS derived from S. mutans and the biofilm biomass was prominently attenuated after β-glucanase treatment for 24 h (Fig. 6a–e). This finding was further confirmed by anthrone method analysis which demonstrated that the β-glucanase treated biofilms displayed conspicuously reduced EPS compared to the untreated groups, and both UA159 and Smurnc+ exhibited more pronounced decrement ratio than Smurnc (Fig. 6f, p < 0.05).
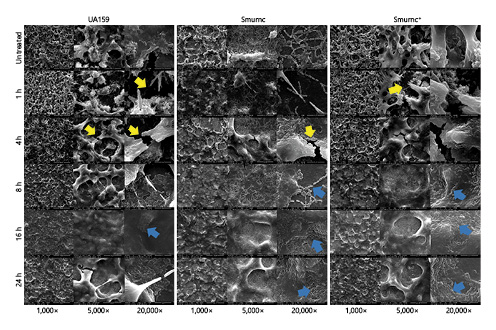
Fig. 5
Dynamics of the morphology of S. mutans biofilms treated with β-glucanase. SEM observations of the S. mutans biofilms which lacked of obvious 3-D structure after enzyme treatment, along with more crannies (yellow arrows) and less compacted EPS matrixes (blue arrows). All images were taken at ×1,000 (scale bars, 100 μm), ×5,000 (scale bars, 20 μm), and ×20,000 (scale bars, 5 μm) magnifications, respectively. S. mutans, Streptococcus mutans; 3D, dimensional; SEM, scanning electron microscopy; Smurnc, S. mutans rnc-deficient strain.
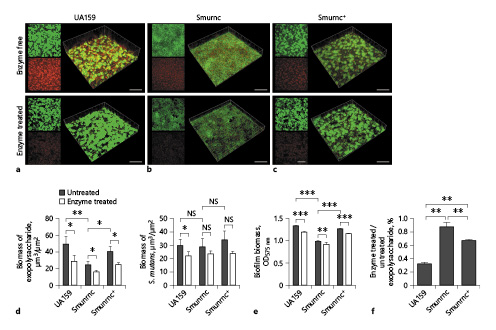
Fig. 6
Changes of the biomass and EPS productions in S. mutans biofilms after β-glucanase treatment. a–c Confocal laser scanning microscopy images of 24-h biofilms of S. mutans. Green, total bacteria (SYTO 9); red, EPS (Alexa Fluor 647). d Quantitative data of bacterial and EPS biomass in the S. mutans biofilms. e Volume of S. mutans biofilms were detected by crystal violet microtiter assay. EPS derived from S. mutans and biofilm biomass were prominently attenuated after β-glucanase treatment. f Anthrone method analysis demonstrated that the β-glucanase-treated biofilms displayed significantly reduced EPS. Results were averaged from 3 independent cultures of different strains, and the data are presented as mean ±SD. *p < 0.05, **p < 0.01, ***p < 0.001. The scale bars of all images were 50 μm. EPS, exopolysaccharide; S. mutans, Streptococcus mutans; SD, standard deviation; Smurnc, S. mutans rnc-deficient strain.
Discussion
EPS derived from S. mutans is a vital virulence factor involved in the pathogenesis of dental caries due to the ability of facilitating the 3D scaffold structure and promoting adhesion of biofilms [Koo et al., 2013; Ellepola et al., 2017]. In the previous research studies, we found that the rnc gene may promote the synthesis and the rearrangement of the structure of EPS, which thus enhances the cariogenicity of S. mutans [Mao et al., 2016, 2018]. Here, our study provided new insights that the rnc gene modulated the conversion of β-glycosidic bonds, which may be essential in regulating the micromolecule structure of extracellular polymeric substances, thus being conducive to the biofilm growth of S. mutans.
For first time, we investigated the effects of rnc on the microstructure of EPS from S. mutans. There were complex regulatory pathways in lactose/galactose catabolism in S. mutans, which affected its cariogenicity and the ability to compete with other species in microecosystem [Abranches et al., 2004; Lei et al., 2020]. The Smurnc mutants lost Gal in water-insoluble polysaccharide and had less proportion of Gal in water-soluble polysaccharide than the UA159 and Smurnc+ (Fig. 1). From this point of view, the variation of Gal metabolism in Smurnc may associate with its attenuated cariogenicity in vitro and in vivo [Mao et al., 2016, 2018]. Contrarily, a larger amount of Glc was found in the water-soluble polysaccharide of Smurnc (Fig. 1b). This phenomenon possibly related to the high expression of a catabolic enzyme gene dexA in Smurnc, which enabled more water-soluble glucans to be degraded and transferred into Glc [Florez Salamanca and Klein, 2018; Mao et al., 2018]. Moreover, the low adhesion ability of Smurnc and Smurnc+ might be relevant to the appearance of Man in water-insoluble EPS (Fig. 4g) [Brambilla et al., 2016]. The different content of the water-insoluble EPS between the UA159 and Smurnc+ may be related to the disparity of post-transcriptional modulation by rnc which was up-regulated in Smurnc+ [Mao et al., 2018]. Additionally, the regulation of homeostasis in S. mutans by the whole genome was possibly disturbed by the exogenous plasmid vector carrying rnc gene, leading to the different characteristics of EPS of Smurnc+ in different conditions compared to the UA159 [Mao et al., 2016].
Glycosidic bonds of monosaccharides were recognized to exist as α- or β-diastereomers [Elferink et al., 2018]. Currently, research studies on EPS of S. mutans mostly focus on the α-polysaccharide-related factors, such as glucosyltransferases [Kim et al., 2018; Lozano et al., 2019; Zhang et al., 2020]. However, we detected β-monosaccharides in the extracellular matrix of UA159 and Smurnc+, whereas both water-soluble EPS and water-insoluble EPS from Smurnc mainly consisted of α-monosaccharides (Fig. 2, 3). According to the probability that α-glycosidic bonds and β-glycosidic bonds of monosaccharides can be converted to each other through epimerization and deoxygenation [Paudel et al., 2013; Giner et al., 2016; Kang et al., 2017], we conjectured that the rnc gene was able to modulate the transformation between α- and β-glycosidic bonds of EPS. The profile of 1H-NMR showed that Smurnc displayed more chemical shifts in the water-insoluble EPS than those from the UA159 or Smurnc+ strains, revealing an essential role of rnc in the displacement of hydrogen bonds (Fig. 3).
We further verified the existence of β-glycosidic bonds in EPS of S. mutans by β-glucanase, which is a kind of hydrolytic enzyme with the property to cleave glucans with β-linkages [Wu et al., 2020; Ye et al., 2020]. After enzyme treatment, the matrix and biomass of S. mutans biofilms became thin and plain with a decrease in the amount of EPS (Fig. 5,6). All the changes in morphology and quantity of EPS were attributed to the degradation of β-glucans [Li et al., 2020], indicating that the composition of EPS from S. mutans comprised glycosidic bonds of β-type which participated in the formation of biofilm.
The β-glycosidic bonds possess greater stability than α-glycosidic bonds owing to the difference in configuration caused by the relative position of hydroxyl and hydrogen at C-1 [Pletikapic et al., 2014; Zhou et al., 2019]. Therefore, isomerization between α-monosaccharides and β-monosaccharides in the EPS may result in changes of biological characteristics of S. mutans. A decrease in β-monosacchrides induced by rnc knockout was accompanied by the attenuated bacterial adhesion force (Fig. 4g), planar structure (Fig. 5), lessened EPS, and reduced biomass of the Smurnc biofilm (Fig. 6), which coincides with our previous investigations that the rnc-deficient strain has weak biofilm-forming ability and cariogenicity [Mao et al., 2016, 2018]. Moreover, the occurrence of β-glucosidic bonds in the polysaccharide exposed that there was potentially β-glycosyl transferase systems in S. mutans, modulating the synthesis of EPS with α-glycosyl transferase collectively. On the one hand, it is suggested that the rnc gene may assist in the hydrolysis of sucrose to conduce to the production of β-glucosidic bonds. On the other hand, rnc was supposed to play a pivotal role in the EPS biosynthesis via the regulatory effects on β-monosaccharides.
In conclusion, the present results demonstrated that deficiency of rnc could alter not only the variety and proportion but also the configuration of monosaccharide in the extracellular matrix by regulating the isomerization between α/β-glycosyl. It was speculated that the rnc gene plays a regulatory role in the environmental adaptation of S. mutans via modulating the β-glycosidic bonds configuration of monosaccharide in the matrix of biofilms. Therefore, β-glycosidic bonds may be new potential micromolecule targets to inhibit EPS synthesis and biofilm formation of S. mutans, providing an alternative avenue for caries prevention and treatment. Furthermore, other than focusing on gene-level research studies, the structural analysis of EPS may be another effective approach to explore the virulence of S. mutans.
Statement of Ethics
The study is exempt from ethical committee approval because there were no experiments involving human or animal subjects.
Conflict of Interest Statement
The authors have no conflicts of interests to declare.
Funding Sources
This work was supported by the National Natural Science Foundation of China (No. 81771068, 81670980, 81800964, and 81900988) and Sichuan Provincial Natural Science Foundation of China (Grant No. 2020YFH0010 and 2018SZ0125). The funding sources had no role in the study design, data acquisition, and analysis or preparation of the manuscript.
Author Contributions
Tao Hu and Yingming Yang designed the experiments, revised, and finalized the manuscript; Yangyu Lu, Hongyu Zhang, and Meng Li performed the experiments, analyzed the data, and wrote and finalized the manuscript; and Lei Lei, Mengying Mao, Jiaqi Song and Yalan Deng contributed to data collection and analysis. All authors gave final approval and agreed to be accountable for all aspects of the work.
Data Availability Statement
All data analysed during this study are included in this published article. More details are available from the corresponding authors on reasonable request.
References
- 1. Abranches J, Chen YY, Burne RA. Galactose metabolism by Streptococcus mutans. Appl Environ Microbiol. 2004 Oct;70(10):6047–52. http://dx.doi.org/10.1128/AEM.70.10.6047-6052.2004.
- 2. Aguado LC, Schmid S, May J, Sabin LR, Panis M, Blanco-Melo D, et al. RNase III nucleases from diverse kingdoms serve as antiviral effectors. Nature. 2017 Jul 6;547(7661):114–7. http://dx.doi.org/10.1038/nature22990.
- 3. Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. http://dx.doi.org/10.1159/000324598.
- 4. Brambilla E, Ionescu AC, Cazzaniga G, Ottobelli M, Samaranayake LP. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation. J Basic Microbiol. 2016 May;56(5):480–92. http://dx.doi.org/10.1002/jobm.201500329.
- 5. Castillo Pedraza MC, de Oliveira Fratucelli ED, Ribeiro SM, Florez Salamanca EJ, da Silva Colin J, Klein MI. Modulation of lipoteichoic acids and exopolysaccharides prevents Streptococcus mutans biofilm accumulation. Molecules. 2020 May 9;25(9):2232. http://dx.doi.org/10.3390/molecules25092232.
- 6. Court DL, Gan J, Liang YH, Shaw GX, Tropea JE, Costantino N, et al. RNase III: Genetics and function; structure and mechanism. Annu Rev Genet. 2013;47:405–31. http://dx.doi.org/10.1146/annurev-genet-110711-155618.
- 7. Du Z, Shi F, Liu D, Ye H, Surhio MM, Li J, et al. Anticoagulant activity of a sulfated Lachnum polysaccharide in mice with a state of hypercoagulability. Bioorg Med Chem Lett. 2016;26(22):5550–6. http://dx.doi.org/10.1016/j.bmcl.2016.09.079.
- 8. Ebisu S, Kato K, Kotani S, Misaki A. Structural differences in fructans elaborated by Streptococcus mutans and Strep. salivarius. J Biochem. 1975;78(5):879–87. http://dx.doi.org/10.1093/oxfordjournals.jbchem.a130993.
- 9. Elferink H, Severijnen ME, Martens J, Mensink RA, Berden G, Oomens J, et al. Direct experimental characterization of glycosyl cations by infrared ion spectroscopy. J Am Chem Soc. 2018;140(19):6034–8. http://dx.doi.org/10.1021/jacs.8b01236.
- 10. Ellepola K, Liu Y, Cao T, Koo H, Seneviratne CJ. Bacterial GtfB augments Candida albicans accumulation in cross-kingdom biofilms. J Dent Res. 2017 Sep;96(10):1129–35. http://dx.doi.org/10.1177/0022034517714414.
- 11. Florez Salamanca EJ, Klein MI. Extracellular matrix influence in Streptococcus mutans gene expression in a cariogenic biofilm. Mol Oral Microbiol. 2018 Apr;33(2):181–93. http://dx.doi.org/10.1111/omi.12212.
- 12. Fujii M, Sato Y, Ito H, Masago Y, Omura T. Monosaccharide composition of the outer membrane lipopolysaccharide and O-chain from the freshwater cyanobacterium Microcystis aeruginosa NIES-87. J Appl Microbiol. 2012 Oct;113(4):896–903. http://dx.doi.org/10.1111/j.1365-2672.2012.05405.x.
- 13. Giner JL, Feng J, Kiemle DJ. NMR tube degradation method for sugar analysis of glycosides. J Nat Prod. 2016 Sep 23;79(9):2413–7. http://dx.doi.org/10.1021/acs.jnatprod.6b00180.
- 14. Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44(2):331–84. http://dx.doi.org/10.1128/mr.44.2.331-384.1980.
- 15. Honma K, Ruscitto A, Sharma A. β-glucanase activity of the oral bacterium tannerella forsythia contributes to the growth of a partner species, Fusobacterium nucleatum, in Cobiofilms. Appl Environ Microbiol. 2018;84(1):e01759–17. http://dx.doi.org/10.1128/AEM.01759-17.
- 16. Ivanov IE, Kintz EN, Porter LA, Goldberg JB, Burnham NA, Camesano TA. Relating the physical properties of Pseudomonas aeruginosa lipopolysaccharides to virulence by atomic force microscopy. J Bacteriol. 2011 Mar;193(5):1259–66. http://dx.doi.org/10.1128/JB.01308-10.
- 17. Kang KB, Ryu J, Cho Y, Choi S-Z, Son M, Sung SH. Combined application of UHPLC-QTOF/MS, HPLC-ELSD and 1H-NMR spectroscopy for quality assessment of DA-9801, a standardised dioscorea extract. Phytochem Anal. 2017 May;28(3):185–94.
- 18. Kim D, Liu Y, Benhamou RI, Sanchez H, Simón-Soro Á, Li Y, et al. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018 Jun;12(6):1427–42. http://dx.doi.org/10.1038/s41396-018-0113-1.
- 19. Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013 Dec;92(12):1065–73. http://dx.doi.org/10.1177/0022034513504218.
- 20. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018 Dec;16(12):745–59. http://dx.doi.org/10.1038/s41579-018-0089-x.
- 21. Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods. 2002;49:193–205. http://dx.doi.org/10.1016/s0167-7012(01)00369-4.
- 22. Lei L, Stipp RN, Chen T, Wu SZ, Hu T, Duncan MJ. Activity of Streptococcus mutans VicR is modulated by antisense RNA. J Dent Res. 2018 Dec;97(13):1477–84. http://dx.doi.org/10.1177/0022034518781765.
- 23. Lei L, Yang Y, Mao M, Li H, Li M, Yang Y, et al. Modulation of biofilm exopolysaccharides by the Streptococcus mutans vicX gene. Front Microbiol. 2015;6:1432. http://dx.doi.org/10.3389/fmicb.2015.01432.
- 24. Lei L, Zhang B, Mao M, Chen H, Wu S, Deng Y, et al. Carbohydrate metabolism regulated by antisense vicR RNA in cariogenicity. J Dent Res. 2020 Feb;99(2):204–13. http://dx.doi.org/10.1177/0022034519890570.
- 25. Li G, Chen S, Wang Y, Xue Y, Chang Y, Li Z, et al. A novel glycosaminoglycan-like polysaccharide from abalone Haliotis discus hannai Ino: purification, structure identification and anticoagulant activity. Int J Biol Macromol. 2011 Dec 1;49(5):1160–6. http://dx.doi.org/10.1016/j.ijbiomac.2011.09.017.
- 26. Li Z, Dong Y, Xiao X, Zhou XH. Mechanism by which β-glucanase improves the quality of fermented barley flour-based food products. Food Chem. 2020 May 1;311:126026. http://dx.doi.org/10.1016/j.foodchem.2019.126026.
- 27. Lim B, Sim M, Lee H, Hyun S, Lee Y, Hahn Y, et al. Regulation of Escherichia coli RNase III activity. J Microbiol. 2015 Aug;53(8):487–94. http://dx.doi.org/10.1007/s12275-015-5323-x.
- 28. Lozano CP, Díaz-Garrido N, Kreth J, Giacaman RA. Streptococcus mutans and Streptococcus sanguinis expression of competition-related genes, under sucrose. Caries Res. 2019;53(2):194–203. http://dx.doi.org/10.1159/000490950.
- 29. Lu Y, Wang T-H, Ding X-L. Induction of production and secretion β(1→4) glucanase with Saccharomyces cerevesiae by replacing the MET10 gene with egl1 gene from Trichoderma reesei. Lett Appl Microbiol. 2009 Dec;49(6):702–7.
- 30. Maeda T, Tanaka Y, Takemoto N, Hamamoto N, Inui M. RNase III mediated cleavage of the coding region of mraZ mRNA is required for efficient cell division in Corynebacterium glutamicum. Mol Microbiol. 2016 Mar;99(6):1149–66. http://dx.doi.org/10.1111/mmi.13295.
- 31. Mao MY, Li M, Lei L, Yin JX, Yang YM, Hu T. The regulator gene rnc is closely involved in biofilm formation in Streptococcus mutans. Caries Res. 2018;52(5):347–58. http://dx.doi.org/10.1159/000486431.
- 32. Mao MY, Yang YM, Li KZ, Lei L, Li M, Yang Y, et al. The rnc gene promotes exopolysaccharide synthesis and represses the vicRKX gene expressions via microRNA-size small RNAse in Streptococcus mutans. Front Microbiol. 2016;7:687. http://dx.doi.org/10.3389/fmicb.2016.00687.
- 33. Mei Y, Zhu H, Hu Q, Liu Y, Zhao S, Peng N, et al. A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohydr Polym. 2015 Jun 25;124:90–7. http://dx.doi.org/10.1016/j.carbpol.2015.02.009.
- 34. Paudel L, Adams RW, Király P, Aguilar JA, Foroozandeh M, Cliff MJ, et al. Simultaneously enhancing spectral resolution and sensitivity in heteronuclear correlation NMR spectroscopy. Angew Chem Int Ed Engl. 2013 Oct 25;52(44):11616–9. http://dx.doi.org/10.1002/anie.201305709.
- 35. Pletikapic G, Lannon H, Murvai U, Kellermayer MSZ, Svetlicic V, Brujic J. Self-assembly of polysaccharides gives rise to distinct mechanical signatures in marine gels. Biophys J. 2014 Jul 15;107(2):355–64.
- 36. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–9. http://dx.doi.org/10.1016/S0140-6736(07)60031-2.
- 37. Senadheera MD, Guggenheim B, Spatafora GA, Huang YC, Choi J, Hung DC, et al. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol. 2005 Jun;187(12):4064–76. http://dx.doi.org/10.1128/JB.187.12.4064-4076.2005.
- 38. Synytsya A, Novák M. Structural diversity of fungal glucans. Carbohydr Polym. 2013 Jan 30;92(1):792–809. http://dx.doi.org/10.1016/j.carbpol.2012.09.077.
- 39. Wen ZT, Nguyen AH, Bitoun JP, Abranches J, Baker HV, Burne RA. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol Oral Microbiol. 2011 Feb;26(1):2–18. http://dx.doi.org/10.1111/j.2041-1014.2010.00581.x.
- 40. Wu CY, Zhou J, Long F, Zhang W, Shen H, Zhu H, et al. Similar hypoglycemic effects of glucomannan and its enzyme degraded products from Amorphophallus albus on type 2 diabetes mellitus in mice and potential mechanisms. Food Funct. 2020 Nov;11(11):9740–51. http://dx.doi.org/10.1039/d0fo02434a.
- 41. Wu F, Yan H, Ma X, Jia J, Zhang G, Guo X, et al. Comparison of the structural characterization and biological activity of acidic polysaccharides from Cordyceps militaris cultured with different media. World J Microbiol Biotechnol. 2012 May;28(5):2029–38. http://dx.doi.org/10.1007/s11274-012-1005-6.
- 42. Yang Y, Mao M, Lei L, Li M, Yin J, Ma X, et al. Regulation of water-soluble glucan synthesis by the Streptococcus mutans dexA gene effects biofilm aggregation and cariogenic pathogenicity. Mol Oral Microbiol. 2019 Apr;34(2):51–63. http://dx.doi.org/10.1111/omi.12253.
- 43. Ye SQ, Zou Y, Zheng QW, Liu YL, Li RR, Lin JF, et al. TMT-MS/MS proteomic analysis of the carbohydrate-active enzymes in the fruiting body of Pleurotus tuoliensis during storage. J Sci Food Agric. 2021 Mar 101;101(5):1879–91. http://dx.doi.org/10.1002/jsfa.10803.
- 44. Zeng L, Chen L, Burne RA. Preferred hexoses influence long-term memory in and induction of lactose catabolism by Streptococcus mutans. Appl Environ Microbiol. 2018;84(14):e00864–18. http://dx.doi.org/10.1128/AEM.00864-18.
- 45. Zhang A, Chen J, Gong T, Lu M, Tang B, Zhou X, et al. Deletion of csn2 gene affects acid tolerance and exopolysaccharide synthesis in Streptococcus mutans. Mol Oral Microbiol. 2020 Oct;35(5):211–221.
- 46. Zhang Y, Dai L, Kong X, Chen L. Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus. Int J Biol Macromol. 2012 Oct;51(3):259–65. http://dx.doi.org/10.1016/j.ijbiomac.2012.05.003.
- 47. Zhao G, Kan J, Li Z, Chen Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr Polym. 2005;61(2):125–31. http://dx.doi.org/10.1016/j.carbpol.2005.04.020.
- 48. Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: structure, bioactivity and associations: a review. Carbohydr Polym. 2019 Mar 1;207:317–32. http://dx.doi.org/10.1016/j.carbpol.2018.11.093.
Yangyu Lu, Hongyu Zhang, and Meng Li contributed equally to this work.

