Introduction
Intracerebral hemorrhage (ICH) accounts for 15–20% of all strokes and belongs to the cerebrovascular diseases with the highest early mortality rate (up to 40%) [-]. Different surgical techniques including microsurgical and endoscopic procedures as well as minimally invasive surgical techniques with the application of recombinant tissue plasminogen activator (rtPA) have been already evaluated as treatment options for patients with spontaneous ICH [-]. Hematoma evacuation via craniotomy in patients with deep-seated as well as lobar hematomas was evaluated in 2 randomized controlled trials (STICH, STICH II) and failed to improve functional outcome at 6-month follow-up compared to conservative treatment alone [, ]. Minimally invasive surgery for hematoma evacuation by application of rtPA with subsequent clot lysis has been extensively investigated in the last years; this therapy facilitates a faster hematoma volume reduction compared to best medical treatment alone [, -]. The efficacy of this technique and its impact on patients’ long-term outcome have been assessed in a randomized controlled study (MISTIE III) which showed a significantly lower mortality rate in the patient group with fibrinolytic therapy compared to the patient group that received best medical treatment only []. Unfortunately, the MISTIE trial was not able to show a significant difference in functional outcome (good functional outcome defined as modified Rankin Scale [mRS] 0–3) 365 days after the bleeding event []. Nevertheless, 80% of all survivors in the MISTIE trial were living at home or in rehabilitation after 365 days, which would rather justify a more aggressive treatment of ICH patients instead of a nihilistic attitude of the treating physicians []. Hence, it can be assumed that a subgroup of ICH patients does benefit from minimally invasive surgical treatment. A definition of reliable selection criteria is of great clinical interest to facilitate better identification of the ICH patients, who are expected to benefit from fibrinolytic therapy. Since no such selection criteria have been established yet, minimally invasive surgical treatment is currently deployed based on individual decision making.
While doing this, a meticulous selection of patients is necessary to avoid the continuation of life-extending measures in patients with most severe primary brain injury inevitably leading to death despite maximal treatment. A reliable estimation of 30-day mortality might be supportive in the process of adequate patient selection for minimally invasive surgical treatment. As the treatment decision in ICH patients usually has to be made at admission, reliable prognostic factors available at admission are needed to proceed to surgery on time, given that surgery is considered indicated. Hemphill et al. [] developed the so-called ICH-score considering 5 known prognostic factors available at admission (age, clinical status, intraventricular hemorrhage, ICH location, and hematoma volume) with the aim to reliably predict the 30-day mortality in conservatively treated ICH patients. In this study, we evaluated the reproducibility of the ICH-score for the estimation of 30-day mortality in ICH patients undergoing fibrinolytic therapy to facilitate a more reliable identification of ICH patients who could benefit from minimally invasive surgery with subsequent clot lysis.
Patients and Methods
Clinical Setting and Patient Population
The data of consecutive patients with spontaneous ICH who underwent fibrinolytic therapy at our department from 2010 to 2016 were retrospectively analyzed. None of the included patients had additional external ventricular drainage. The included patients were treated exclusively with fibrinolytic therapy, and none of the patients had additional hematoma evacuation via craniotomy. The medical records of the included patients were reviewed, and the data concerning the patient characteristics, the hematoma-related data, and the mortality data were documented. A complete data could be gathered for all included patients. The ICH was diagnosed by unenhanced computed tomography (CT) scan within 24 h after symptom onset. In lobar hematomas, secondary bleeding causes such as vascular pathologies were ruled out by additionally performing a CT angiography. The catheter placement into the hematoma was performed using the neuronavigation. The fibrinolytic therapy with rtPA (Actilyse® Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany) was applied on 1–3 consecutive days starting with the day of ICH diagnosis. In every patient, a CT scan was performed postoperatively to confirm an intrahematomal catheter position, followed by injection of rtPA and clamping of the drainage for 30 min. The drainage was then reopened, and the liquefied hematoma was drained against a negative pressure gradient. A CT scan was repeated 24 h after the first rtPA application. In case of relevant residual hematoma and still intrahematomal catheter position, rtPA injection was repeated, followed by a CT scan 24 h later and a third rtPA injection if needed. The rtPA dosage was calculated based on the hematoma volume with 1 mg per 1 cm hematoma diameter as described before []. The 30-day mortality was retrospectively evaluated. Then, the ICH-score was applied to match the mortality of our patients with the mortality predicted by the ICH-score. The ICH-score (range 1–5) is based on parameters available at the time of ICH diagnosis: age (≤80 years = 0 vs. >80 years = 1), hematoma volume (≤30 mL = 0 vs. >30 mL = 1), intraventricular expansion (no = 0, yes = 1), and initial clinical status according to the Glasgow Coma Scale (GCS) score (GCS 3–4 = 2, GCS 5–12 = 1, and GCS 13–15 = 0). The ICH-score predicts the following mortality rates in conservatively treated patients with ICH: 0 = 0%, 1 = 13%, 2 = 26%, 3 = 72%, 4 = 97%, 5 = 100%.
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 []. An Ethics Committee approval was obtained for this study (Number 15/9/18). Due to the retrospective study design, an informed consent was waived.
Statistical Analysis
For the statistical analysis statistics software, GraphPad Prism Version 7.0 was used. Descriptive analysis was used to depict the patient characteristics. To evaluate the correlation of 30-day mortality with the individual prognostic parameters and with the ICH-score, a Chi-square test was applied with a calculation of ORs and 95% CI. The considered significance level was <0.05.
Results
Patient Population
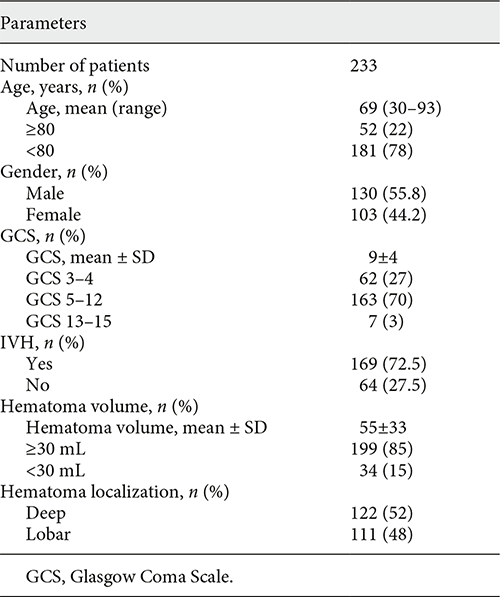
A total of 233 patients with ICH treated by fibrinolytic therapy were analyzed. The mean age of the patients was 69 years (range 30–93), 130 (55.8%) were male and 103 (44.2%) female. Mean GCS at admission was 9 ± 4. The mean initial hematoma volume was 55 ± 33 mL, whereas an extension to the ventricular system (intraventricular hemorrhage, IVH) was present in 169 (72.5%) of the patients. In 122 patients (52%), the hematoma was deep-seated, and 111 patients (48%) had a lobar hematoma. The patients’ characteristics are summarized in Table 1.
The 30-Day Mortality Dependent on the Parameters Included in the ICH-Score
The 30-day mortality rate was 30% (70/233 patients). An age of ≥80 years was associated with a significantly higher 30-day mortality rate (OR 2.26, 95% CI 1.14–4.50, chi-square test p = 0.01). A hematoma volume of ≥30 mL led significantly more often to 30-day mortality compared to hematomas with <30 mL (OR 3.72, 95% CI 1.18–13.03, chi-square test p = 0.01). The 30-day mortality was significantly higher in the patients with IVH compared to the patients without IVH (OR 2.97, 95% CI 1.34–6.73, chi-square test p = 0.003).
ICH-Score
The distribution of the ICH-score in our cohort was as followed: ICH-score 1 was found in 5.6% (13/233), ICH-score 2 in 21.9% (51/233), ICH-score 3 in 35.2% (82/233), ICH-score 4 in 13.3% (31/233), and ICH-score 5 in 24% (56/233).
The ICH-score was significantly associated with 30-day mortality. The mortality rate increased with an increasing score (chi-square test p < 0.0001). The ICH-score estimated the following 30-day-mortality rates in our cohort: 1 = 0% (0/13), 2 = 0% (0/51), 3 = 1.3% (1/82), 4 = 43% (13/31), and 5 = 100% (56/56). Compared to the estimated 30-day mortality by applying the ICH-score in the conservatively treated ICH patient cohort by Hemphill et al. [], the 30-day mortality in the ICH patients treated with fibrinolytic therapy was 13% lower if the ICH-score was 1, 26% lower if the ICH-score was 2, 70.7% lower if the ICH-score was 3, 54% lower if the ICH-score was 4, and unchanged if the ICH-score was 5. Figure 1 shows a comparison of the distribution of the ICH-score in our study cohort with the distribution of the ICH-score in the original study cohort.
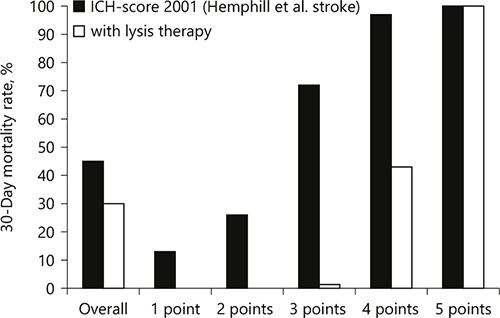
Fig. 1
Comparison of the estimated 30-day mortality rates in our study with the reported 30-day mortality in the study of Hemphill et al. []. ICH, intracerebral hemorrhage.
Discussion
In this study, we retrospectively applied the ICH-score as a prognostic tool in a patient population treated with fibrinolytic therapy and estimated the 30-day mortality in comparison to the reported mortality of conservatively treated ICH patients by Hemphill et al. []. The 30-day mortality was significantly lower in all ICH-grades except for ICH-grade 5. The overall 30-day mortality in our patient population was 30%, which was significantly lower compared to the reported mortality rate of 45% in the conservatively treated patients in the study of Hemphill et al. [] Considering the results of our study, the ICH-score might be a helpful tool for patient selection for fibrinolytic therapy: patients with an ICH-scores 1–4 seem to be suitable candidates for fibrinolytic therapy.
In contrast to the original ICH-score (range 0–6) in conservatively treated patients, the ICH-score in our study had a range from 1 to 5, because fibrinolytic therapy was not performed in patients with infratentorial ICH and with small supratentorial ICH. The other parameters (age, mean GCS) of the patient population in our study were comparable with the study cohort in the original ICH-score paper.
The ICH-score was elaborated using a logistic regression model to detect independent outcome predictors. The GCS was the strongest early mortality predictor and therefore represents the main part of the ICH-score. This is in line with the data of our study. The cutoff of 30 mL hematoma volume was defined by Broderick et al. [] who described it as a strong predictor of 30-day mortality with high sensitivity and specificity (96 and 98%, respectively) when combined with GCS, which was then validated in the ICH-score []. For the hematoma volume assessment, the ABC/2 formula (a formula for ellipsoids with A for the greatest hematoma diameter, B for the diameter 90° to A and C for the approximate number of CT slices multiplied by the slice thickness) based on initial CT scan was used, which is easy to perform in an emergency setting. Although the accuracy of this method was shown in previous studies [], the ABC/2 formula bears the risk of hematoma volume overestimation, which increases in irregularly shaped hematomas, cerebellar, or brain stem hematomas []. An overestimation of the hematoma volume might lead to an overprediction of 30-day mortality in some patients. Webb et al. [] evaluated the ABC/2 formula in the cohort of the 3 randomized trials on ICH (MISTIE III, CLEAR III, and CLEAR IVH) in comparison to a computer-assisted hematoma volume measurement and attested this method a sufficient accuracy to guide treatment decisions under consideration of its limitations in large and irregular hematomas. The consideration of age as a prognostic factor is a matter of discussion. On one side, a higher age implicates a higher risk for multimorbidity and a higher age is assumed to be associated with lower recovery capacity after brain injury. On the other side, there are interindividual differences between the patients at same age concerning comorbidities. Therefore, putting a cutoff age is methodologically difficult. However, in the ICH-score, a cutoff age of 80 years was a significant outcome predictor.
The ICH-score has been validated in several retrospective studies including different ICH patient populations which confirmed its robustness and reliability for predicting the 30-day mortality after acute ICH [, ]. In the context of mortality estimation, a possible impact of the decision of medical support withdrawal has to be discussed. An early do-not-resuscitate decision is independently associated with higher short- and long-term mortality []. On the other hand, the decision for withdrawal of treatment is often made as a consequence of failure to improve and when dead was considered inevitable, as a post hoc analysis of the INTERACT2 study has revealed []. Hemphill et al. [] have also prospectively evaluated the correlation of the ICH-score with the 1-year functional outcome according to the mRS and showed that an increasing ICH-score is related to a lower likelihood of favorable outcome. An additional important finding was that the ability of ICH-score to predict functional outcome was not affected by the withdrawal of support []. Indeed, due to the retrospective nature of the study, we cannot exclude a possible impact of the withdrawal of therapy on the mortality rate. But, even if the decision of therapy-withdrawal has had an impact on the mortality in our study, this would consequently mean that the real mortality rate (without withdrawal therapy) could be even lower than the estimated mortality rate in our patent population.
A couple of modifications of the original ICH-score should be mentioned. Godoy et al. [] developed a modified ICH-score (mICH-score) by adding the Graeb’s score for the ascertainment of the amount and distribution of intraventricular hemorrhage and also by stratifying the parameters such as age, GCS, and hematoma volume into more subgroups. The mICH-score was not able to improve the prediction of 30-day mortality, but it was better in predicting the outcome at 6 months follow-up. The focus in our study was the mortality; therefore, the ICH-score and not the mICH-score was applied. Cheung and Zou [] have modified the original ICH-score by replacing the GCS by the National Institutes of Health Stroke Scale, which is an established outcome predictor in ischemic stroke. That score was slightly better in predicting good outcome (mRS ≤2) at 30 days compared to the original ICH-score and showed similar results concerning the 30-day mortality. The authors recommended using their score when the prediction of a good outcome is the main target [].
Up to now, effective treatment options for ICH are missing []. The extent of brain damage after ICH determines the prognosis and depends on the hematoma volume and its extension to the ventricular system, leading to the assessment of hematoma evacuation/volume reduction as a therapeutic target in several randomized controlled trials [, , , ]. When it comes to long-term functional outcome of the patients neither a hematoma evacuation via craniotomy nor a hematoma volume reduction by clot lysis had proven to be superior to the best medical treatment so far [, , , , ]. The most recently published MISTIE III trial, unfortunately, failed to show an improvement in functional outcome by fibrinolytic therapy according to mRS 365 days after the bleeding []. There are some possible explanations for these results: 1-The number of patients with lobar hematomas, which are deemed to have a better overall prognosis, was higher in the conservatively treated group compared to the MISTIE group, 2-the treatment aim of reaching an end-of-treatment hematoma volume of <15 mL could not be achieved in all patients (the mean end-of-treatment volume was 16 mL) []. According to the results of the MISTIE trial, fibrinolytic therapy cannot be recommended as a suitable treatment for all ICH patients. But still, a subgroup of patients might benefit from this minimally invasive treatment. Since the outcome of ICH patients remains poor and is far from being satisfactory, it is of great clinical interest to work on developing clinical tools, which could help to identify patients who would possibly benefit from fibrinolytic therapy. A definition of clear criteria for a reliable patients’ selection is essential to identify the patients who would profit from this treatment, which highlights, even more, the relevance of evaluating the ICH-score, which parameters are available at admission, as a tool for patient selection for fibrinolytic therapy.
Strengths and Limitations of the Study
The strength of our study is the relatively high number of patients treated with fibrinolytic therapy. Since the management of ICH patients at our center did not change during the study period, we can assume a data consistency considering fibrinolytic therapy, which is another strength of the study. The main limitation of our study is the retrospective nature of data collection.
Conclusion
The ICH-score not only allows a reliable estimation of the 30-day mortality in ICH patients treated conservatively but also in patients treated by clot lysis. Fibrinolytic therapy leads to lower 30-day mortality in patients with ICH-score 1–4 compared to the reported mortality rates in conservatively treated patients with supratentorial ICH. Patients with ICH-score 5 do not benefit from fibrinolytic therapy and should no longer be considered to be candidates for this treatment option. The use of the ICH-score in clinical practice might be a supportive tool during the decision-making process with the advantage of standardization of clinical decisions.
Future prospective studies are needed to show if a patient selection for fibrinolytic therapy based on the ICH-score would be able to achieve not only lower mortality but also an improvement in functional outcome of the patients compared to best medical treatment alone.
Statement of Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 []. The study was approved by the local Ethics Committee of the Georg-August-University Göttingen (number 15/9/18).
Disclosure Statement
I declare that I have no conflicts of interest. D.M., V.R., and B.I. declare that they have no conflicts of interest.
Funding Sources
This study was not funded or otherwise financially supported.
Author Contributions
V.M. has contributed to the conception and design of the work, analysis and interpretation of data for the work, drafting and critically revising the work for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and solved. B.I. has contributed to the acquisition, analysis and interpretation of data for the work. D.M. has contributed to interpretation of data for the work, for revising it critically for important intellectual content, and final approval of the version to be published. V.R. has contributed to the interpretation of data for the work, revising the manuscript for important intellectual content, for final approval of the version to be published, and for agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
References
- 1. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. <X00_Journal>J Stroke</X00_Journal>. 2017 Jan;19(1):3–10.
- 2. Lee JY, King C, Stradling D, Warren M, Nguyen D, Lee J, et al. Influence of hematoma location on acute mortality after intracerebral hemorrhage. <X00_Journal>J Neuroimaging</X00_Journal>. 2014 Mar-Apr;24(2):131–6.
- 3. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. <X00_Journal>Stroke</X00_Journal>. 2015 Jul;46(7):2032–60.
- 4. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al.; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. <X00_Journal>Lancet</X00_Journal>. 2005 Jan;365(9457):387–97.
- 5. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. <X00_Journal>Lancet</X00_Journal>. 2013 Aug;382(9890):397–408.
- 6. Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF. Surgical trials in intracerebral hemorrhage. <X00_Journal>Stroke</X00_Journal>. 2013 Jun;44(6 Suppl 1):S79–82.
- 7. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al.; MISTIE Investigators. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. <X00_Journal>Lancet Neurol</X00_Journal>. 2016 Nov;15(12):1228–37.
- 8. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al.; MISTIE III Investigators. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. <X00_Journal>Lancet</X00_Journal>. 2019 Mar;393(10175):1021–32.
- 9. Goyal N, Tsivgoulis G, Malhotra K, Katsanos AH, Pandhi A, Alsherbini KA, et al. Minimally invasive endoscopic hematoma evacuation vs best medical management for spontaneous basal-ganglia intracerebral hemorrhage. <X00_Journal>J Neurointerv Surg</X00_Journal>. 2019 Jun;11(6):579–83.
- 10. Yao Z, Hu X, You C, He M. Effect and feasibility of endoscopic surgery in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. <X00_Journal>World Neurosurg</X00_Journal>. 2018 May;113:348–356.e2.
- 11. Schaller C, Rohde V, Meyer B, Hassler W. Stereotactic puncture and lysis of spontaneous intracerebral hemorrhage using recombinant tissue-plasminogen activator. <X00_Journal>Neurosurgery</X00_Journal>. 1995 Feb;36(2):328–33.
- 12. Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G; Multicenter randomized controlled trial (SICHPA). Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). <X00_Journal>Stroke</X00_Journal>. 2003 Apr;34(4):968–74.
- 13. Thiex R, Rohde V, Rohde I, Mayfrank L, Zeki Z, Thron A, et al. Frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. <X00_Journal>J Neurol</X00_Journal>. 2004 Dec;251(12):1443–50.
- 14. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. <X00_Journal>Acta Neurochir Suppl (Wien)</X00_Journal>. 2008;105:147–51.
- 15. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al.; MISTIE Investigators. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. <X00_Journal>Stroke</X00_Journal>. 2013 Mar;44(3):627–34.
- 16. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. <X00_Journal>Stroke</X00_Journal>. 2001 Apr;32(4):891–7.
- 17. Millum J, Wendler D, Emanuel EJ. The 50th anniversary of the Declaration of Helsinki: progress but many remaining challenges. <X00_Journal>JAMA</X00_Journal>. 2013 Nov;310(20):2143–4.
- 18. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. <X00_Journal>Stroke</X00_Journal>. 1993 Jul;24(7):987–93.
- 19. Webb AJ, Ullman NL, Morgan TC, Muschelli J, Kornbluth J, Awad IA, et al.; MISTIE and CLEAR Investigators. Accuracy of the ABC/2 score for intracerebral hemorrhage: systematic review and analysis of MISTIE, CLEAR-IVH, and CLEAR III. <X00_Journal>Stroke</X00_Journal>. 2015 Sep;46(9):2470–6.
- 20. Huttner HB, Steiner T, Hartmann M, Köhrmann M, Juettler E, Mueller S, et al. Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. <X00_Journal>Stroke</X00_Journal>. 2006 Feb;37(2):404–8.
- 21. Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC 3rd. External validation of the ICH score. <X00_Journal>Neurocrit Care</X00_Journal>. 2004;1(1):53–60.
- 22. Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martínez JJ, González-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. <X00_Journal>Stroke</X00_Journal>. 2007 May;38(5):1641–4.
- 23. McCracken DJ, Lovasik BP, McCracken CE, Frerich JM, McDougal ME, Ratcliff JJ, et al. The intracerebral hemorrhage score: a self-fulfilling prophecy? <X00_Journal>Neurosurgery</X00_Journal>. 2019 Mar;84(3):741–8.
- 24. Muñoz Venturelli P, Wang X, Zahuranec DB, Lavados PM, Stapf C, Lindley R, et al.; INTERACT2 Investigators. Withdrawal of active treatment after intracerebral haemorrhage in the INTERACT2 study. <X00_Journal>Age Ageing</X00_Journal>. 2017 Mar;46(2):329–32.
- 25. Hemphill JC 3rd, Farrant M, Neill TA Jr. Prospective validation of the ICH Score for 12-month functional outcome. <X00_Journal>Neurology</X00_Journal>. 2009 Oct;73(14):1088–94.
- 26. Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: can modification to original score improve the prediction? <X00_Journal>Stroke</X00_Journal>. 2006 Apr;37(4):1038–44.
- 27. Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. <X00_Journal>Stroke</X00_Journal>. 2003 Jul;34(7):1717–22.
- 28. Williams JR. The Declaration of Helsinki and public health. <X00_Journal>Bull World Health Organ</X00_Journal>. 2008 Aug;86(8):650–2.