Introduction
Acute cerebral infarction of cardioembolic origin (mainly reported in patients with atrial fibrillation [AF] or patent foramen ovale) has been associated with lesions involving the cortex and with multiple lesions in single or multiple cerebral circulation territories on magnetic resonance imaging (MRI), although other studies have failed to find such association [-]. To the best of our knowledge, imaging characteristics in acute infarction patients with intracardiac thrombus (ICT) have never been reported.
Our aim was to analyse the acute infarction MRI pattern in patients with ICT and to compare them with stroke patients with AF. Recently, our group showed that small cortical cerebellar infarctions (SCCIs) were associated with acute infarction of cardioembolic origin [, ]. Therefore, in addition to acute infarction location, number, size, and volume, the presence of acute SCCI and non-SCCI was also assessed.
Methods
We performed a retrospective study (http://www.clinicaltrials.gov NCT04456309) analysing brain MRI scans of consecutive patients presenting with acute symptomatic cardioembolic infarction associated with ICT who were recruited and registered in the stroke database between June 2018 and November 2019 of our centre (Nîmes University Hospital, France). These patients were compared with consecutive cardioembolic stroke patients with known or newly discovered AF (in the absence of ICT on echocardiography), who were recruited during the same time period. Inclusion criteria were brain MRI (including diffusion-weighted imaging [DWI]) performed within 1 week of symptom onset; complete etiological work up including intracranial (time of flight, gadolinium-enhanced magnetic resonance angiography, or computed tomographic angiography) and extracranial (gadolinium-enhanced magnetic resonance angiography, computed tomographic angiography, or duplex ultrasound) vessel imaging; transthoracic or transoesophageal echocardiography; and ≥24-h ECG monitoring (acute stroke unit monitoring and/or Holter ECG monitoring). In our centre, work up for stroke patients includes transthoracic echocardiography in all patients (i.e., independent of the suspected underlying stroke cause).
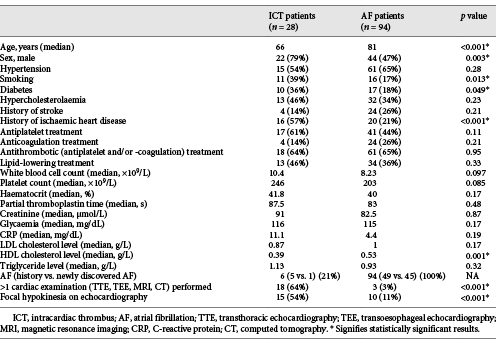
Analysed baseline characteristics were sex, age, common cardiovascular risk factors (arterial hypertension, diabetes, hypercholesterolaemia, and smoking), stroke history, and history of ischaemic heart disease. Biological characteristics on admission including white blood cell and platelet count, haematocrit, partial thromboplastin time, creatinine, glycaemia, C-reactive protein, and cholesterol and triglycerides levels were also assessed. The presence of focal hypokinesia on echocardiography was analysed, together with the number of cardiac examinations (transthoracic echocardiography, transoesophageal echocardiography, cardiac MRI, or computed tomography [CT]) performed. For the ICT patients, we assessed the examination modality showing the ICT (transthoracic echocardiography, transoesophageal echocardiography, cardiac MRI or CT), the thrombus size and localisation, the associated echocardiographic abnormalities, the presence of associated AF, and the time interval between brain MRI and ICT identification.
Brain MRI was performed with a 1.5T magnet (Ingenia, Philips, The Netherlands; DWI b-values =0 and 1,000 s/mm2, TR 4,280 ms, and TE 97 ms). In the case of technical problems of the 1.5T MRI scanner, a 3T magnet (Skyra; Siemens, Erlangen, Germany) was used. DWI parameters for both scanners were set as follows: field of view of 230 × 230 and 240 × 240 mm2, matrix size of 224 × 224 and 288 × 288, resolution equal to 1.02 × 1.02 × 6 and 0.88 × 0.88 × 5.5 mm3, 24 and 30 slices in each volume, repetition time of 3562.1 and 4285.4 ms, and echo time of 99.3 and 96.9 ms.
Analyses of acute infarction MRI characteristics were based on axial DWI and performed by an experienced rater (CT) blinded to clinical data and MRI sequences other than DWI. Analysed acute infarction characteristics were location (anterior, middle, or posterior cerebral artery territory; anterior, posterior, or mixed anterior-posterior circulation; presence of multiterritorial infarctions [mixed anterior-posterior circulation or bilateral anterior circulation]; brainstem; cerebellum; SCCI or non-SCCI in the case of cerebellar infarction; cortical, subcortical, or cortico-subcortical location), number of infarction lesions, size of subcortical lesion (< or >15 mm), and total infarction volume. An infarction lesion was considered as a separate lesion when a DWI lesion was clearly and completely separated from another DWI lesion by a brain area appearing normal on DWI. For the analysis of the total infarction volume on DWI, DICOM files for each subject were converted into an NIfTI format using MRIcro software []. The NIfTI files corresponding to DWI were anonymised before manual segmentation. The volume of segmented brain regions was computed under Analysis of Functional NeuroImages toolboxes by means of the number of voxels and respective voxel size []. Ethics approval was obtained according to local regulations, and informed consent was obtained from all patients. Categorical variables are expressed as counts and percentages and continuous variables as medians and interquartile range according to their distribution. Cerebral infarction MRI characteristic comparisons between patients with ICT versus patients with AF were assessed using the χ2 test or Fisher’s exact test for qualitative variables and the Wilcoxon-Mann-Whitney test for quantitative variables. Multivariate logistic regression was used to identify risk factors for ICT. Risk factors significant at the threshold of 10% in univariate analysis were retained in the multivariate model. Forward stepwise selection was performed to select risk factors at the threshold of 5%. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
We included 28 ICT and 94 AF patients. Baseline, biological, and cardiac characteristics of both groups are shown in Table 1. Compared with AF, ICT patients were younger (median age 66 vs. 81 years, p < 0.001), more frequently male (79 vs. 47%, p = 0.003), and smokers (39 vs. 17%, p = 0.013) and had more frequent history of diabetes (36 vs. 18%, p = 0.049) and ischaemic heart disease (57 vs. 21%, p < 0.001). Lower HDL cholesterol levels (median 0.39 vs. 0.53 g/L, p < 0.001) were observed in the ICT group in the absence of other biological differences between both groups. Six of the 28 ICT patients (21%) had associated AF. ICT patients had more cardiac examinations (multiple examinations 64 vs. 3%, p < 0.001) and more frequent focal hypokinesia on echocardiography (54 vs. 11%, p < 0.001) than AF patients.
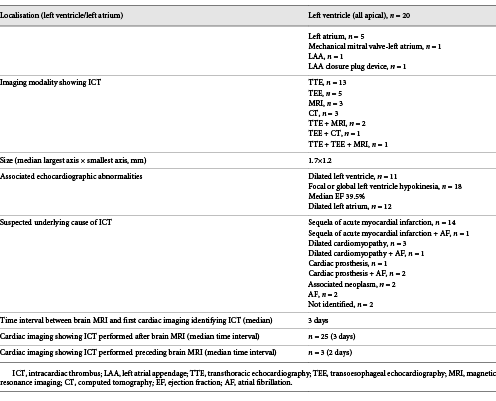
ICT characteristics are shown in Table 2. The ICT was located in the left ventricle in the majority of cases (71%), observed on transthoracic echocardiography in almost half of the cases (46%), and frequently associated with other echocardiographic abnormalities, with a median interval of 3 days between brain MRI and ICT identification. The suspected underlying cause of ICT was the sequela of acute myocardial infarction in half of the patients.
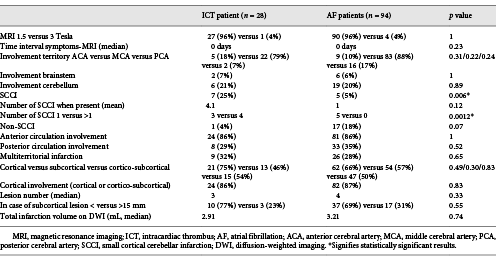
Acute infarction MRI characteristics are shown in Table 3. ICT patients showed more frequent SCCI (25 vs. 5%, p = 0.006) than the AF patients. For those with an SCCI presence, multiple SCCIs were more frequent than single SCCI (p = 0.0012) in the ICT group when compared with AF patients. In the ICT group, 5 out of 7 SCCI patients had multiple SCCIs (Fig. 1), whereas all 5 AF SCCI patients showed single SCCI. No other differences were observed between the groups for infarction localisation, number, size, or volume.
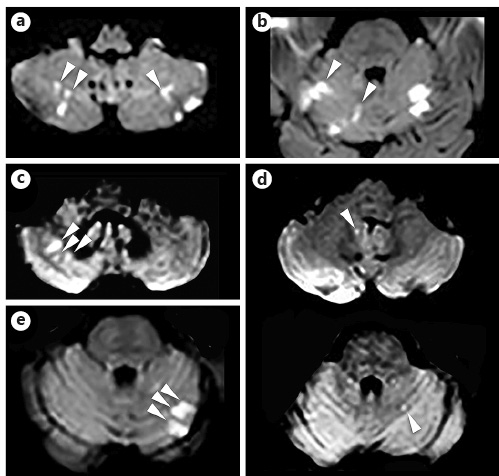
Fig. 1
DWI showing the presence of multiple SCCI (arrowheads) in 5 patients of the ICT group. ICT, intracardiac thrombus; DWI, diffusion-weighted imaging; SCCI, small cortical cerebellar infarction.
On multivariate analysis, younger age (p < 0.001), history of ischaemic heart disease (p < 0.001), and low HDL cholesterol levels (p = 0.01) were significantly associated with ICT. Results approaching statistical significance were observed for SCCI (more frequent in the ICT group, p = 0.053) and non-SCCI (more frequent in the AF group, p = 0.053) on MRI.
Since stroke risk is considered much higher in ICT than in AF, the 6 patients with both ICT and AF were included in the ICT group. We performed additional analyses taking into account only the 22 patients with ICT without AF and compared them with the 94 AF patients. Identical baseline, biological, and MRI parameters were significantly associated with ICT presence on uni- and multivariate analyses including the 6 patients with both ICT and AF in the ICT group. No additional differences in characteristics were observed in the ICT + AF (n = 6) versus isolated ICT (n = 22) or versus isolated AF (n = 94) group. All 7 SCCI patients in the ICT group were ICT patients without associated AF.
There was no significant association between lipid-lowering treatment and HDL levels (p = 0.6). Patients with a history of ischaemic heart disease and focal hypokinesia on echocardiography were slight younger but without statistical significance (median age 74 vs. 76, p = 0.5, and median age 70 vs. 75, p = 0.28, respectively).
Discussion
Our study showed that stroke patients with ICT, compared with AF patients, were younger, more frequently male, and smokers; had more frequent history of diabetes and ischaemic heart disease; and showed lower HDL cholesterol levels. On MRI, the only difference between the groups was the presence of more frequent SCCI in the ICT group.
Our study had several limitations: MRI analyses were retrospective, the vast majority of patients only had transthoracic echocardiography (especially in the AF group), and the groups had different numbers of cardiological examinations performed. The vast majority of AF patients and nearly half of ICT patients only had transthoracic echocardiography, probably leading to an underestimation of ICT identification, especially of left atrium and atrial appendage ICT, better visualised on transoesophageal echocardiography. The higher number of cardiac examinations performed in the ICT group was probably explained by the fact that these patients were younger and often known to have a history of associated ischaemic heart disease, leading to more extensive cardiac work up to identify possible ICT. In our study, a relative high proportion of ICT patients (compared with AF) was included probably because our hospital is a tertiary cardiological and neurological care centre for high-risk patients (including stroke patients with ICT), whereas classic AF-related stroke patients were generally managed in surrounding secondary care centres (with possibly minor differences in work-up and cardiac imaging for stroke patients). This probably created a selection bias in our study.
More frequent history of ischaemic heart disease and focal hypokinesia on echocardiography in the ICT group probably explained the association with male sex, smoking, and diabetes. Interestingly, the presence of ICT was associated with lower HDL cholesterol levels in our study, despite younger age. In earlier reports, experimental models have shown that low HDL cholesterol levels were associated with increased platelet-dependent thrombogenic potential and that in ST-segment elevation myocardial infarction patients, low HDL cholesterol levels were associated with thrombus presence on angiography and with high thrombus burden [-]. Low HDL cholesterol levels, especially in the presence of high LDL cholesterol levels, have been shown to be associated with increased platelet adhesion, activation, and aggregation [-]. This is due to modulatory effects of lipoproteins on platelets by altered cholesterol and phospholipid content of platelet membranes with downstream alteration in enzyme activities or receptor-mediated alteration of agonist-induced signal transduction pathways. However, the effect size and clinical significance of low HDL cholesterol levels as a risk factor for ICT have to be confirmed and determined in future studies.
SCCI, typically occurring in a distal arterial territory and referred to as end-territorial infarctions, has been recently reported to be associated with acute infarction of cardioembolic origin [, ]. In our study, SCCI was observed in one-quarter of the ICT patients. Since diffusion restriction on DWI persists for about 1 week on MRI, SCCI observed in the ICT patients in our study may represent asymptomatic infarction preceding the symptomatic infarction, leading to hospital admission by hours or days (related to the elevated embolic potential of ICT). Another hypothesis is that small thrombus fragments originating from the ICT accompany larger fragments and that SCCI represent these smaller thrombi destined to the cerebellum for rheological reasons or that they are more easily identified radiologically in the cerebellum (vs. supratentorial cortical microinfarctions due to the susceptibility artefacts from adjacent skull bone interfaces).
Identifying biomarkers for ICT is important because of the high risk of stroke recurrence in the case of ICT (especially well studied in patients with recent acute myocardial infarction, cardiomyopathy, and valvular heart disease), with also important consequences in regard to timing and choice of anticoagulation therapy []. Based on our study, early and extensive cardiac imaging has to be performed in search for ICT in stroke patients presenting with acute SCCI on brain MRI. Further studies are needed to identify in detail brain MRI characteristics in acute stroke patients at high risk for ICT in order to select patients acquiring extensive cardiac work up trying to identify these ICT associated with high stroke recurrence risk.
Acknowledgments
We would like to thank Dr. Sarah Kabani (Service de Biostatistique, Epidémiologie Clinique, Santé Publique et Innovation en Méthodologie (BESPIM), CHU de Nîmes, 4 Rue du Professeur Debré, 30029 Nîmes Cedex 09) for proofreading our manuscript.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The subjects have given their written informed consent. The Local Ethics Committee (Nîmes University Hospital, France) approved the study protocol and provided the reference number (Approval Number IRB 20.05.09).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
None of the authors received funding of any kind.
Author Contributions
All authors (all fulfilling the ICMJE criteria) contributed to the acquisition and analyses of data, and revised and agreed with the entire content of the manuscript.
References
- 1. Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60:1730–4.http://dx.doi.org/10.1001/archneur.60.12.1730.
- 2. Li SY, Yang XM, Zhao XQ, Liu LP, Wang YL, Jiang Y, et al. Newly detected atrial fibrillation is associated with cortex-involved ischemic stroke. Chin Med J. 2019;132:2053–8.http://dx.doi.org/10.1097/CM9.0000000000000390.
- 3. Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke. 2013;44:3350–6.http://dx.doi.org/10.1161/STROKEAHA.113.002459.
- 4. Azeem MU, Nagy M, Miller MM, Ghasemi M, Mikati A, Silver B, et al. Prevalence of a multiple territory stroke pattern after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2020;29:104700.http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2020.104700.
- 5. Bernstein RA, Di Lazzaro V, Rymer MM, Passman RS, Brachmann J, Morillo CA, et al. Infarct topography and detection of atrial fibrillation in cryptogenic stroke: results from CRYSTAL AF. Cerebrovasc Dis. 2015;40:91–6.http://dx.doi.org/10.1159/000437018.
- 6. Ter Schiphorst A, Tatu L, Thijs V, Demattei C, Thouvenot E, Renard D. Small obliquely oriented cortical cerebellar infarctions are associated with cardioembolic stroke. BMC Neurol. 2019;19:100.http://dx.doi.org/10.1186/s12883-019-1328-0.
- 7. Renard D, Ion I, Ricci JE, Mura T, Thouvenot E, Wacongne A. Chronic small cortical cerebellar infarctions on MRI are associated with patent foramen ovale in young cryptogenic stroke. Cerebrovasc Dis. 2020;49:105–9.http://dx.doi.org/10.1159/000505959.
- 8. Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47–56.http://dx.doi.org/10.1016/j.jneumeth.2016.03.001.
- 9. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73.http://dx.doi.org/10.1006/cbmr.1996.0014.
- 10. Naqvi TZ, Shah PK, Ivey PA, Molloy MD, Thomas AM, Panicker S, et al. Evidence that high-density lipoprotein cholesterol is an independent predictor of acute platelet-dependent thrombus formation. Am J Cardiol. 1999;84:1011–7.http://dx.doi.org/10.1016/s0002-9149(99)00489-0.
- 11. Wang P, Wang Y, Ma W, Li H, Chen H. High-density lipoprotein cholesterol and intracoronary thrombosis burden. Coron Artery Dis. 2013;24:1–5.http://dx.doi.org/10.1097/MCA.0b013e32835aab80.
- 12. Arısoy A, Altunkaş F, Karaman K, Karayakalı M, Çelik A, Ceyhan K, et al. Association of the monocyte to HDL cholesterol ratio with thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2017;23(8):992–7.http://dx.doi.org/10.1177/1076029616663850.
- 13. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et alAmerican Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236.