Introduction
Oral anticoagulant therapy (OAT) saves lives and prevents stroke-associated disability in patients with atrial fibrillation (AF). The novel non-vitamin K antagonist (VKA) oral anticoagulants (NOAC) have shown at least non-inferior efficacy compared to VKA in preventing ischemic strokes and a greater safety especially with regard to OAT-associated intracerebral hemorrhage (ICH) [] and are being used increasingly []. With the dabigatran-binding humanized antibody fragment idarucizumab [], a first specific antidote for NOAC has very recently been approved by the regulatory authorities and antidotes to the factor Xa inhibitors will follow suit. Availability of these antidotes will especially benefit 2 patient groups that pose serious problems in the emergency management of stroke patients. One is the group of patients with NOAC-associated ICH, the other one the group of patients presenting within the therapeutic time window for thrombolysis but reporting to be on active OAT with a NOAC. In the latter group, one could imagine that thrombolysis could be safe after administration of a fast-acting drug that induces complete reversal of the anticoagulant's activity. We set out to estimate the quantitative need of NOAC antidote doses on a typical stroke unit.
Methods
We retrieved data from a large prospective stroke inpatient quality assurance registry covering the entire federal state of Hesse in Germany (6,000,000 inhabitants). Data entry is compulsory by a federal contract and the registry achieves a nearly 100% completion, verified by administrative hospital data. We collected information on discharge diagnoses ICH or ischemic stroke (ICD-10: I61 or I63), age, sex, risk factors, known AF and OAT reported on admission for 3 subsequent years (2012-2015). Since the specific OAT drug was not recorded in the registry before 2015, we invited 10 stroke units with the highest patient volume (treating 867-1,855 patients with the discharge diagnoses of ischemic stroke, ICH and transitory ischemic attack in 2014). Eight of these 10 stroke units contributed to the study. Together, these 8 stroke units treated 44.8% of ischemic stroke patients and 52.2% of ICH patients in the state of Hesse in 2014. We reviewed patient charts for the actual OAT on admission. In 2015, the differentiation between VKA and NOAC became a mandatory item of the database, which allows us to provide information on the type of OAT of all stroke patients treated in the state of Hesse during these 12 months, circumventing the selection bias of being treated in a large hospital. The characteristics of stroke patients treated in the 8 large stroke units and the stroke patients that were treated in all other hospitals are given in table 1. Based on this information, we estimated the number of acute ischemic stroke and ICH patients suitable for anticoagulation reversal treatment.
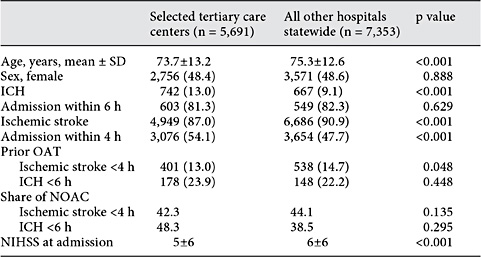
Table 1 Patient characteristics of acute ICH and ischemic stroke patients admitted in 2015 to 8 of the 10 largest stroke centers in the federal state of Hesse within 24 h of symptom onset in comparison to those patients who were treated at all other hospitals statewide
Results
Antidote Treatment in NOAC-Associated Hemorrhage
Incidence rate of hospitalized acute ICH (<24 h after symptom onset) in Hesse was 24.5/100,000 per year. Of these patients, 64% (16.0/100,000 per year) arrived within 6 h of symptom onset, and 18% (4.5/100,000 population) arrived with an unknown time point of symptom onset (fig. 1a). Incidence of ICH occurring under OAT with the patient hospitalized within 6 h or with an unknown time point of symptom onset was 4.5/100,000 per year. The proportion of OAT-associated ICH among all ICH patients rose from 2012 to 2015 (fig. 1b). Patients with OAT-associated ICH were significantly older (76.8 vs. 71.5 years, p < 0.001), more often had AF (18.2 vs. 13.8%, p = 0.002) and had a significantly higher burden of risk factors (online suppl. table 1; for all online suppl. material, see http://www.karger.com/doi/10.1159/000447952).
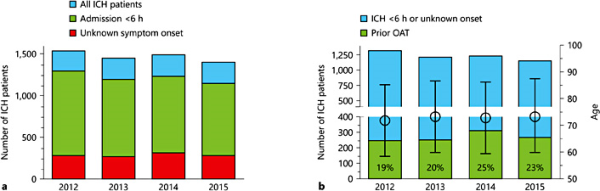
Fig. 1
a ICH patient numbers during the observation period. We included all patients with acute ICH (symptom onset <24 h). b Shares of OAT-associated ICH among ICH patients. OAT as reported by patient orally or via the medication list. Mean age and SD are given on the right y axis.
In the selected 8 stroke units, the proportion of NOACs rose from 7% in 2012 to 20% in 2013 among patients with anticoagulation-associated ICH. Given that the incidence of ICH occurring under OAT that is hospitalized within 6 h or with an unknown time point of symptom onset is 4.5/100,000 and all NOAC ICH patients will be treated with a specific antidote, 0.9 reversal treatments have to be expected if the NOAC share at admission is 20%. In 2015, the NOAC share among ICH patients hospitalized within 6 h or with unknown onset was 48% in the selected 8 stroke units and 39% in the ICH patients treated in all other hospitals in the federal state. The corresponding NOAC-associated ICH incidences for a NOAC share of 40 and 60% are 1.8 and 2.7/100,000 population, respectively. In other words, if the NOAC share among OAT-associated ICH is 40%, as it is currently the case in our federal state in Germany, a stroke unit serving a city of 200,000 inhabitants has to expect about 50 ICH cases per year and about 4 ICH-related NOAC reversals per year (7% of all hospitalized acute ICH patients).
While there is no high quality evidence for the efficacy of reversal of the coagulopathy to improve clinical outcomes, the need for an operation would be an absolute indication to use a reversal agent. Among the 120 cases of NOAC-associated ICH that were recorded in 2015 in our federal state, 20 patients (17%) underwent an operation (external ventricular drain (n = 13), decompressive surgery (n = 6), hematoma evacuation (n = 8) or a combination thereof).
Antidote Treatment Prior to Thrombolysis in Ischemic Stroke
So far, it is not yet established whether thrombolysis can be administered safely in anticoagulated patients who receive an antidote that immediately and completely reverses the anticoagulant's activity without pro-coagulant effects. However, patients who arrive in the hospital with functionally relevant stroke symptoms on OAT and are not suitable for thrombectomy (i.e., because of a lack of large vessel occlusion) cause a therapeutic dilemma that may in the future be overcome by the NOAC-specific antidote systems. Incidence rate of hospitalized acute (<24 h after symptom onset) ischemic stroke in Hesse was 198.2/100,000 per year. Of these patients, 47% (93.4/100,000 population per year) arrived within 4 h of symptom onset (fig. 2a). Incidence of ischemic stroke occurring under OAT in patients hospitalized within 4 h was 12.4/100,000 population per year without relevant changes throughout the observation period (fig. 2b). Patients who presented on OAT were significantly older (77.1 vs. 73.1 years, p < 0.001) and had a higher prevalence of known AF (32.1 vs. 19.9%, p < 0.001) but did not differ significantly in sex and vascular risk factors (online suppl. table 2). Thrombolysis rate in the non-anticoagulated patients arriving within 4 h of symptom onset was 37% (30.4/100,000 population). Interestingly, also 14% (1.8/100,000 population) of anticoagulated patients arriving within 4 h received thrombolysis, probably according to the guideline-based practice to thrombolyse patients with an INR <1.7 or patients on NOAC who report an interval since last drug intake >24 h. This led to an overall thrombolysis rate of 34% of all patients arriving within the 4-hour time window.
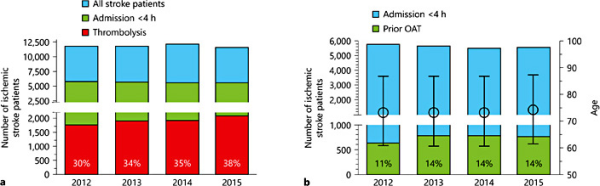
Fig. 2
a Ischemic stroke patient numbers during the observation period. We included all patients with acute stroke (symptom onset <24 h). Thrombolysis rates are given as percentage of patients admitted within the therapeutic time window (admission <4 h after symptom onset). b Share of thrombolysis candidates reporting OAT. OAT as reported by patient orally or via the medication list. Mean age and SD are given on the right y axis.
In the selected 8 stroke units, the proportion of NOACs rose from 6% in 2012 to 26% in 2013 among the patients with ischemic stroke admitted on anticoagulation. Given that the incidence of ischemic stroke occurring under OAT in patients hospitalized within 4 h of symptom onset is 12.4, and 1.8/100,000 hereof are already thrombolysed despite anticoagulation, 10.6/100,000 remain as potential candidates for thrombolysis after NOAC reversal. Assuming that the thrombolysis rate in non-anticoagulated patients in the 4-hour time window of 37% also applies to this subgroup, this will lead to 1 additional tissue plasminogen activator (t-PA) treatment per 100,000 per year if the NOAC share at admission is 25% (3.4% increase in overall thrombolysis rate). In 2015, the NOAC share among thrombolysis candidates hospitalized within 4 h was 42% in the selected 8 stroke units and 44% in the thrombolysis candidates treated in all other hospitals in the federal state. The corresponding values for a NOAC share of 40 and 60% are 1.6/100,000 (5.4% increase) and 2.4/100,000 population (8.2% increase), respectively. In other words, if the NOAC share is 50%, which will probably soon be a realistic scenario, a stroke unit serving a city of 200,000 inhabitants has to expect about 60 t-PA treatments per year and up to 4 additional t-PA treatments after NOAC reversal per year (1% of all hospitalized acute ischemic stroke cases) if the principle of reversing NOAC-OAT prior to thrombolysis appears safe, and alternative therapies, especially mechanical thrombectomy, are not feasible.
Discussion
The proportion of patients reporting to be on OAT when admitted for ischemic stroke or ICH was not negligible in our patient sample. The proportion of NOAC intake in patients admitted with an ICH or ischemic stroke on active OAT rose steeply from below 10% in 2012 to approximately 45% in 2015 and has probably not reached a steady state yet, rendering the acute stroke treatment of these patients a frequent problem for neurologists and emergency care physicians. In the REVERSE-AD trial assessing the safety and reversal capacity of idarucizumab for dabigatran reversal, 18 of the 51 patients with life-threatening bleeding under dabigatran presented with an ICH as qualifying event [].
To date, there is no evidence from randomized controlled trials proving that normalization of coagulation parameters in patients with OAT-associated ICH is effective in terms of ameliorating patient outcomes. However, current guidelines acknowledge that in view of the dire clinical course of VKA-associated ICH in comparison to ICH in patients with intact coagulation [], it is current practice to do so and provide recommendations for the treatment of VKA-associated ICH []. A recent report on 61 patients with NOAC-associated ICH from the RASUNOA-pilot registry shows that this entity also has a poor prognosis with high mortality and a high rate of hematoma expansion []. Whether specific reversal agents can alter the course of the disease is not yet clear. Since doubts have been voiced on the feasibility of sufficiently powered randomized controlled trials to compare treatment regimens for OAT-associated ICH [], data from registries such as the ongoing RASUNOA-prime (NCT02533960), NOACISP (NCT02353585) and RADOA (NCT01722786) registries will most likely provide the most valid data on this issue. Retrospective observations stress the importance of the factor time in OAT reversal of VKA-associated ICH []. Patients with NOAC-associated ICH should also receive the antidote as early as possible after symptom onset. The need for speed collides with the time-consuming process of elaborated coagulation assays to assess NOAC activity. Point-of-care testing (POCT) of NOAC activity will probably be available within a few years. In the meantime, it does not seem reasonable to await the results of non-routine coagulation assays before administering the antidote. Due to the high cost of the novel specific reversal agents and the assumption that a relevant proportion of ICH patients with reported NOAC intake probably will not have full anticoagulant activity when admitted to the hospital and therefore not require OAT reversal, POCT systems will probably be cost-effective.
Currently, thrombolysis is largely withheld from patients reporting NOAC intake on admission. Even though experimental data from translational animal experiments [,,], several case reports and a large study pooling data from different thrombolysis registries [] insinuate that patients on NOAC appear to be at a potentially lower risk for thrombolysis-associated ICH than patients treated with VKA OATs, thrombolysis of patients exposed to OAT is certainly not standard practice. We show that NOAC use among patients admitted with ischemic stroke is clearly on the rise, not only in patients with newly diagnosed AF but also in stroke patients reporting OAT on admission. The second part of our analysis is founded on the assumption that it may be feasible to reverse the NOAC-induced coagulopathy by a specific antidote in patients suffering a stroke under OAT and subsequently administer thrombolysis. This approach has not yet been systematically studied in humans, neither for VKA nor for NOACs. We could show in translational animal experiments that administration of prothrombin complex concentrate prior to t-PA treatment reduces hemorrhagic transformation in VKA-anticoagulated mice subjected to middle cerebral artery occlusion []. A very recent first case report of a patient receiving t-PA treatment under dabigatran anticoagulation after OAT reversal with idarucizumab showed no hemorrhagic complications and hinted at a positive thrombolytic effect [].
We need to make it clear that we present hypothetical models based on assumptions. While both approaches appear well-founded on pathophysiological considerations, it is neither known whether OAT reversal of a NOAC by a specific antidote will improve clinical outcome after NOAC-associated ICH nor whether the application of a fast-acting antidote will allow the safe thrombolysis of patients who suffer an ischemic stroke on active NOAC anticoagulation. These questions will have to be addressed by registries in the future and their results may impact on the numbers of required antidote doses. Concerning ICH, some hematomas will be deemed fatal after the initial brain imaging and active treatment may be withheld from these patients in the context of a change in therapy goals to a palliative care setting. This should also be considered when interpreting our estimates. We are aware that the assumptions underlying our analysis are a moving target. For example, endovascular thrombectomy, which is probably safe in patients with NOAC use, is on the rise and the marketing of POCT systems may allow the identification of patients who are not exposed to therapeutic NOAC levels. Medication errors are frequent under NOAC treatment []. Different NOACs with upcoming antidotes are competing for market shares. Currently, trials are underway to assess NOACs in secondary prevention after cryptogenic stroke. They may be used for stroke prevention in cervical artery dissection and cerebral venous sinus thrombosis []. If NOACs prove to be effective for these entities as well, the prevalence of anticoagulation may rise even further.
Currently, stroke specialists are hoping for a broad availability of appropriate antidotes. With the marketing of the first specific antidote, they may be pondering the future quantitative need for antidote doses. Our calculations are based on the assumptions that all patients with ICH of acute (<6 h) or unclear onset and all ischemic stroke patients arriving in the time window who are eligible for thrombolysis (currently 37% in the non-anticoagulated patients) will receive a NOAC antidote. These assumptions yield the maximal antidote demand. From our data, we can extrapolate that a stroke unit serving a population with a comparable demographic structure should prepare to administer NOAC antidotes to up to 1% of all hospitalized acute ischemic stroke patients and 7% of all hospitalized acute ICH patients.
Disclosure Statement
D.F., D.N., B.I., C.H., M.A., S.T., A.R. and B.M. report no conflicts of interest. W.P. has received research funding from Novartis Pharma and honoraria from Bayer Healthcare and Boehringer Ingelheim. A.S. has received funding and honoraria from Bayer HealthCare, Boehringer Ingelheim, Desitin Arzneimittel, Eisai, Pfizer, Sage Therapeutics and UCB Pharma. G.H. received travel grants from Boehringer Ingelheim und Bayer HealthCare. T.N.-H. received speaker's honoraria from Boehringer Ingelheim, Bayer, Pfizer and a travel grant from Bayer. O.C.S. has received honoraria from Bayer Healthcare and Pfizer and research funding from Bayer Healthcare. A.F. received speaker's honoraria from Boehringer Ingelheim, Bayer, Pfizer, Daiichi Sankyo. T.S. received speaker's honoraria from Boehringer Ingelheim, Bayer, Pfizer, Daiichi Sankyo. H.S. received honoraria from Bayer, Boehringer Ingelheim and Sanofi, as well as from Elsevier, Springer and Thieme Publishers. C.F. has received funding and a travel grant from Boehringer Ingelheim.
References
- 1. Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ: Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012;110:453-460.
- 2. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma C, Zint K, Elsaesser A, Bartels DB, Lip GY; GLORIA-AF Investigators: Antithrombotic treatment patterns in patients with newly diagnosed nonvalvular atrial fibrillation: the GLORIA-AF registry, phase II. Am J Med 2015;128:1306-1313.e1.
- 3. Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI: Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-520.
- 4. Flaherty ML, Haverbusch M, Sekar P, Kissela BM, Kleindorfer D, Moomaw CJ, Broderick JP, Woo D: Location and outcome of anticoagulant-associated intracerebral hemorrhage. Neurocrit Care 2006;5:197-201.
- 5. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M; European Stroke Organisation: European stroke organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014;9:840-855.
- 6. Purrucker JC, Haas K, Rizos T, Khan S, Wolf M, Hennerici MG, Poli S, Kleinschnitz C, Steiner T, Heuschmann PU, Veltkamp R: Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol 2016;73:169-177.
- 7. Flaherty ML, Adeoye O, Sekar P, Haverbusch M, Moomaw CJ, Tao H, Broderick JP, Woo D: The challenge of designing a treatment trial for warfarin-associated intracerebral hemorrhage. Stroke 2009;40:1738-1742.
- 8. Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Jüttler E, Grau A, Palm F, Röther J, Michels P, Hamann GF, Hüwel J, Hagemann G, Barber B, Terborg C, Trostdorf F, Bäzner H, Roth A, Wöhrle J, Keller M, Schwarz M, Reimann G, Volkmann J, Müllges W, Kraft P, Classen J, Hobohm C, Horn M, Milewski A, Reichmann H, Schneider H, Schimmel E, Fink GR, Dohmen C, Stetefeld H, Witte O, Günther A, Neumann-Haefelin T, Racs AE, Nueckel M, Erbguth F, Kloska SP, Dörfler A, Köhrmann M, Schwab S, Huttner HB: Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824-836.
- 9. Pfeilschifter W, Bohmann F, Baumgarten P, Mittelbronn M, Pfeilschifter J, Lindhoff-Last E, Steinmetz H, Foerch C: Thrombolysis with recombinant tissue plasminogen activator under dabigatran anticoagulation in experimental stroke. Ann Neurol 2012;71:624-633.
- 10. Sun L, Zhou W, Ploen R, Zorn M, Veltkamp R: Anticoagulation with dabigatran does not increase secondary intracerebral haemorrhage after thrombolysis in experimental cerebral ischaemia. Thromb Haemost 2013;110:153-161.
- 11. Ploen R, Sun L, Zhou W, Heitmeier S, Zorn M, Jenetzky E, Veltkamp R: Rivaroxaban does not increase hemorrhage after thrombolysis in experimental ischemic stroke. J Cereb Blood Flow Metab 2014;34:495-501.
- 12. Seiffge DJ, Hooff RJ, Nolte CH, Béjot Y, Turc G, Ikenberg B, Berge E, Persike M, Dequatre-Ponchelle N, Strbian D, Pfeilschifter W, Zini A, Tveiten A, Næss H, Michel P, Sztajzel R, Luft A, Gensicke H, Traenka C, Hert L, Scheitz JF, De Marchis GM, Bonati LH, Peters N, Charidimou A, Werring DJ, Palm F, Reinhard M, Niesen WD, Nagao T, Pezzini A, Caso V, Nederkoorn PJ, Kägi G, von Hessling A, Padjen V, Cordonnier C, Erdur H, Lyrer PA, Brouns R, Steiner T, Tatlisumak T, Engelter ST; NOACISP Study Group: Recanalization therapies in acute ischemic stroke patients: impact of prior treatment with novel oral anticoagulants on bleeding complications and outcome. Circulation 2015;132:1261-1269.
- 13. Pfeilschifter W, Spitzer D, Czech-Zechmeister B, Steinmetz H, Foerch C: Increased risk of hemorrhagic transformation in ischemic stroke occurring during warfarin anticoagulation: an experimental study in mice. Stroke 2011;42:1116-1121.
- 14. Schäfer N, Müller A, Wüllner U: Systemic thrombolysis for ischemic stroke after antagonizing dabigatran with idarucizumab - a case report. J Stroke Cerebrovasc Dis 2016;pii:S1052-3057(16)30061-1.
- 15. Pfeilschifter W, Luger S, Brunkhorst R, Lindhoff-Last E, Foerch C: The gap between trial data and clinical practice - an analysis of case reports on bleeding complications occurring under dabigatran and rivaroxaban anticoagulation. Cerebrovasc Dis 2013;36:115-119.
- 16. Caprio FZ, Bernstein RA, Alberts MJ, Curran Y, Bergman D, Korutz AW, Syed F, Ansari SA, Prabhakaran S: Efficacy and safety of novel oral anticoagulants in patients with cervical artery dissections. Cerebrovasc Dis 2014;38:247-253.
